1 Introduction
Mesoporous Silica Nanoparticles (MSN) have attracted considerable interest in life and materials science [1–6]. Their unique properties such as high specific surface area (800–1000 m2g−1), monodisperse diameters (50–200 nm) and tunable pore size (2 to 4 nm) offer a large scope of applications such as drug delivery [7–11], photodynamic therapy [12–14], MRI [15–18], cancer cells targeting [19–21], catalysis [22,23], antireflective coatings [24]… The functionalization of MSN is the subject of tremendous efforts and many different functions such as amine [12], epoxide [25] or thiol [26] are routinely used with MSN to construct more complex structures. In this field, the isocyanate function has also been used with the condensation of isocyanatopropyltriethoxysilane to the mesoporous silica framework. However, as shown by Radu et al. [27], the conditions of the condensation of the triethoxysilane moiety (toluene at high temperature) affects the isocyanate group with the partial hydrolysis of this later. To avoid this drawback, we turned to 3-isocyanatopropyltrichlorosilane as the reagent of choice. Indeed we have previously shown that 10-isocyanatodecyltrichlorosilane was suitable for the preparation of isocyanato-functionalized self-assembled monolayers on silicon wafers without alteration of the isocyanate function [28–31]. We now present our work on the functionalization of MSN using isocyanatopropyltrichlorosilane and the subsequent reactivity of the isocyanate function with different nucleophiles.
2 Results and discussion
Isocyanatopropyltrichlorosilane was synthesized by hydrosilylation of allylisocyanate with HSiCl3 using Karstedt's catalyst [28]. Isocyanatopropyltrichlorosilane was then grafted on the surface of MSN at RT in toluene or trichloroethylene in the presence of an excess of diisopropylethylamine, to lead to isocyanate-functionalized MSN. Diisopropylethylamine is necessary to trap HCl in order to avoid its subsequent addition to the isocyanate group. After 1 h, an aliquot was centrifuged in order to analyze the reaction. The isocyanate function was detected at 2273 cm−1 on the IR spectrum (KBr pellet, Fig. 1).
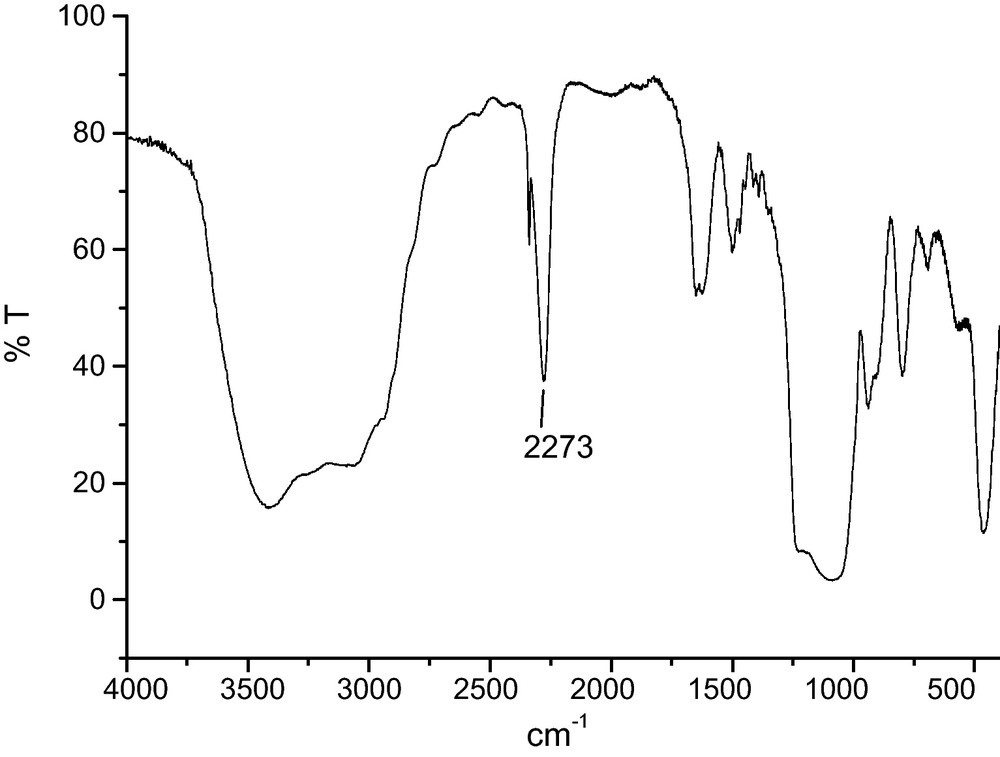
IR spectrum of isocyanate group grafted on Mesoporous Silica Nanoparticles (MSN) (KBr pellet).
Thus, the reaction was much faster than with isocyanatopropyltriethoxysilane which required 12 h at the reflux of toluene for its grafting at the surface of MSN [27]. This isocyanate function of precursor 1 (Scheme 1) was particularly prompt to hydrolysis, thus the subsequent reactions were carried out without isolation of the nanoparticles. To assess the accessibility of the isocyanate on the surface and its reactivity, nucleophiles bearing primary amino groups were used. (Scheme 1).
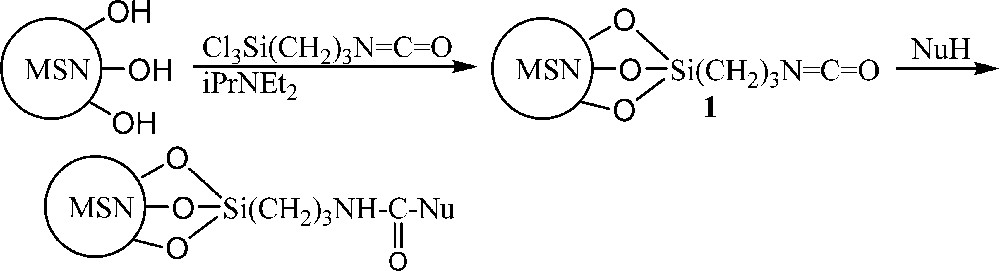
Reaction of Mesoporous Silica Nanoparticles (MSN) with isocyanatopropyltrichlorosilane to form precursor 1 and subsequent addition of nucleophiles.
Aliphatic or aromatic nucleophiles reacted with isocyanate-functionalized MSN overnight. Nanoparticles were isolated by centrifugation, washed with EtOH. IR spectra (KBr pellets) showed a total disappearance of the isocyanate function and the formation of a urea bond as attested by amide I and amide II bands (Table 1).
Data of the Mesoporous Silica Nanoparticles (MSN) after reaction with isocyanatopropyltrichlorosilane and subsequent addition of NuH to the isocyanate function.
| NuH | Amide I (cm−1) | Amide II (cm−1) | Specific Surface Area (m2g−1) | Loading (mmol/g) | DLS before grafting (nm) | DLS after grafting (nm) |
| p-Toluidine | 1646 | 1556 | 294 | 1.80 | 168 | 225 |
| p-Anisidine | 1650 | 1556 | 204 | 1.75 | 162 | 219 |
| Dodecylamine | 1695 | 1540 | 464 | 1.10 | 168 | 244 |
| Butylamine | 1691 | 1540 | 208 | 1.30 | 159 | 205 |
| BOC-hydrazine | 1687 | 1540 | 295 | 1.40 | 168 | 221 |
| 1650 | 1556 | 434 | 0.86 | 162 | 351 |
Solid-state 29Si direct polarization (DP) MAS NMR showed that the condensation method led to T3 (minor), T2 and T1 (major) species, in agreement with a covalent anchoring of the trichlorosilane group on the MSN surface. A representative spectrum is presented in Fig. 2.
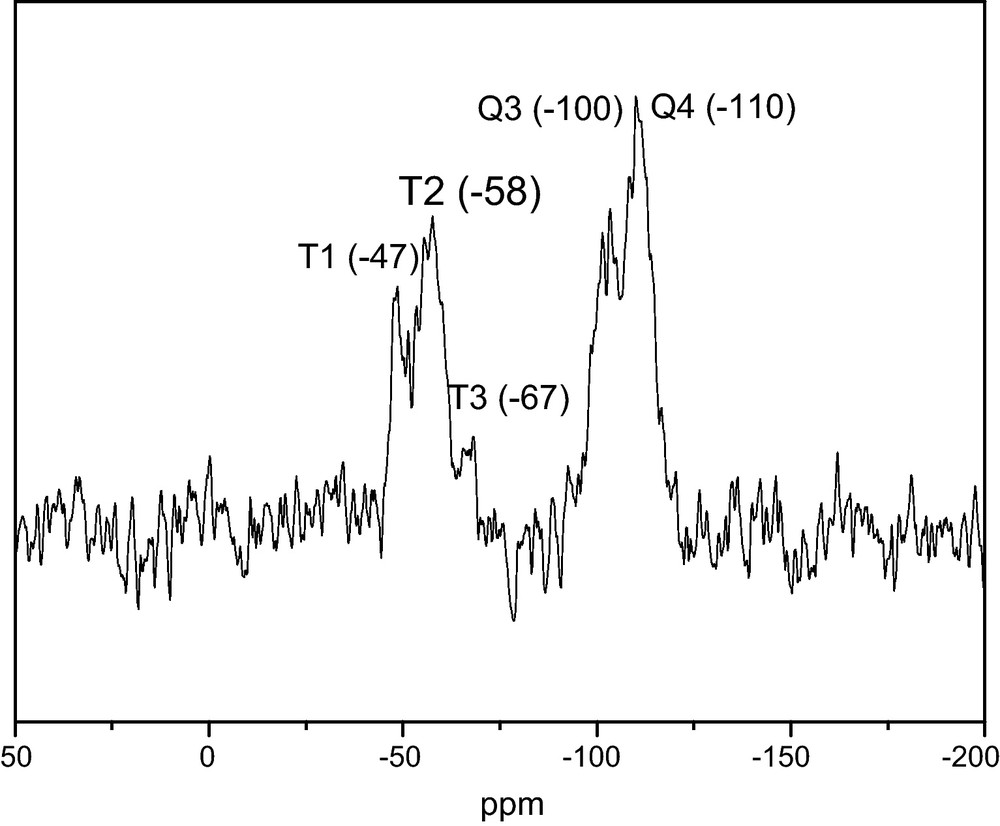
Solid-State 29Si DP MAS NMR of Mesoporous Silica Nanoparticles (MSN) functionalized with isocyanatopropyltrichlorosilane and dodecylamine.
Small molecules such as p-anisidine, p-toluidine, BOC-hydrazine, or butylamine were grafted on the surface of MSN and inside the pores as shown by the low N2 adsorption-desorption specific surface area obtained (BET) [32]. Alternatively larger molecules such as dodecylamine or biphotonic photosensitizer 1 [33] were preferentially grafted on the surface of MSN as an increase of the N2 adsorption-desorption specific surface area was noticed (Fig. 3).
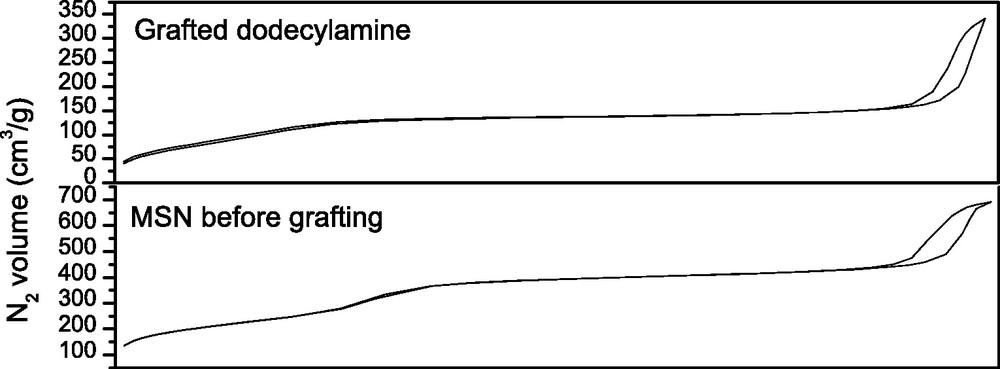
N2 adsoprtion-desorption experiments before and after grafting of dodecylamine.
The loading of the molecules determined by microanalysis (%N) was high (0.86–1.80) mmol g−1 which correspond to a monolayer coverage of the MSN [34] and a partial grafting inside the pores of the MSN in agreement with the BET results. Molecule 2 gave the lowest loading due to its steric hindrance. Dynamic light scattering experiments were performed in EtOH before and after the isocyanate grafting and the reaction with NuH. With small molecules (p-anisidine, p-toluidine, BOC-hydrazine, butylamine), an increase of the hydrodynamic diameter of 46–57 nm was observed whereas larger molecules (Dodecylamine, 2) gave 76–89 nm of the increase of the hydrodynamic diameter.
The UV–vis spectrum of grafted biphotonic photosensitizer 2 is reported in Fig. 4 showing that the mild conditions of grafting did not damage its structure. The condensation of the isocyanate group with BOC-hydrazine led to the protected semi-carbazide function in mild conditions. This function is very useful for the further grafting of biomolecules such as α-oxoaldehyde-functionalized peptides on nanoparticles [35].
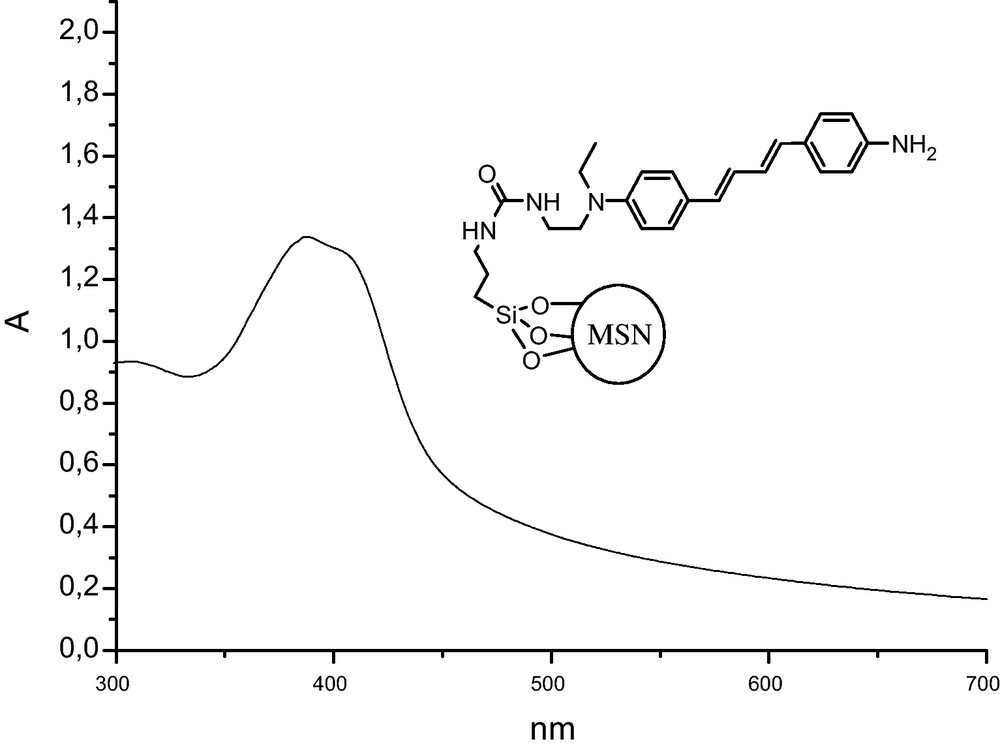
UV–vis spectrum of biphotonic photosensitizer 2 grafted on Mesoporous Silica Nanoparticles (MSN), recorded in EtOH (5 mg mL−1).
3 Conclusion
We have demonstrated that isocyanatopropyltrichlorosilane was the reagent of choice for the functionalisation of MSN with the isocyanate group without damaging this function, due to the high reactivity of the tricholorosilane moiety in mild conditions. Isocyanate-functionalized MSN was then successfully reacted with nucleophiles bearing primary amino groups showing the accessibility of the isocyanate group at the surface of MSN. A monolayer coverage of the MSN was observed with a partial grafting inside the pores of the MSN. Work is in progress to apply this chemistry to the preparation of MSN with gatekeeper for triggered drug delivery (Supplementary data).
4 Experimental
In a typical experiment, MSN (50 mg, 900 m2g−1 of specific surface area, 100 nm diameter) prepared as described [12], were suspended in toluene or trichloroethylene (2 mL) using ultrasounds (30 min). The suspension was cooled to 0 °C. Diisopropylethylamine (1 mmol) then isocyanatopropyltrichlorosilane (0.2 mmol) were successively added and the reaction was stirred for 1 h. Nucleophile bearing amino group (1 mmol) dissolved in toluene or trichloroethylene (2 mL) was then added and the reaction was stirred at RT overnight. MSN were isolated by centrifugation (20,000 rpm, 10 min) which allowed removing excess reagents and salts. Redispersion in EtOH (ultrasounds 15 min), then centrifugation three times afforded the functionalized MSN.
Acknowledgements
Wassim El Malti thanks the ministère de l’Éducation nationale de la Recherche et de la Technologie for an MENRT grant. We thank X. Dumail for the synthesis of biphotonic photosensitizer 2.


