1 Introduction
Michael addition of heteroatom nucleophiles to conjugated alkenes constitutes an important strategy in carbon-heteroatom bond forming reactions [1]. It opens an easy route for the pivotal synthetic intermediates like β-amino carbonyls, esters, nitriles and amides which find applications in the synthesis of natural products, chiral auxiliaries, bioactive compounds, pharmaceuticals, fine chemicals, etc. [2]. Classical Mannich reaction of enolates with imines is a useful alternative for the introduction of such functionality; however, its limited scope coupled with harsh reaction conditions makes it a less attractive route in the synthesis of complex molecules [3,4]. In contrast, the addition of nitrogenous nucleophiles to electron deficient alkenes (aza-Michael reaction) has attracted much attention from organic chemists principally due to its operational simplicity and high atom economy. Over the past few years, a good number of protocols have been reported for this important reaction [5a–h]. These efficient protocols are well suited to primary aliphatic as well as secondary amines; however, aromatic amines being weakly nucleophilic, fail to undergo the Michael addition reaction.
It is well known that, nucleophilicity of aromatic amines is highly solvent dependent [6] and they are twice more nucleophilic in aqueous medium [7]. Thus, Michael addition of anilines was explored earlier in aqueous medium [8a–g]. These water-mediated protocols often require longer reaction time, thermal or MW activation and furnish the desired products in poor to moderate yields. To alleviate these problems, in recent years Michael addition of anilines has also been investigated using YNO3, SiO2.AlCl3, SiCl4, RuCl3 / PEG, I2, etc. as catalysts [9a–f]. It is worth mentioning that, despite remarkable success of these protocols, their scope has been limitedly explored for intermolecular aza-Michael addition reaction. Consequently, the development of a versatile and an efficient protocol applicable to inter- as well as the intramolecular Michael addition of anilines, leading preferably to biologically active compounds is highly desirable. In continuation with our ongoing studies on the development of new synthetic methodologies [10], herein we report our studies on aza-Michael reaction with special reference to aromatic amines as Michael donors (Scheme 1).
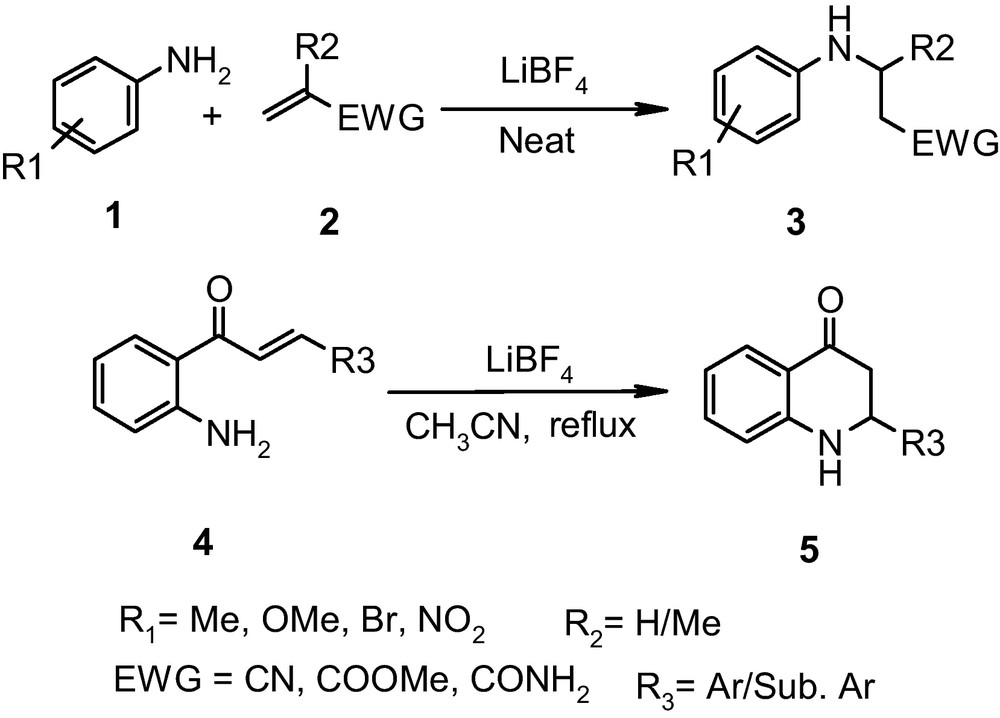
2 Results and discussion
A systematic study was undertaken to develop the most suitable conditions for the reaction between aniline and methyl vinyl ketone as model substrates. Accordingly, an equimolar mixture of aniline and methyl vinyl ketone was stirred together at ambient temperature under solvent-free condition. The reaction was exorgenic and furnished the desired β-amino ketone in excellent yield, in a short time (30 min, 93%, GCMS).1 To demonstrate the generality of the reaction conditions, various ring-substituted anilines were initially reacted with methyl vinyl ketone and, having obtained the satisfactory results, the studies were extended to other conjugated ketones. It is worth mentioning that the reactions of anilines with ethyl vinyl ketone were very smooth however; under the established reaction condition their reaction with cyclohexenone was unprecedented2 while chalcone furnished corresponding β-amino ketone in fair yield. In examining the scope of the protocol, it was further noticed that anilines failed to undergo addition to other Michael acceptors such as methyl acrylate, acrylonitrile as well as acrylamide. These observations clearly project the essentiality of an appropriate activator viz. energy source, solvent or catalyst for successful aza-Michael reaction with wide scope.
From a mechanistic viewpoint, the success of the aza-Michael reaction relies either on the increase in nucleophilicity of anilines or the electrophilicity of conjugated alkenes, and the use of a Lewis acid catalyst is one of the keys in performing the aza-Michael reaction efficiently. It is well accepted that the metal ion from Lewis acid forms a strong coordinate bond with the electron withdrawing groups in conjugated alkenes and thereby increase their electrophilicity to facilitate the addition of nitrogenous nucleophiles [9a].
Lithium tetrafluoroborate is a well-known mild Lewis acid catalyst available commercially and unlike lithium perchlorate it is non-explosive, non-oxidizing as well as a non-nucleophilic agent. It acts as a slow release source of boron trifluoride and provides a convenient route to effect organic transformations under neutral conditions [11]. It has been used earlier by us in the synthesis of α-aminonitriles [10c] and by others in the synthesis of homoallylic acetates [12a], in intramolecular Diels alder reactions [12b], etc. A literature survey revealed that, although a variety of Lewis acids have been used earlier in Michael addition of aniline to conjugated alkenes, to the best of our knowledge, there are no reports on the use of lithium tetrafluoroborate for either the inter- or intramolecular aza-Michael reaction. With reference to our earlier experience [10c], we planned to explore the suitability of LiBF4 in Michael addition of anilines to electron deficient alkenes.
During the initial exploratory reactions, addition of aniline to methyl acrylate was studied as a model reaction using LiBF4 (10 mol %) as catalyst. The reaction was too sluggish at room temperature but it went to completion in acceptable time (30 min) on heating the reaction mixture up to 70 °C under solvent-free condition. The studies on the effect of catalyst loading as well as change in the reaction temperature did not exhibit any appreciable change in the yield of desired Michael addition product. Thus, under the established reaction conditions for the aniline in hand, various substituted anilines were screened for their addition to methyl acrylate and the protocol was then extended towards other Michael acceptors viz. methyl methacrylate, acrylonitrile as well as acrylamide. In extending the scope of the protocol, it was observed that aniline can also undergo addition to poorly electrophilic chalcone under the same reaction condition (entry 14, Table 1) This initial success to intermolecular aza-Michael addition reaction prompted us to explore the suitability of LiBF4 in intramolecular aza-Michael addition using 2’-aminochalcone as a model substrate. This is because, the applicability of the resultant 2-aryl-2,3-dihydroquinoline-4-(1H) one, as a valuable precursor in the synthesis of medicinally important compounds is well documented [13,14].
Lithium tetrafluoroborate catalyzed Michael addition of anilines to conjugated alkenes.
| Entry | M. Donor (1) | M. acceptor (2) | Catalyst (mol %) | Temp (°C) | Time (min) | Yield (%)a |
| 1 2 3 4 5 6 | 1a 1b 1c 1d 1e 1f | 2a | – | RT | 30 30 40 30 30 50 | 93 92 90 91 87 67 |
| 7 8 9 10 | 1a 1b 1c 1d | 2b | – | RT | 30 30 30 | 90 85 93 84 |
| 11 12 13 | 1a 1b 1c | 2c | – | RT | 20 25 25 | 85 81 82 |
| 14 | 1a | 2d | 10 | RT 70 | 30 30 | 20 55 |
| 15 16 17 18 19 | 1a 1b 1c 1d 1e | 2e | 10 | 70 | 15 25 10 20 10 | 90 95 93 91 90 |
| 20 | 1a | 2f | 10 | 70 | 20 | 87 |
| 21 22 23 | 1a 1c 1d | 2g | 10 | 70 | 20 15 25 | 94 95 85 |
| 24 25 26 | 1b 1c 1e | 2h | 10 | 70 | 30 30 10 | 94 95 85 |
a Isolated yields; all compounds gave satisfactory spectroscopic data; 1a: Aniline; 1b: 4-Methyl aniline; 1c: 4-Methoxy aniline; 1d: 4-Chloro aniline; 1e: 4-bromoaniline; 1f: 3-nitro aniline.
To test the feasibility of the protocol, to a stirred solution of 2-amino chalcone, 4a, in acetonitrile was added LiBF4 (10 mole %) and stirring was continued at room temperature. Timely analysis of the reaction mixture (comparison with authentic sample) revealed the formation of desired quinolones (20%, tlc, 4 h). However, the reaction furnished the desired dihydroquinolone, 5a on heating the reaction mixture (70 °C) for a short time (20 min). Without modifying the reaction conditions, the scope of the protocol was examined using a variety of 2-aminochalcones. In all the studied cases, corresponding 2-aryl-2,3-dihydroquinoline-4(1H)-one was obtained in excellent yield and purity (Table 2). There are only a few reports on the synthesis of such quinolones [15]. However, the protocol developed by us is operationally simple and high yielding.
3 Conclusion
In conclusion, we have demonstrated for the first time the use of LiBF4 as a highly efficient catalyst in promoting inter- as well as intramolecular aza-Michael addition of aromatic amines to a range of Michael acceptors.
4 Experimental
Various aromatic amines, Michael acceptors and LiBF4 (Aldrich/Lancaster) were used as received while various 2’-amino chalcones were prepared by potassium phosphate catalyzed condensation of 2-amino acetophenone with a range of aldehydes. IR spectra were recorded as neat or as KBr disc on Perkin-Elmer [FT-IR-783] spectrometer. 1H (300 MHz) and 13C (75.4 MHz) NMR spectra were recorded on Bruker-AC-300 spectrometer as CDCl3 solutions of samples using TMS as internal standard and δ values are expressed in ppm.
4.1 General experimental procedure
4.1.1 Intermolecular aza-Michael reaction
To the stirred solution of aniline (2 mmol) and Michael acceptor (except conjugated ketone) (2 mmol) was added lithium tetrafluoroborate (10 mol %) and the mixture was stirred at appropriate temperature (Table 1) till completion of the reaction (TLC). Water (10 mL) was added and the product was extracted with ethyl acetate (3 × 15 mL). The combined organic extract was washed with water, dried (anhy. Na2SO4) and solvent removed. Purification of the resultant product by short column chromatography over silica gel (60–120 mesh) furnished the desired Michael addition product.
4.1.2 Intramolecular aza-Michael reaction
A mixture of 2’-amino chalcone (2 mmol) and lithium tetrafluoroborate (10 mol %) was stirred at 70 °C until completion of the reaction (TLC). Water (10 mL) was added and the product was extracted with ethyl acetate (3 × 15 mL). The combined organic extract was washed with water, dried (anhy. Na2SO4) and solvent removed. Purification of the resultant product by short column chromatography over silica gel (60–120 mesh) furnished the desired 2-aryl-2,3-dihydroquinolin-4-(1H)-one.
Most of the intra as well as intermolecular aza-Michael addition products are known compounds and their structures were confirmed by comparison of the observed spectral data with that reported earlier. The spectral data of the representative compounds is summarized below.
4.2 Spectral data of representative compounds
4.2.1 2-[4-(Propan-2-yl phenyl)]-2,3-dihydroquinolin-4(1H)-one, 5d
1H NMR (300 MHz, CDCl3,): δ 1.27 (d, 6H, J = 6.9 Hz, 2 x CH3), 2.73–2.98 (m, 2H, CH2), 4.52 (brs, 1H, NH), 4.73 (dd, 1H, J = 13.8 & 3.9 Hz, CH), 6.68–7.40 (m, 7H, ArH), 7.88 (dd, 1H, J = 7.8 & 1.4 Hz, ArH); 13C NMR (75 MHz, CDCl3,): δ 23.97, 33.86, 46.40, 58.22, 115.87, 118.34, 118.99, 126.64, 127.01, 127.61, 135.37, 138.33, 149.30, 151.61, 193.52 ppm; ESIMS: m/z = 266 (M + H).
4.2.2 2-(2,6-Dimethylphenyl)-2,3-dihydroquinolin-4(1H)-one,5h
1H NMR (300 MHz, CDCl3): δ 2.5–2.62 (m, 7H, 2 x CH3 & 1H from CH2), 3.19–3.30 (m, 1H, 1H from CH2), 4.52 (brs, 1H, NH), 4.73 (dd, 1H, J = 15.6 & 3.6 Hz, CH), 6.70–7.37 (m, 6H, ArH), 7.92 (dd, 1H, J = 7.8 & 1.4 Hz, ArH); 13C NMR (75 MHz, CDCl3): δ 20.50, 21.42, 54.16, 115.95, 118.02, 118.65, 127.88,127.98, 129.72, 129.91, 135.28, 135.36, 137.02, 151.93, 193.84 ppm; ESIMS: m/z = 252 (M + H).
4.2.3 2-Furanyl-2,3-dihydroquinolin-4(1H)-one, 5i
1H NMR (300 MHz, CDCl3): δ 2.92 (dd, 1H, J = 16.1 & 5.4 Hz, CH), 3.00 (dd, 1H, J = 16.3 & 9.8 Hz, CH), 4.79–4.84 (m, 2H, CH & NH), 6.25 (d, 1H, J = 3.2 Hz, ArH), 6.32 (m, 2H, ArH), 7.37–7.58 (brs, 3H, ArH), 7.84 (dd, 1H, J = 7.9 & 1.2 Hz, ArH); 13C NMR (75 MHz, CDCl3): δ 41.9, 50.7, 106.8, 110.3, 116.0, 118.5, 119.1, 127.3, 135.4, 142.4, 150.4, 153.3, 192.6 ppm; ESIMS: m/z = 214 (M + H).
4.2.4 2-(3,4-dimethoxyphenyl)-2,3-dihydroquinolin-4(1H)-one,5f
1H NMR (300 MHz, CDCl3): δ 2.87 (m, 2H, CH2), 3.86 (s, 6H, 2 x OCH3), 4.48 (brs, 1H, NH), 4.78 (dd, 1H, J = 13.2 & 4.0 Hz, CH), 6.70–7.33 (m, 6H, ArH), 7.85 (dd, 1H, ArH). 13C NMR (75 MHz, CDCl3): δ 46.7, 56.02, 58.4, 56.06, 109.5, 111.3, 116.0, 118.5, 119.02, 119.0, 127.6, 133.6, 149.1, 149.3, 151.7, 135.5, 193.5 ppm.
Acknowledgments
Authors (UVD and MAK) thank UGC, New Delhi for financial assistance. We are also thankful to DST and UGC, New Delhi for providing NMR and elemental analysis facilities to the Chemistry Department of Shivaji University, Kolhapur under FIST and SAP program.
1 The reaction between alkyl vinyl ketone with anilines is exothermic and we suppose that the heat liberated during the reaction is one of the driving forces of the reaction. In support of this supposition, computational studies on the calculation of Gibbs free energy as well as heat of formation of the products is currently on and these results will be communicated separately.
2 The resultant products decompose upon standing.


