1 Introduction
Zinc and magnesium organometallics have strongly influenced the development of organic synthesis over the last century [1]. In recent years, it became important to prepare highly functionalized organometallics for the construction of complex organic target molecules [2]. Zinc organometallics were especially well suited for such a purpose, due to the low reactivity of the carbon-zinc bond and to the excellent response of this carbon-metal bond for participating in various transition metal catalysis [3]. New preparation methods also allowed the synthesis of functionalized organomagnesium reagents [4]. Both of these metals display a low toxicity and are moderately priced. This short review describes two general methods for the synthesis of these useful organometallics: the first method involves a direct metal insertion and uses organic halides of type 1 as substrates, whereas the second method is based on a C–H activation of aromatic (or heterocyclic structures such as 2) (Scheme 1). Applications of these polyfunctional organometallics for carbon-carbon bond forming reactions will also be described.
Polyfunctional organometallics in organic synthesis.
2 Preparation of polyfunctional Zn- and Mg-organometallics
2.1 Insertion reactions to organic halides
Whereas the insertion of zinc dust to aryl iodides can be accomplished only by using highly reactive zinc powder (Rieke zinc) [5] or polar solvents, the reaction of commercial zinc dust with ethyl 4-iodobenzoate (3) in THF is very sluggish and provides the desired zinc reagent 4 in less than 5% after 24 h at 70 °C. However, in the presence of LiCl (0.3–2 equiv) a fast reaction takes place at 25 °C and leads to the desired zinc reagent 4 in ca. 98% yield. The role of LiCl is to solubilize the aryl zinc halide (ArZnX) produced on the zinc surface (Scheme 2) [6].

LiCl-mediated preparation of functionalized organozinc reagents.
This reaction is very versatile and allows the synthesis of various aromatic and heterocyclic zinc reagents bearing sensitive functional groups like an ester, an aldehyde, or a ketone (Scheme 3).

General Preparation of Functionalized Organozinc Reagents.
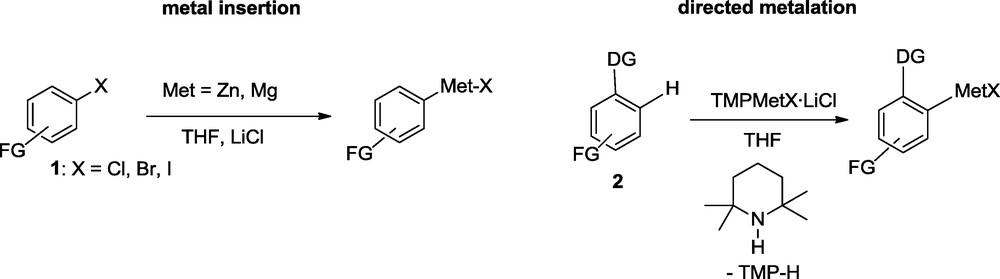
This LiCl-activation of the zinc surface is quite general and a number of metals like Al, In, or Mg can be efficiently activated by addition of LiCl in THF. Thus, the treatment of the mixed carbonate 4 with Mg powder in the presence of LiCl provides the expected Grignard reagent 5 in 91% yield. The insertion reaction is complete at −20 °C within 1 h. Quenching with an aldehyde provides the expected alcohol 6 in 90% yield [7]. In the presence of more sensitive functional groups such as a methyl ester (7), the Mg-insertion is performed in the presence of ZnCl2, so that the intermediate Mg-reagent (8) is converted in situ into the corresponding zinc reagent (9). Copper-catalyzed allylation affords the diester 10 in 83% yield (Scheme 4).
Chemoselective preparation of functionalized arylmagnesium reagents.
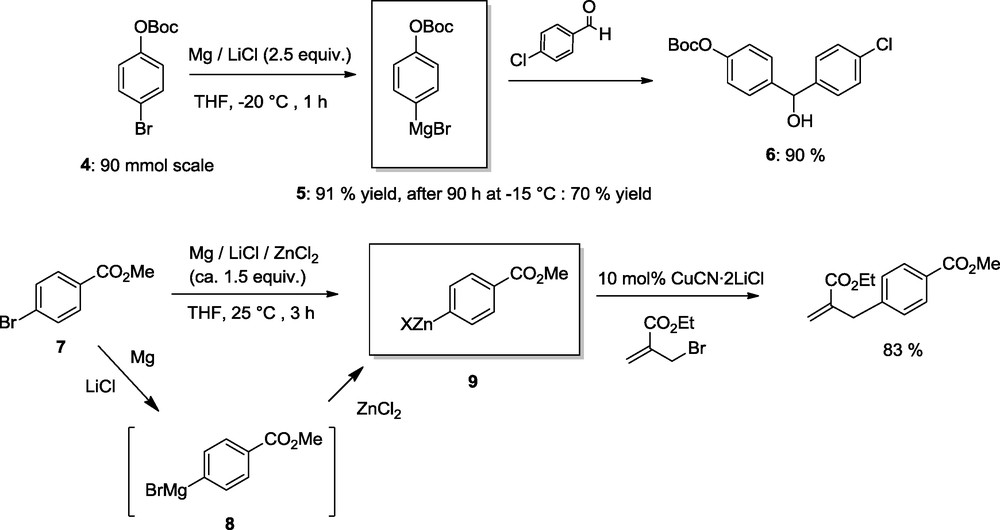
Benzylic chlorides are excellent substrates for preparing the corresponding Zn-reagent, either by a direct reaction with Zn dust in the presence of LiCl or by using Mg-powder in the presence of ZnCl2 and LiCl-. This second method allows an expeditive synthesis of the zinc reagent 11 (45 min us. 24 h using only Zn and LiCl) [8]. The presence of MgCl2 generated during the formation of a benzylic zinc reagent using Mg/ZnCl2/LiCl has an additional beneficial effect. It enhances greatly the reactivity of the zinc species. Thus, whereas the zinc compound 12 does not react with the aldehyde 13 in the absence of MgCl2, in its presence a fast reaction occurs producing the alcohol 14 in 80% isolated yield [9] (Scheme 5).
New preparation of benzylic zinc reagents using Mg as reducing agent.
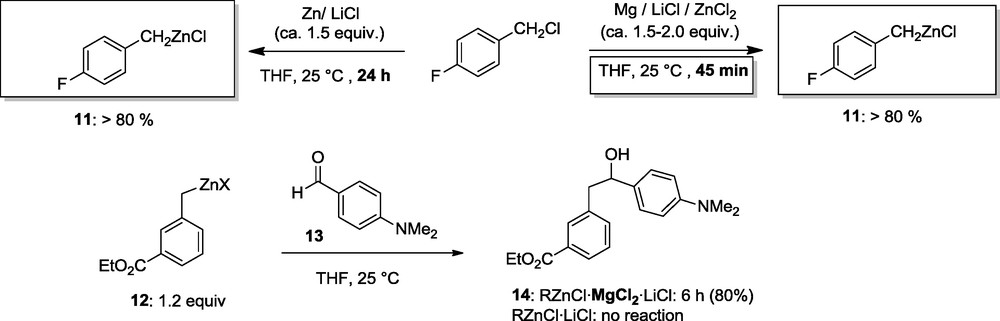
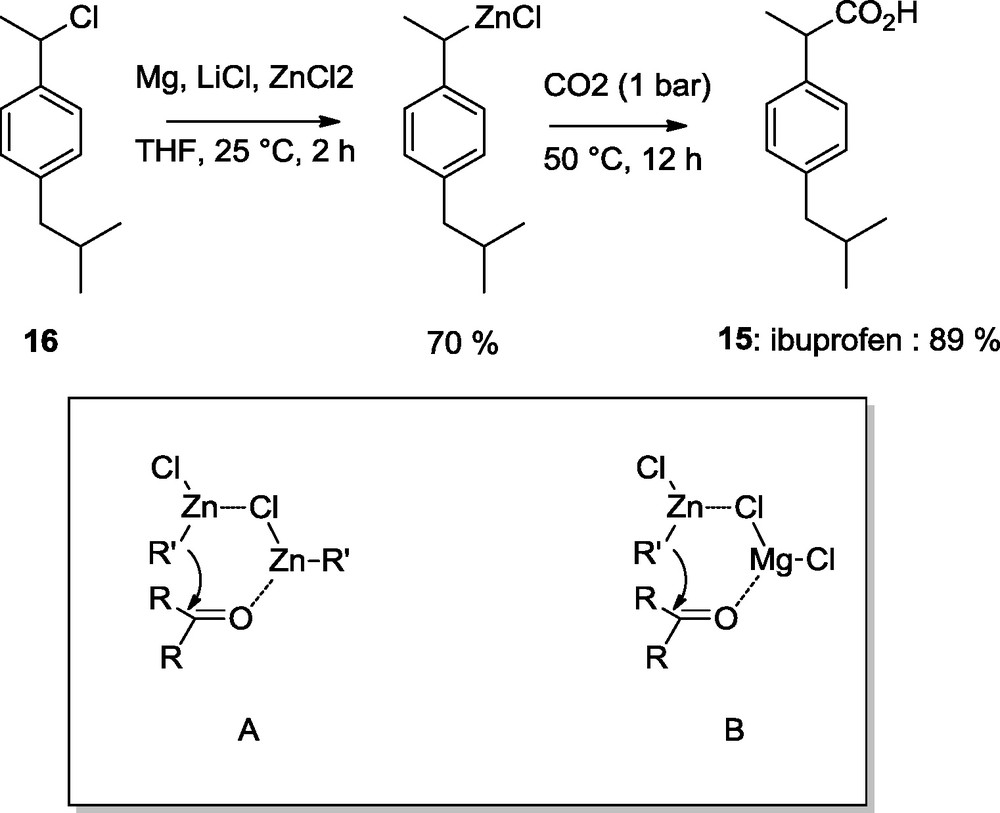
The presence of MgCl2 facilitates the addition CO2 to the zinc reagent, a reaction which otherwise proceeds only in polar solvents [10]. This allowed us to prepare ibuprofen (15) in 89% yield in a one-pot procedure starting from the benzylic chloride (16) [11]. The rate acceleration due to MgCl2 is best explained by assuming that the usual transition state A for the addition of a zinc organometallic to a carbonyl compound is now rather of the type B where the carbonyl group is coordinated to MgCl2 which is a much stronger Lewis-acid than R’ZnCl (Scheme 6) [9].
MgX2-addition of organic reagents to carbonyl derivatives.
2.2 Directed deprotonation using LiCl-solubilized TMP-bases
The use of sterically hindered 2,2,6,6-tetramethylpiperidyl (TMP)-metal amides solubilized by LiCl allows the direct magnesiation or zincation of a range of polyfunctional aromatics and heterocycles. Thus, TMPMgCl·LiCl (17) which is readily prepared by the reacting of 2,2,6,6-tetramethylpiperidine with iPrMgCl·LiCl has a monomeric structure in solution as shown by García-Álvarez et al. [11]. It has excellent solubility in THF (1.2 M) and deprotonates positions in heterocycles otherwise difficult to metalate. Thus, the furan (18) reacts readily at −78 °C with TMPMgCl·LiCl (17) producing the magnesium reagent 19 which after transmetalation with ZnCl2 undergoes a smooth cross-coupling with an aryl iodide providing the cross-coupling product 20 in 79% yield (Scheme 7) [12].

Synthesis of magnesiated furans and pyrroles.
The corresponding zinc base TMPZnCl·LiCl (21) is prepared in a similar way starting from TMP-H using BuLi and a subsequent transmetalation with ZnCl2. The base (21) has also an excellent solubility in THF (ca. 1.3 M) and shows a unique chemoselectivity in deprotonation reactions. Thus, the heterocycles 22 and 23 which bear respectively an aldehyde and an nitro group are readily zincated at 25 °C with TMPZnCl·LiCl (21) within a few minutes (Scheme 8) [13].

TMPZnCl·LiCl: a chemoselective base for the directed zincation of sensitive aromatics and heteroaromatics.
Also, a sensitive heterocycles such as the purine (24) are zincated at 25 °C with TMPZnCl·LiCl (21) leading to a selective zincation at the imidazole ring of the purine skeleton. Very sensitive substrates like the furan (25) which bears both a nitro group and an ester function is smoothly zincated with TMPZnCl·LiCl (21) at the α-position to the methyl ester group. After allylation with 3-bromocyclohexene the expected furan 26 is obtained in 76% yield (Scheme 9) [13].

Zincations in the presence of ester, nitro groups and aldehydes.
Remarkably, TMPZnCl·LiCl is also able to zincate functionalized olefins such as the trifluoromethyl ketone derivative 27 or nitro olefins of type 28. The resulting zinc reagents 29 and 30 can either be acylated or allylated providing polyfunctional compounds like 31 and 32 (Scheme 10) [14].
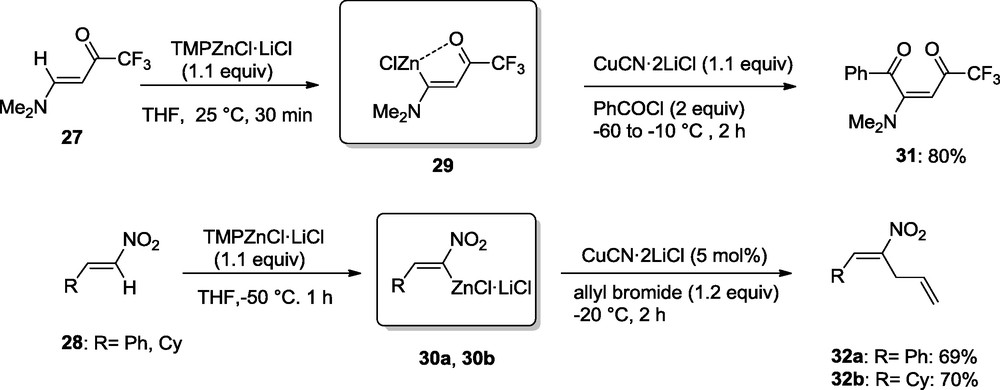
Zincation of electron-poor Olefins.
The metalation of pyridines is an important synthetic challenge and Kessar et al. have shown that BF3·OEt2 enables a low temperature lithiation of pyridines with TMPLi [15]. We could show that LiCl-solubilized TMP-bases such as TMPMgCl·LiCl (17) or TMPZnCl·LiCl (21) are compatible with BF3·OEt2 at temperature below −20 °C. Thus, 3-fluoropyridine (33) can be readily metalated in position 4 by the reaction with BF3·OEt2 followed by the addition of TMPMgCl·LiCl (17) [16]. After a Pd-catalyzed cross-coupling with an aryl iodide the desired 4-substituted pyridine (34) is obtained in 74% yield. In contrast with the absence of BF3·OEt2, a selective magnesiation occurs in position 2 providing the 2-arylated pyridine 35 [16]. A similar behaviour is observed for a number of pyridines bearing an electron-withdrawing substituent in position 3. Thus, 3-cyanopyridine (36) can be substituted with complete regioselectivity either in position 2 in the absence of BF3·OEt2 or in position 4 in the presence of BF3·OEt2 (Scheme 11) [16].
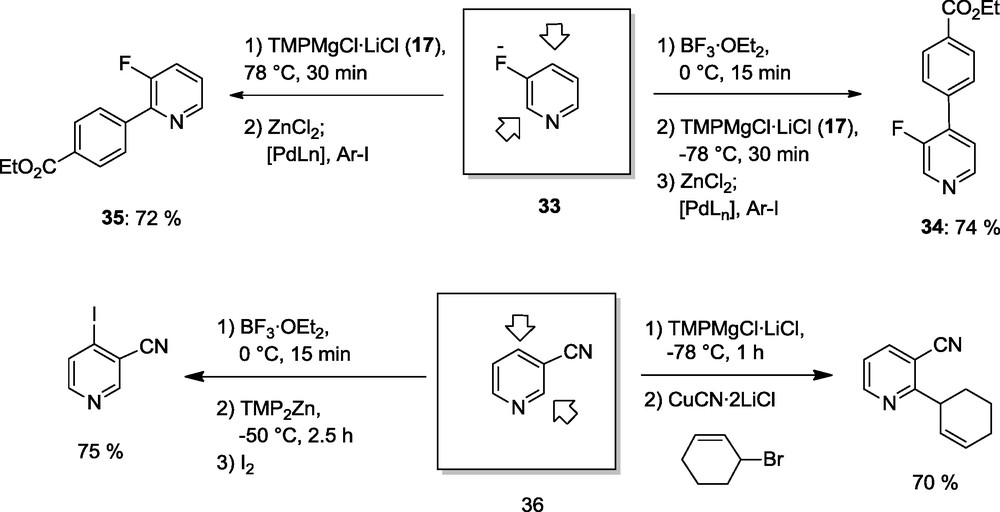
BF3-triggered selective metalations.
A full functionalization of the pyridine scaffold was performed using LiCl-solubilized TMP-bases of Zn and Mg. Thus, 4-cyanopyridine (37) is successively functionalized in position 3, then position 2, then position 5 and finally in position 6. This demonstrates the versatility of the TMP-bases and although all functionalizations of a given pyridine cannot be achieved in each case, a significant progress has been made with these new TMP-bases in combination with BF3·OEt2 (Scheme 12) [17].
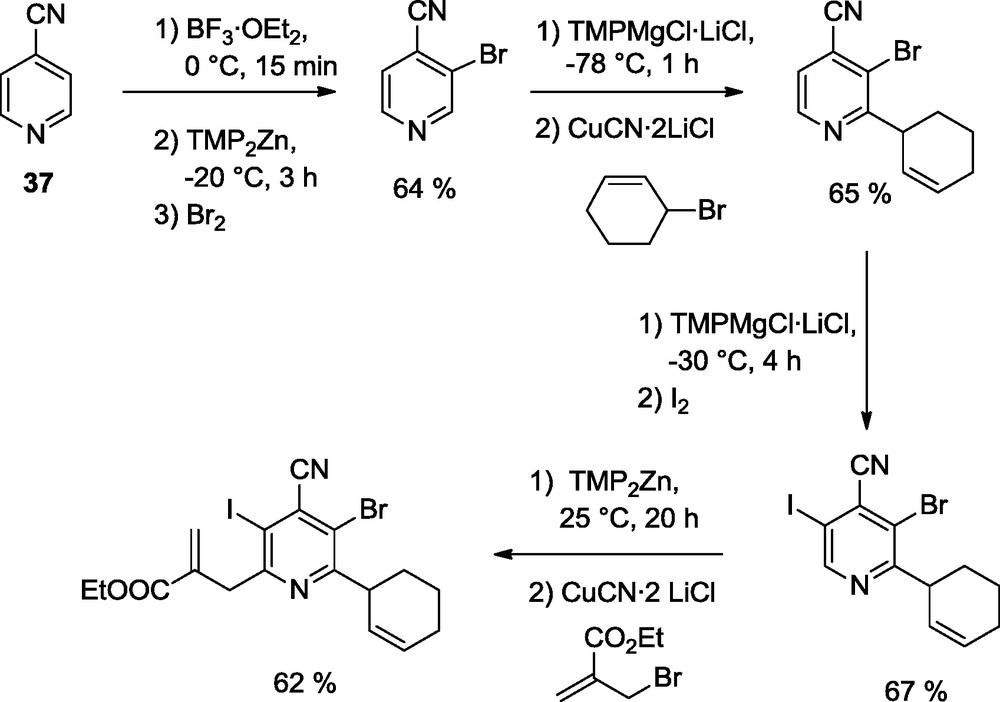
Preparation of fully substituted pyridines.
Organozincs were used for the synthesis of new types of heterocycles or for improving current heterocycle syntheses. Thus, the Fisher-indole synthesis requires strong acidic conditions, uses non-commercial aryl hydrazines as substrates and provides often a mixture of regioisomeric indoles. These problems can be solved by performing a zinc mediated Fischer-indole synthesis. This one-pot procedure requires readily available aryldiazonium salts and tolerate a range of functional groups. Thus, various zinc reagents such as 38 and 39 add under mild conditions to the aryldiazonium salts 40 and 41 providing after a [3,3]-sigmatropic shift at 125 °C (microwave irradiation) the indoles 42 and 43 in 75–90% yields (Scheme 13) [18].
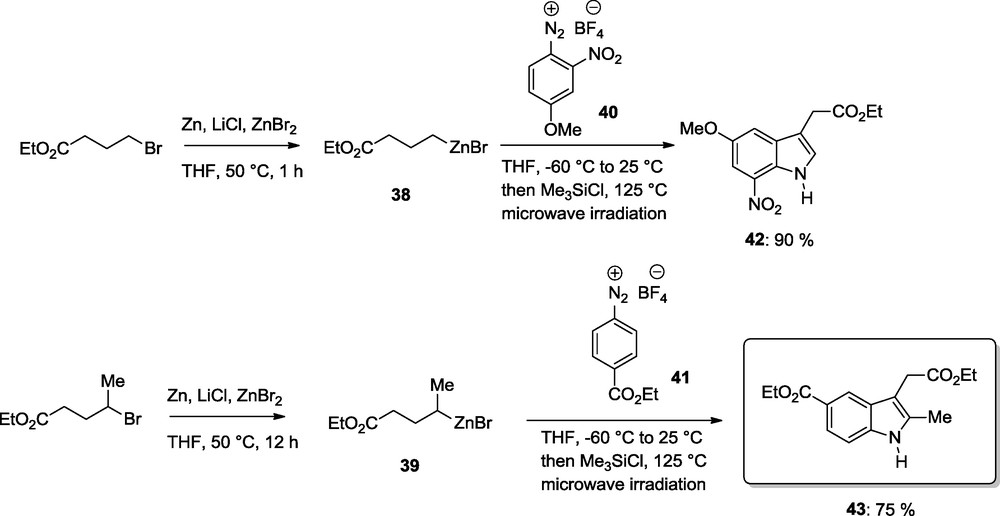
Zinc-mediated Fischer indole synthesis.
Secondary cycloalkylzinc reagents can be used as well. These reagents produce annulated indoles such as 44 and 45 in 81–88% yields. This method has also be applied to the synthesis of biologically active indoles such as iprindole 46 which is prepared in a efficient procedure starting from cyclohexylzinc bromide which is obtained by the direct zinc insertion to cyclohexyl bromide using Zn dust and LiCl in THF (50 °C, 1 h); Scheme 14 [18].
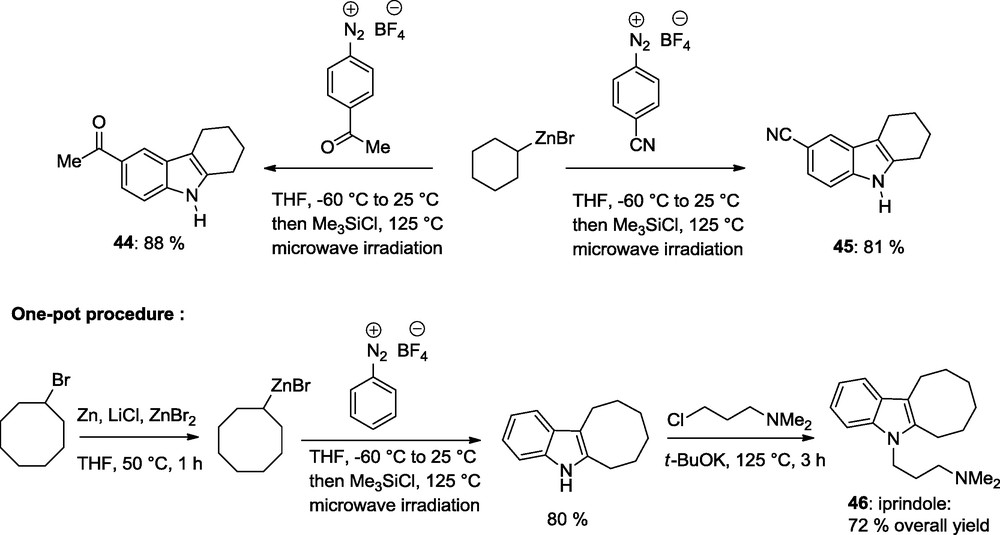
Synthesis of iprindole.
2.3 Diastereoselective cross-couplings
The preparation of diastereomerically pure compounds by using cross-coupling reactions is an important synthetic method. Starting from menthyl iodide (47), we have found that the resulting diastereomeric zinc reagent react in a stereoconvergent way with an arylpalladium(II)-halide providing only one Pd(II)-intermediate bearing all the substituents in a equatorial position (48). After reductive elimination, arylated menthyl derivatives of type 49 are obtained with high diastereoselectivity (Scheme 15) [19].
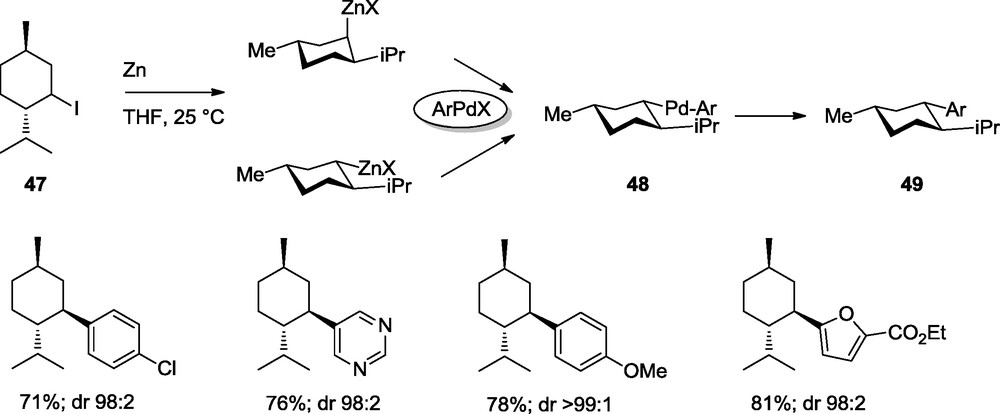
Diastereoselective Negishi cross-couplings.
Remarkably, this diastereoselective cross-coupling can be extended to synthesis bearing a substituent in position 2, 3 or 4. Thus, cyclohexylzinc reagent such as 50–52 undergo highly diastereoselective cross-couplings with methyl 4-iodobenzoate leading to the arylated cyclohexane derivatives 53–55 in 72–82% yields and dr > 95:5; Scheme 16 [19].
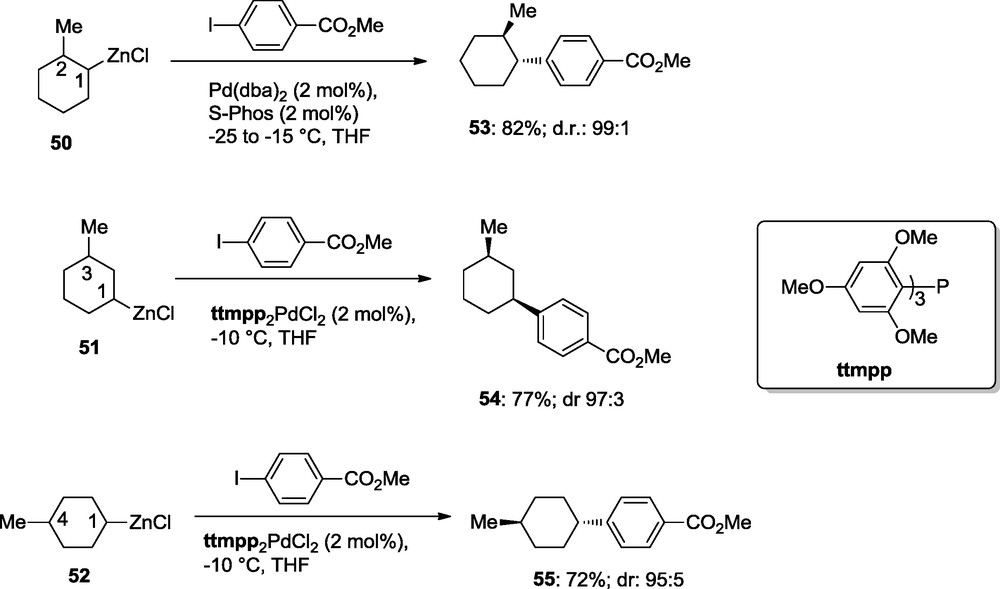
Diastereoselective cross-coupling reactions.
It was also possible to extend these cross-coupling to the arylation of Boc-protected piperidines of type 56. Their metalation with s-BuLi and transmetalation with ZnCl2 provides the zinc reagents 57 which after reaction with an aryl iodide (Ar-I) are furnishing the 2,4-disubstituted piperidines 58a–d in diastereoselectivities > 99:1; Scheme 17 [20].
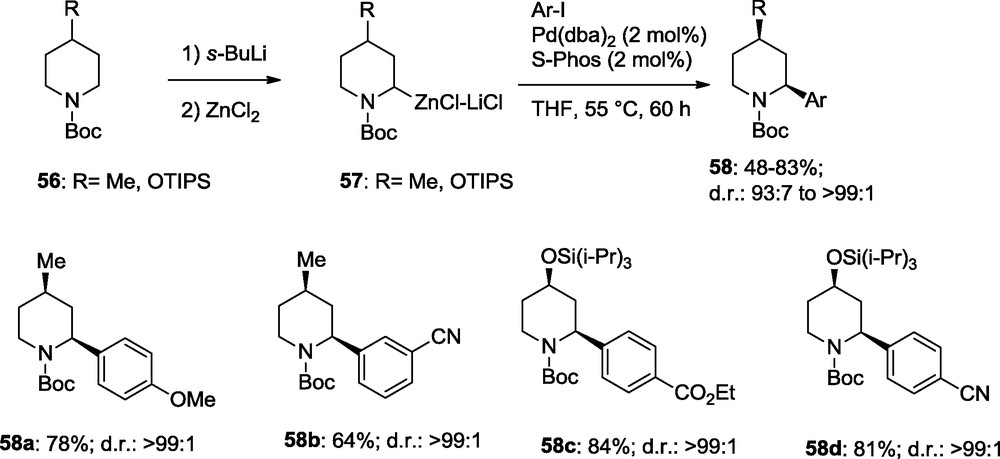
Diastereoselective cross-coupling reactions with piperidylzinc reagents.
By using 2,6-disubstituted zincated piperidines of type 59, a Pd-catalyzed rearrangement takes place via an elimination and readdition of ArPd-H leading to 2,5-disubstituted piperidines of type 60. Thus, the arylated piperidines 60a–c are obtained with diastereoselectivities better than 88:12 (Scheme 18) [20].
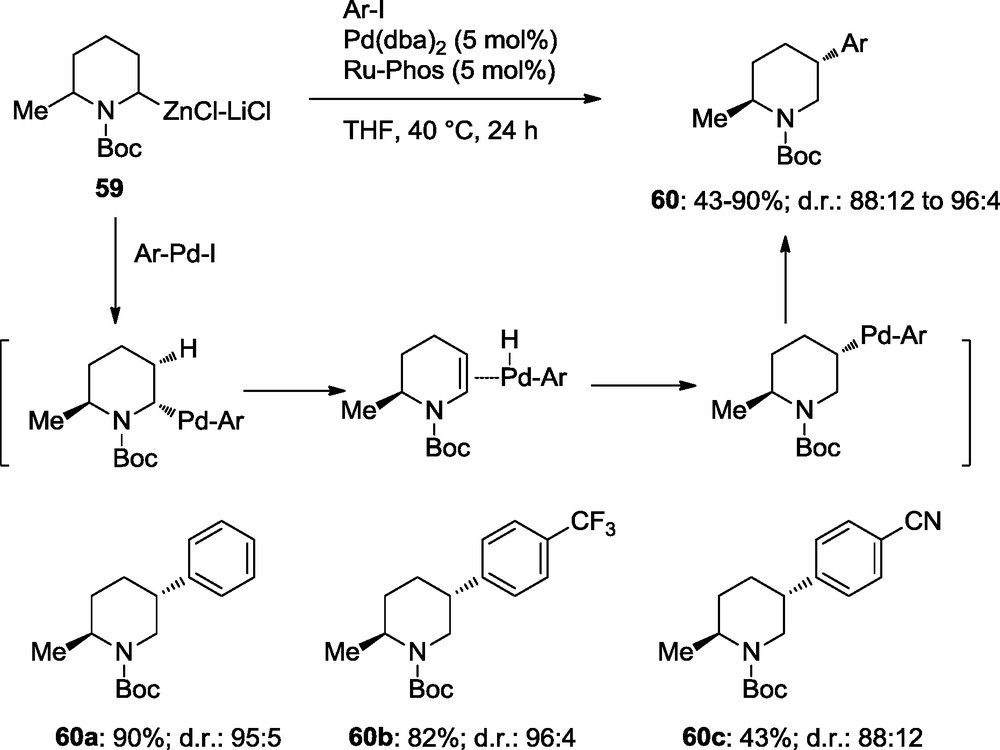
Rearrangements in diastereoselective cross-coupling reactions.
The preparation of alkynylcyclohexane derivatives in a diastereoselective fashion can also be realized via a Pd-catalyzed cross-coupling. Interestingly, although most Pd-catalyses require a phosphine ligand, it was possible to perform Csp3-Csp cross-coupling of cyclohexylzinc reagents with alkynyl bromides using neocuproine (61) as a ligand. The cross-coupling furnish the alkynylated products of type 62 in good yields and high diastereoselectivities (up to 98:2); Scheme 19 [21].

Diastereoselective Csp-Csp3 cross-coupling reactions.
3 Conclusion
In conclusion, we have reported in this review article, a new range of general synthetic methods allowing the preparation of polyfunctional organometallics of magnesium and zinc. The low toxicity of these metals, excellent availability and exceptional chemoselectivity make these reagents very versatile tools for organic synthesis.


