1 Introduction
Pyrazoles derivatives are synthetic targets of utmost importance in the pharmaceutical industry, since such a heterocyclic moiety represents the core structure of numerous drugs. Furthermore, recent reports indicate of a number of highly potent inhibitors of coagulation factors Xa [1]. A number of compounds containing the pyrazole core have been examined for antidepressant activity through screening against monoamine oxidases [2], treatment of obesity as cannabinoid-1 antagonists [3], antiviral activity against the West Nile virus [4], and multidrug resistance modulators in tumor cells [5]. Pyrazoles are extremely powerful reagents for the construction of nitrogen containing substance [6]. The 1,3-dipolar cycloaddition reaction is a classical and widely used method for the construction of 2-pyrazolines [7]. The oxidation of 2-pyrazoline, is, in fact, the pyrazolenine which applies to many synthetic strategies. A large number of procedures are found in the literature to accomplish this fundamental setup. Several reagents are available for oxidation of the 2-pyrazolines, the nature of the product depending on the choice of oxidant and the substitution pattern of the substrate. Various efforts have been made previously in the oxidation of 2-pyrazolines with a variety of reagents including Zr(NO3)4 [8], Pd/C [9], Co(II) and oxygen [10], iodobenzene diacetate [11], lead tetraacetate [12], MnO2 [13], potassium permanganate [14] and NBS [15], for the preparation of pyrazolenines. The combination of dimethylsulfoxide (DMSO) with an electrophilic species to form “activated DMSO” [16] has been widely exploited for the oxidation of alcohols to their respective carbonyl compounds. However, many of these methods are subject to certain drawbacks such as longer reaction times, low yields and toxicity due to the presence of some elements embodied in the reagents utilized. So still there is need for development of new catalysts which overcome all these drawbacks.
2 Results and discussion
The reaction of dienes 1a–d and 2-diazopropane furnishes the 1-pyrazoline 3a–d, which undergoes spontaneous tautomerization to afford the 2-pyrazoline 4a–d in good yield (Scheme 1) [17].
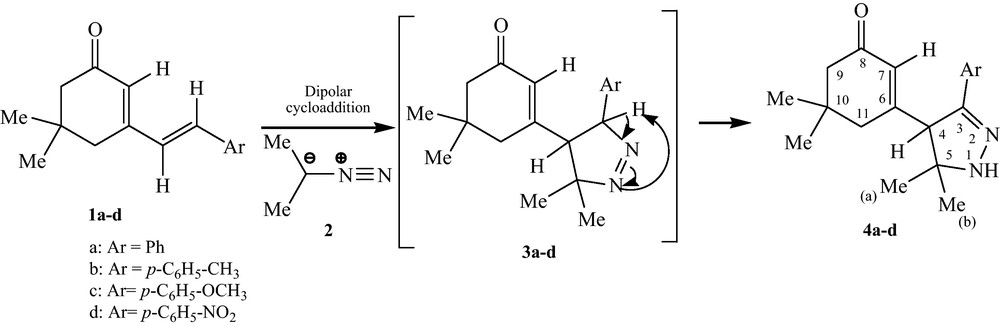
Preparation of 2-pyrazolines.
The FAB mass and two-dimensional NMR spectra of these adducts are identical and correspond to diastereoisomeric structures indiscernible by spectroscopic analyses. However, regiochemical assignments of all adducts were deduced from their HMBC 2D-NMR spectra. H-7 and H-11 protons correlate only with the carbon atom C-4 (58.9–59.5 ppm). Also, methyl protons (a) and (b) correlate only with two carbon atoms C-4 and C-5 and each other suggesting that they are directly linked to the quaternary carbon C-5. In a similar manner, aromatic protons correlate with the carbon C-3 which is directly bonded to the aryl group. Consequently, this latter correlation shows Ar-C3-C4-C5-(Me(a),Me(b)) linkages indicative of a “inverse” regiochemistry which is generally observed in 1,3-dipolar cycloaddition reactions of simple diazoalkanes with α,β-unsaturated ketones [18].
On the other hand the same reaction is carried out under similar operating conditions with α,β-unsaturated ketones 5 has led to a 2-pyrazoline cycloadduits 6 (Scheme 2) [19].
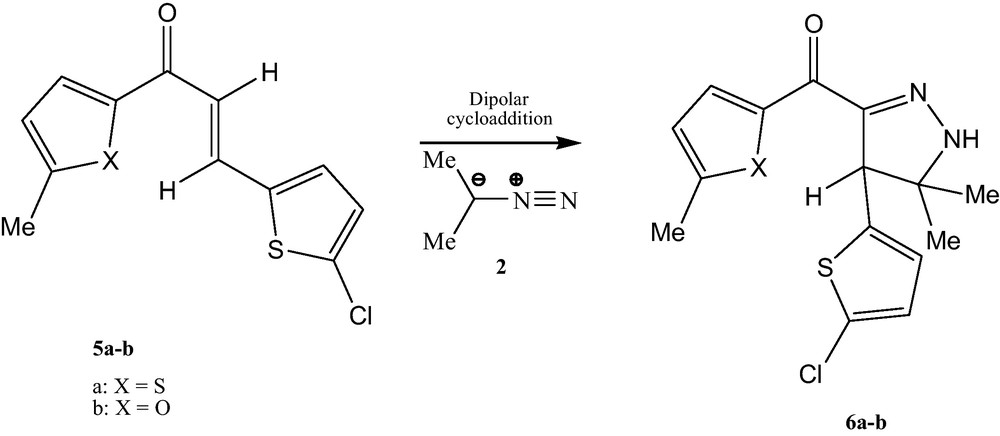
Synthesis of 2-pyrazolines.
As shown in Scheme 3, the reaction between 2-pyrazoline derivatives 4a–d and 6a–b and dimethylsulfoxide under Swern conditions gave good yields of pyrazolenine derivatives 7a–d and 8a–b which can be considered as suitable precursors to gem-dimethylcyclopropenes after photochemical nitrogen extrusion [20].
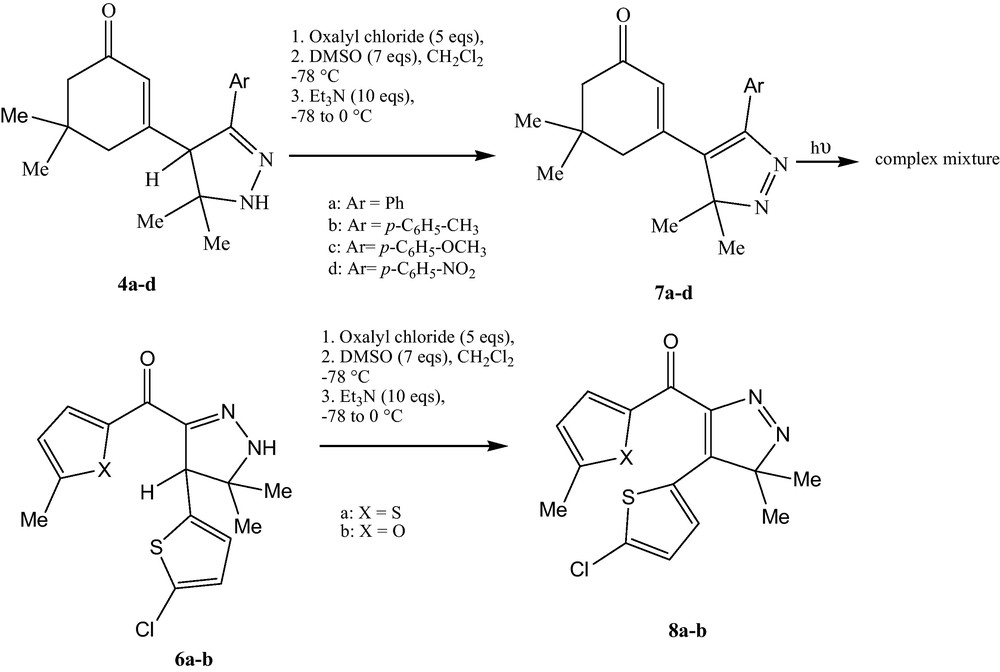
Formation of pyrazolenines.
In the approach to the Swern oxidation, oxalyl chloride is added to a solution of the 2-pyrazoline 4 and dimethylsulfoxide, while maintaining the temperature at −78 °C. Under these conditions the “activated” DMSO intermediate 9 is formed as a transient species which is rapidly consumed by the 2-pyrazoline 4 to form the alkoxysulfonium chloride 10. Addition of triethylamine afforded the pyrazolenine 7 [21] (Scheme 4).
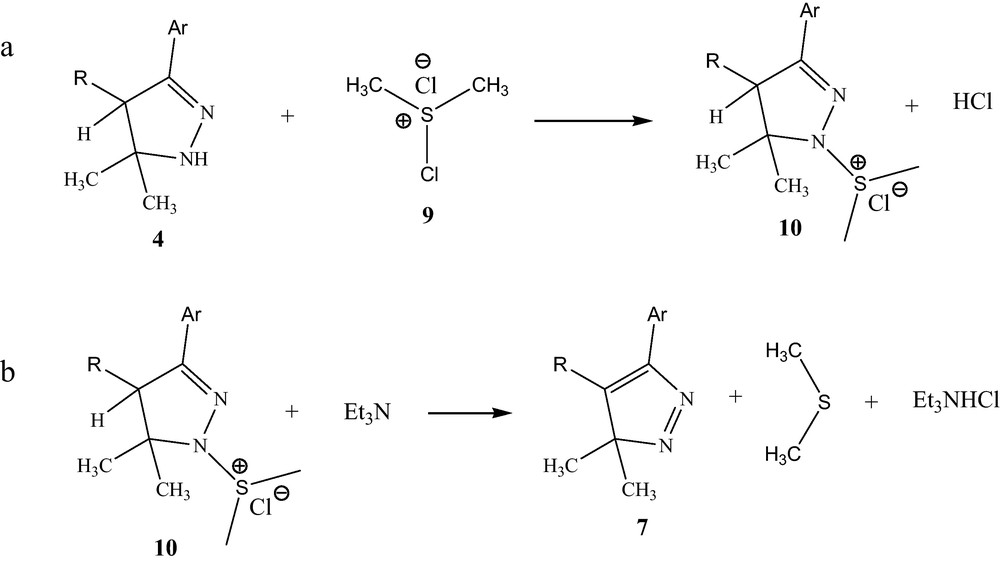
Swern oxidation reaction steps.
Irradiation of an ethereal solution of the pyrazolenine 7 at 0–5 °C using 350 nm lamps gave a complex mixture of products.
3 Conclusion
In conclusion, we have described the synthesis of new pyrazolines derivatives with complete regioselectivity. The Swern conditions, involving the use of very simple and inexpensive reagents, allow the one-pot transformation of 2-pyrazolines with a pyrazolenines into synthetically valuable. These findings constitute a significant addition to the growing list of synthetic applications of activated dimethylsulfoxide.
4 Experimental
General Methods. Chromatography was performed with silica gel 60 (230–400 mesh), and silica gel F254 plates were used for preparative TLC. The IR spectra frequencies are gives in cm−1. NMR spectra were determined in CDCl3 solutions at 300 and 75.5 MHz for 1H and 13C NMR, respectively; chemical shifts (δ) were reported in ppm and J values are gives in hertz.
4.1 1,3-Dipolar Cycloaddition of 2-diazopropane with dienes (1) and α,β-unsaturated ketones (5)
To a solution of dipolarophiles 1a–d or 5a–d (1.0 mmol) in diethyl ether, cooled at −20 °C, was added portionwise 2.6 M ethereal solution of 2-diazopropane. The reaction was kept at the same temperature during 1 h. The solvent was removed and chromatography (SiO2; ethyl acetate/petroleum ether, 1:3) to afford compounds 4a–d and 6a–d.
4.1.1 2-Pyrazoline (4a)
Yield (0.192 g, 65%), colourless solid. M.p 180–181 °C, FAB-MS m/z (%): 297 (MH+,100), Anal. Calcd. For C19H24N2O: C, 76.99; H, 8.16; N, 9.45%; Found: C, 76.87; H, 8.14; N, 9.51%. IR (KBr): N = N 1540; C = O 1680; NH 3250 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.85 (s, 3H, CH3), 1.00 (s, 3H, CH3), 1.06 (s, 3H, CH3), 1.33 (s, 3H, CH3), 2.21 (s, 2H, H9), 2.65 (s, 2H, H11), 3.76 (s, 1H, H4), 5.74 (s, 1H, H7), 5.83 (br s, 2H, H1), 6.90–7.30 (m, 5H, Harom). 13C{1H}NMR (75 MHz, CDCl3) δ: 22.82 (CH3), 28.29 (CH3), 29.40 (CH3), 33. 41(C-10), 38.94 (C-11), 51.50 (C-9), 59.54 (C-4), 67.40 (C-5), 125.40 (C-7), 127.43–136.32 (Carom), 150.15 (C-6), 153.31 (C-3), 200.45 (C-8).
4.1.2 2-Pyrazoline (4b)
Yield (0.217 g, 70%), colourless solid. M.p 170–171 °C, FAB-MS m/z(%): 311 (MH+,100), Anal. Calcd. For C20H26N2O: C, 77.38; H, 8.44; N, 9.02%; Found: C, 77.30; H, 8.39; N, 9.08%. IR (KBr): C = N 1550; C = O 1690; NH 3250 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.86 (s, 3H, CH3), 1.01 (s, 3H, CH3), 1.06 (s, 3H, CH3), 1.32 (s, 3H, CH3), 2.22 (s, 2H, H9), 2.30 (s, 3H, CH3), 2.66 (s, 2H, H11), 3.73 (s, 1H, H4), 5.75 (s, 1H, H7), 5.81 (br s, 2H, H1), 6.85; 7.08 (AA’BB’, 4H, Harom, J = 8.7 Hz); 13C{1H}NMR (75 MHz, CDCl3) δ: 21.20 (CH3), 22.90 (CH3), 28.30 (CH3), 29.40 (CH3), 33.50 (C-10), 39.00 (C-11), 51.60 (C-9), 59.30 (C-4), 67.30 (C-5), 125.40 (C-7), 128.00–137.00 (Carom), 150.20 (C-6), 153.50 (C-3), 201.01 (C-8).
4.1.3 2-Pyrazoline (4c)
Yield (0.261 g, 80%), colourless solid. M.p 210–211 °C, FAB-MS m/z(%): 327 (MH+,100), Anal. Calcd. For C20H26N2O2: C, 73.59; H, 8.03; N, 8.58%; Found: C, 73.50; H, 8.11; N, 8.51%. IR (KBr): C = N 1550; C = O 1680; NH 3330 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.75 (s, 3H, CH3), 0.93 (s, 3H, CH3), 0.99 (s, 3H, CH3), 1.21 (s, 3H, CH3), 2.22 (s, 2H, H9), 2.66 (s, 2H, H11), 3.72 (s, 1H, H4), 3.78 (s, 3H, OCH3), 5.76 (s, 1H, H7), 5.80 (br s, 1H, H1), 6.81; 6.88 (AA’BB’, 4H, Harom, J = 9 Hz); 13C{1H}NMR (75 MHz, CDCl3) δ: 22.80 (CH3), 28.00 (CH3), 28.20 (CH3), 29.20 (CH3), 33.30 (C-10), 39.00 (C-11), 51.60 (C-9), 55.19 (OCH3), 58.90 (C-4), 67.30 (C-5), 125.40 (C-7), 114.00–158.90 (Carom), 150.10 (C-6), 153.50 (C-3), 201.20 (C-8).
4.1.4 2-Pyrazoline (4d)
Yield (0.238 g, 70%), colourless solid. M.p 202–203 °C, FAB-MS m/z(%): 342 (MH+,100), Anal. Calcd. For C19H23N3O3: C, 66.84; H, 6.79; N, 12.31%; Found: C, 66.76; H, 6.82; N, 12.39%. IR (KBr): C = N 1550; C = O 1680; NH 3330 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.76 (s, 3H, CH3), 0.94 (s, 3H, CH3), 0.98 (s, 3H, CH3), 1.26 (s, 3H, CH3), 2.23 (s, 2H, H9), 2.64 (s, 2H, H11), 3.70 (s, 1H, H4), 5.78 (s, 1H, H7), 5.81 (br s, 1H, H1), 7.81; 8.12 (AA’BB’, 4H, Harom, J = 8.7 Hz); 13C{1H}NMR (75 MHz, CDCl3) δ: 22.81 (CH3), 27.01 (CH3), 28.22 (CH3), 29.16 (CH3), 33.27 (C-10), 38.50 (C-11), 51.62 (C-9), 58.91 (C-4), 67.29 (C-5), 125.39 (C-7), 124.10–171.5 (Carom), 150.09 (C-6), 152.97 (C-3), 202.19 (C-8).
4.1.5 2-Pyrazoline (6a)
Yield (0.287 g, 85%), yellow solid. M.p 140–141 °C, Anal. Calcd. For C15H15ClN2OS2: C, 53.16; H, 4.46; N, 8.27%; Found: C, 53.23; H, 4.35; N, 8.21%. IR (KBr): C = N 1520; C = O 1640; NH 3300 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.87 (s, 3H, CH3), 1.29 (s, 3H, CH3), 2.41 (s, 3H, CH3), 4.10 (s, 1H, H4), 6.15 (br s, 1H, H1), 6.67; 7.09 (2H, Hth, J = 3.6 Hz); 6.82; 7.51 (2H, Hth, J = 3.9 Hz). 13C{1H}NMR (75 MHz, CDCl3) δ: 15.90 (CH3), 22.51 (CH3), 29.02 (CH3), 57.94 (C-4), 68.00 (C-5), 152.55 (C-3), 125.2–153.04 (Cth), 187.80 (C = O).
4.1.6 2-Pyrazoline (6b)
Yield (0.226 g, 70%), yellow solid. M.p 149–150 °C, Anal. Calcd. For C15H15ClN2O2S: C, 55.81; H, 4.68; N, 8.68%; Found: C, 55.89; H, 4.59; N, 8.47%. IR (KBr): C = N 1525; C = O 1650; NH 3320 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.89 (s, 3H, CH3), 1.28 (s, 3H, CH3), 2.41 (s, 3H, CH3), 4.09 (s, 1H, H4), 6.35 (br s, 1H, H1), 6.13; 6.63 (2H, Hfu, J = 3 Hz); 6.69; 7.13 (2H, Hth, J = 3.6 Hz). 13C{1H}NMR (75 MHz, CDCl3) δ: 15.79 (CH3), 22.47 (CH3), 28.99 (CH3), 57.87 (C-4), 67.93 (C-5), 151.94 (C-3), 108.15–153.65 (Cfu,th), 186.41 (C = O).
4.2 Dehydrogenation of 2-Pyrazolines
To a solution of oxalyl chloride (5 equiv) in dry CH2Cl2 (10 mL), at −78 °C under an argon atmosphere, was added DMSO (7 equiv). The solution was stirred for 15 min, until effervescence ceased. A solution of the 2-pyrazolines 4a–d or 6a–d (1 mmol) in dry CH2Cl2 (5 mL) was added dropwise, and the solution was stirred for 10 min at −78 °C. Triethylamine (10 equiv) was then added and the solution was left to warm to 0 °C for 30 min, while stirred. The reaction mixture was diluted with CH2Cl2 (20 mL) and washed with saturated aqueous NH4Cl (3 × 20 mL). The organic layer was dried (MgSO4) and evaporated, and the residue was purified by chromatography (SiO2; ethyl acetate/petroleum ether, 1:4) to afford compounds 7a–d and 8a–d.
4.2.1 Pyrazolenine (7a)
Yield (0.235 g, 80%), colourless solid. Mp 121–122 °C, Anal. Calcd. For C19H22N2O: C, 77.52; H, 7.53; N, 9.52%; Found: C, 77.44; H, 7.56; N, 9.45%. IR (KBr): C = C–N = N 1615; C = O 1690 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.95 (s, 6H, CH3), 1.25 (s, 6H, CH3), 2.20 (s, 2H, H9), 2.63 (s, 2H, H11), 5.71 (s, 1H, H7), 6.89–7.35 (m, 5H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 20.50 (CH3), 27.62 (CH3), 33. 35 (C-10), 38.91 (C-11), 51.51 (C-9), 147.34 (C-4), 163.20 (C-5), 125.37 (C-7), 127.36–136.47 (Carom), 150.14 (C-6), 153.28 (C-3), 201.21 (C-8).
4.2.2 Pyrazolenine (7b)
Yield (0.277 g, 90%), colourless solid. M.p 110–111 °C, Anal. Calcd. For C20H24N2O: C, 77.89; H, 7.84; N, 9.08%; Found: C, 77.91; H, 7.81; N, 9.06%. IR (KBr): C = C–N = N 1610; C = O 1690 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.91 (s, 6H, CH3), 1.25 (s, 6H, CH3), 2.31 (s, 3H, CH3), 2.20 (s, 2H, H9), 2.59 (s, 2H, H11), 5.71 (s, 1H, H7), 6.85; 7.08 (AA’BB’, 4H, Harom, J = 8.7 Hz); 13C{1H}NMR (75 MHz, CDCl3) δ: 20.53 (CH3), 21.09 (CH3), 26.98 (CH3), 33.51 (C-10), 38.55 (C-11), 51.61 (C-9), 147.33 (C-4), 162.83 (C-5), 125.40 (C-7), 128.11–138.01 (Carom), 151.13 (C-6), 154.10 (C-3), 200.03 (C-8).
4.2.3 Pyrazolenine (7c)
Yield (0.275 g, 85%), colourless solid. M.p 123–124 °C, Anal. Calcd. For C20H24N2O2: C, 74.04; H, 7.46; N, 8.64%; Found: C, 74.00; H, 7.43; N, 8.71%. IR (KBr): C = C-N = N 1615; C = O 1685 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.88 (s, 6H, CH3), 1.27 (s, 6H, CH3), 2.19 (s, 2H, H9), 2.65 (s, 2H, H11), 5.71 (s, 1H, H7), 3.77 (s, 3H, OCH3), 6.81; 6.88 (AA’BB’, 4H, Harom, J = 9 Hz); 13C{1H}NMR (75 MHz, CDCl3) δ: 20.45 (CH3), 26.88 (CH3), 32.91 (C10), 39.13 (C11), 51.58 (C-9), 55.18 (OCH3), 146.11 (C-4), 163.01 (C-5), 125.38 (C-7), 115.11–158.87 (Carom), 151.04 (C-6), 152.77 (C-3), 200.82 (C-8).
4.2.4 Pyrazolenine (7d)
Yield (0.254 g, 75%), colourless solid. M.p 98–99 °C, Anal. Calcd. For C19H21N3O3: C, 67.24; H, 6.24; N, 12.38%; Found: C, 67.38; H, 6.17; N, 12.45%. IR (KBr): C = C-N = N 1610; C = O 1685 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 0.87 (s, 6H, CH3), 1.28 (s, 6H, CH3), 2.18 (s, 2H, H9), 2.63 (s, 2H, H11), 5.68 (s, 1H, H7), 7.80; 8.22 (AA’BB’, 4H, Harom, J = 8.7 Hz); 13C{1H}NMR (75 MHz, CDCl3) δ: 20.38 (CH3), 26.56 (CH3), 32.88 (C10), 39.09 (C11), 51.51 (C-9), 146.17 (C-4), 163.21 (C-5), 124.66 (C-7), 123.89–169.57 (Carom), 150.36 (C-6), 151.69 (C-3), 200.54 (C-8).
4.2.5 Pyrazolenine (8a)
Yield (0.218 g, 65%), yellow solid. Mp 101–102 °C, Anal. Calcd. For C15H13ClN2OS2: C, 53.48; H, 3.89; N, 8.32%; Found: C, 53.53; H, 3.99; N, 8.42%. IR (KBr): C = C-N = N 1615; C = O 1690 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 1.60 (s, 6H, CH3), 2.39 (s, 3H, CH3), 6.55; 7.07 (2H, Hth, J = 3.6 Hz); 6.67; 7.74 (2H, Hth, J = 3.9 Hz). 13C{1H}NMR (75 MHz, CDCl3) δ: 16.02 (CH3), 21.81 (CH3), 97.67 (C-3), 138.15 (C-4), 157.29 (C-5), 121.02–153.19 (Cth), 190.13 (C = O).
4.2.6 Pyrazolenine (8b)
Yield (0.257 g, 80%), yellow solid. Mp 107–108 °C, Anal. Calcd. For C15H13ClN2O2S: C, 56.16; H, 4.08; N, 8.73%; Found: C, 56.11; H, 4.00; N, 8.58%. IR (KBr): C = C-N = N 1610; C = O 1690 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 1.63 (s, 6H, CH3), 2.42 (s, 3H, CH3), 6.19; 6.62 (2H, Hth, J = 3 Hz); 6.55; 7.15 (2H, Hfu, J = 3.9 Hz). 13C{1H}NMR (75 MHz, CDCl3) δ: 15.92 (CH3), 22.03 (CH3), 97.55 (C-3), 137.87 (C-4), 157.12 (C-5), 114.00–153.11 (Cfu,th), 190.22 (C = O).


