1 Introduction
Despite several reports on the application of metalloporphyrins in the oxidation of organic compounds [1–8], there are few reports on their applications as Lewis acid catalysts. High-valent metalloporphyrins, in which the metal is in its highest oxidation state, can be used as mild Lewis acids. In this respect, electron-deficient porphyrins of Cr(III), Fe(III), Sn(IV) and V(IV) have been used in organic transformations [9–26].
Proctection of hydroxy compounds is of great importance in organic synthesism especially in the multi step transformations [27–29]. Tetrahydropyranylation with DHP, methoxymethylation with FDMA (formaldehyde dimethyl acetal), trimethylsilylation with HMDS (hexamethyldisilazane) and acetylation with Ac2O are the most frequently used methods for protection of hydroxy compounds [30,31]. Due to the remarkable stability of tetrahydropyranyl ethers under a variety of conditions such as alkaline media, Grignard reagents, alkyl lithiums, metal hydrides, oxidative reagents, and alkylating and acylating reagents, tetrahydropyranylation has attracted much attention in comparison with the others [30]. A variety of catalysts including protonic and Lewis acid catalysts such as p-toluene sulfonic acid (PTSA) [32], bis[trimethylsilyl]sulfate [33], (CH3)3SiI [34], CuCl2 [35], DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) [36], Ru(CH3)3(triphosphine)](OTf)2 [37], I2 [38], AlCl3.6H2O [39], In(OTf)3 [40], ZrCl4 [41], K5CoW12O40.3H2O [42], ionic liquids [43], Bu4N+Br3− [44], and LiOTf [45], K-10 clay [46], alumina impregnated with ZnCl2 [47], silica chloride [48], AlCl3@polystyrene [49], silica-based sulfonic acid [50,51], H6P2W18O62 [52], PdCl2(CH3CN)2 [53], vanadyl(IV) acetate [54], BF3-etherate [55] and homogeneous and heterogeneous [SnIV(TPP)(OTf)2] [56,57] have been developed for tetrahydropyranylation of alcohols and phenols.
Vanadium is required for normal health, and could act in vivo either as a metal cation or as a phosphate analogue, depending on the oxidation state, V(lV) or V(V), respectively. In proteins, vanadium is a cofactor in an algal bromoperoxidase and in certain prokaryotic nitrogenases. However, its oxidation state within organisms seems to be highly variable. The biochemistry of vanadium potentially involves four oxidation states that are relatively stable in aqueous solution. These are V2+, V3+, VO2+ and VO2+ (the oxidation states 2, 3, 4, and 5, respectively) [58].
In the present work, highly efficient tetrahydropyranylation of alcohols and phenols with 3,4-dihydro-2H-pyran catalyzed by reusable electron-deficient tetraphenylporphyrinatovanadium(IV) trifluoromethanesulfonate, [VIV(TPP)(OTf)2], is reported (Scheme 1).
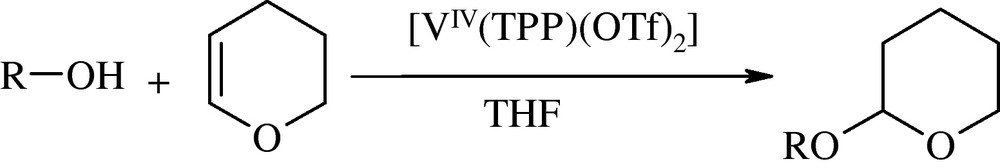
Tetrahydropyranylation of alcohols and phenols with DHP catalyzed by [VIV(TPP)(OTf)2].
2 Experimental
Chemicals were purchased from Fluka and Merck chemical companies. 1HNMR spectra were recorded in CDCl3 solvent on a Bruker-Avance 400 MHz spectrometer. Infrared spectra were run on a Philips PU9716 or Shimadzu IR-435 spectrophotometer. Elemental analysis was carried out with a LECO, CHNS-932 instrument. UV-Vis spectra were recoreded on a Shimadzu-160 spectrophotometer. All analyses were performed on a Shimadzu GC-16A instrument with a flame ionization detector using silicon DC-200 or Carbowax 20 M columns. Tetraphenylporphyrin, [VO(TPP)] and [V(TPP)Cl2] were prepared according to the literature [59–61].
2.1 Preparation of tetraphenyporphyrinatovanadium(IV) trifluoromethanesulfonate, [VIV(TPP)(OTf)2]
To a solution of V(TPP)Cl2 (0.97 g, 1 mmol) in THF (50 mL), at 55 °C, AgCF3SO3 (0.54 g, 2 mmol) was added. The solution was stirred at 55 °C for 30 min. The AgCl precipitate was filtered through a 0.45 μM filter. The resulting solution was evaporated at room temperature. Then, the [VIV(TPP)(OTf)2] was extracted with CH2Cl2 and the crystals were obtained after evaporation of solvent at room temperature.
Visible spectrum: 456 (Soret), 585, 628 nm; ν(KBr): 1030, 1168, 1224, 1298 cm−1 (belong to SO3 groups and porphyrin ring); CHNS analyses: Calcd. C, 57.44; H, 2.93; N, 5.83; S, 6.67 found: C, 57.35; H, 2.88; N, 5.91, S, 6.65.
2.2 General procedure for tetrahydropyranylation of alcohol and phenols with DHP catalyzed by [VIV(TPP)(OTf)2]
A solution of alcohol or phenol (1 mmol) and DHP (2 mmol per OH group) in THF (0.5 mL) was prepared. The [VIV(TPP)(OTf)2] (1 mol%) was added to this solution and stirred at room temperature. The progress of the reaction was monitored by GC. After completion of the reaction, the solvent was evaporated, Et2O (10 mL) was added and the catalyst was filtered. The filtrates were washed with brine, dried over Na2SO4 and concentrated under reduced pressure to afford the crude product.
2.3 Catalyst recovery and reuse
The reusability of the catalyst was checked in the sequential pyranylation of benzyl alcohol. At the end of each reaction, the solvent was evaporated, Et2O (10 mL) was added and the catalyst was filtered. The catalyst was used with fresh benzyl alcohol, DHP and THF.
3 Results and discussion
3.1 Investigation of catalytic activity of high-valent [VIV(TPP)(OTf)2] in the tetrahydropyranylation of alcohol and phenols with DHP
The preparation route for [VIV(TPP)(OTf)2] is shown in Scheme 2. The [VIVO(TPP)], which can be obtained commercially or prepared in laboratory, was converted to [VIV(TPP)Cl2] with SOCl2, which in turn was converted to [VIV(TPP)(OTf)2] with AgOTf. The catalytic activity of these three catalysts was checked in the tetrahydropyranylation of benzyl alcohol with DHP. The results showed that the catalytic activity has the following order: [VIV(TPP)(OTf)2](100%) > [VIV(TPP)Cl2] (52%) > [VIVO(TPP)] (17%). These data showed that introducing of OTf−groups has a crucial effect on the catalytic activity of [VIV(TPP)(OTf)2]. This may be due to the electron withdrawing nature of these non-coordinated ligands which in turn increases the electron-deficiency of metal center and therefore, the catalytic activity increases. Despite of high oxophilicity of VIV species, [VIV(TPP)(OTf)2] is a stable catalyst and can be used under air. Therefore, [VIV(TPP)(OTf)2] was chosen as catalyst for tetrahydropyranylation of alcohols and phenols. In this manner, the reaction parameters were optimized in the reaction of benzyl alcohol with DHP. As can be seen in Table 1, the best conditions are alcohol (1 mmol), DHP (2 mmol), catalyst (1 mol%) and THF as solvent. Under the optimized conditions a wide range of alcohols including primary, secondary and tertiary alcohols were converted to their corresponding THP-ethers in good to excellent yields and short reaction times. The results, which are summarized in Table 2, showed that all primary, secondary and tertiary alcohols including aromatic, aliphatic and cyclic ones were converted efficiently to their corresponding tetrahydropyranyl ethers in 3 to 8 min. In the case of aromatic alcohols, the nature of substituents has no significant effect on the product yield.
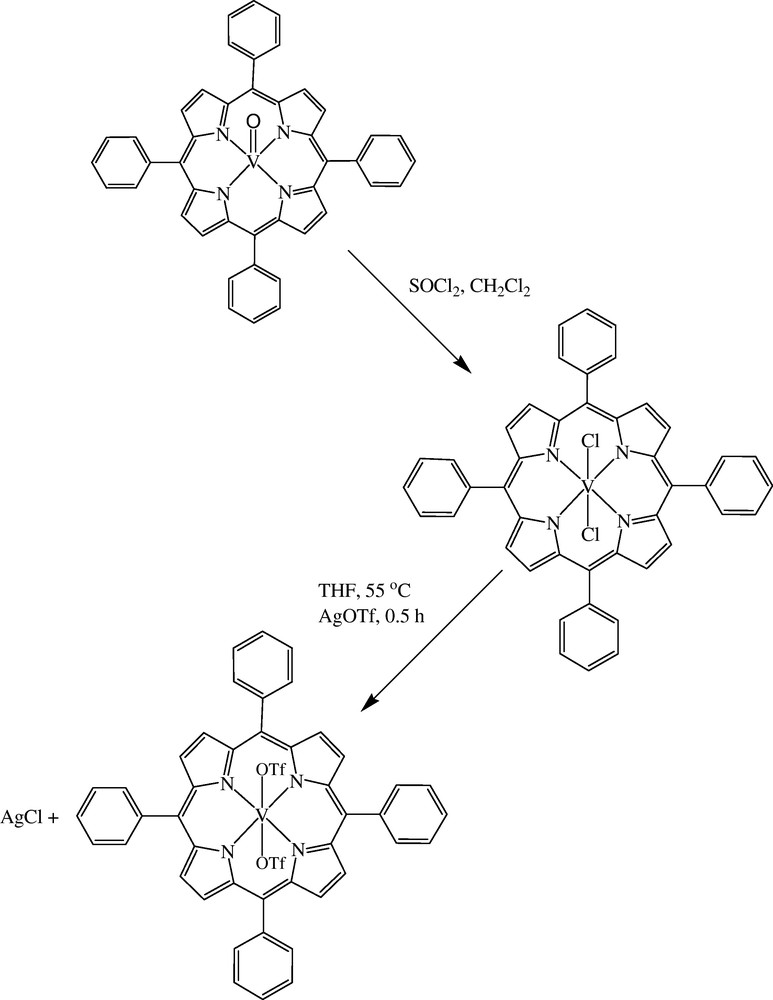
The preparation route for the catalyst.
Optimization of reaction parameters in the tetrahydropyranylation of benzyl alcohol (1 mmol) with DHP catalyzed by [VIV(TPP)(Otf)2] at room temperature.
| Entry | Catalyst (mol%) | DHP (mmol) | Solvent | Time (min) | Yield (%)a |
| 1 | 0.5 | 2 | THF | 3 | 74 |
| 2 | 1 | 2 | THF | 3 | 100 |
| 3 | 2 | 2 | THF | 3 | 100 |
| 4 | 1 | 1 | THF | 3 | 46 |
| 5 | 1 | 2 | CH3CN | 3 | 69 |
| 6 | 1 | 2 | CH2Cl2 | 3 | 58 |
| 7 | 1 | 2 | CHCl3 | 3 | 35 |
| 8 | 1 | 2 | n-Hexane | 3 | 19 |
a GC yield.
Tetrahydropyranylation of alcohols catalyzed by [VIV(TPP)(Otf)2] at room temperaturea.
| Entry | Hydroxy compound | THP ether | Time (min) | Yield (%)b |
| 1 | 3 | 100 | ||
| 2 | 2 | 95 | ||
| 3 | 2 | 94 | ||
| 4 | 3 | 95 | ||
| 5 | 2 | 93 | ||
| 6 | 5 | 97 | ||
| 7 | 4 | 97 | ||
| 8 | 4 | 94 | ||
| 9 | 8 | 100 | ||
| 10 | 8 | 94 | ||
| 11 | 8 | 85 | ||
| 12 | 4 | 94 | ||
| 13 | 4 | 92 | ||
| 14 | 5 | 100 | ||
| 15 | 8 | 91 | ||
| 16 | 6 | 85 | ||
| 17 | 8 | 85 | ||
| 18 | 5 | 96 | ||
| 19 | 5 | 95 |
a Reaction conditions: alcohol (1 mmol), DHP (2 mmol), catalyst (1 mol%), THF (0.5 mL).
b GC yield.
The ability of this catalyst in the tetrahydropyranylation of phenols was also investigated. The results showed that this catalyst efficiently converted different phenols to their corresponding THP-ethers in 3 to 5 min (Table 3).
Tetrahydropyranylation of phenols with DHP catalyzed by [VIV(TPP)(Otf)2] at room temperaturea.
| Entry | Phenol | THP ether | Time (min) | Yield (%)b |
| 1 | 4 | 90 | ||
| 2 | 4 | 92 | ||
| 3 | 4 | 85 | ||
| 4 | 5 | 90 | ||
| 5 | 5 | 90 |
a Reaction conditions: phenol (1 mmol), DHP (2 mmol), catalyst (1 mol%), THF (0.5 mL).
b GC yield.
Since the catalyst was active in the tetrahydropyranylation of primary, secondary and tertiary alcohols as well as phenols, a set of competitive reactions was performed to investigate the chemoselectivity of this method. In this manner, an equimolar mixture of benzyl alcohol (primary alcohol) with benzhydrol (secondary alcohol) and 2-methyl-3-phenyl-2-propanol (tertiary alcohol), and phenol was subjected to tetrahydropyranylation with DHP (2 mmol) in the presence of [VIV(TPP)(OTf)2]. The results indicated that primary alcohol was more reactive in the presence of secondary and tertiary ones, and phenol (Table 4). When the same reactions were carried out in the presence of 4 mmole of DHP, the same results were obtained.
Selective pyranylation of alcohols and phenols catalyzed by [VIV(TPP)(Otf)2] in THFa.
| Row | ROH | THP ether | Time (min) | Yield (%)b,c |
| 1 | 3 | 90 (100) | ||
| 30 (37) | ||||
| 2 | 3 | 90 (100) | ||
| 24 (34) | ||||
| 3 | 3 | 92 (100) | ||
| 47 (51) | ||||
| 4 | 3 | 90 (100) | ||
| 14 (25) |
a Reaction conditions for a binary mixture: 1 mmole of each alcohol or phenol, DHP (2 mmol), catalyst (1 mol%), THF (0.5 mL).
b GC yield.
c Yields in the parentheses refer to the yields in the presence of 4 mmole of DHP.
The results obtained by this catalytic system were compared with some of those reported in the literature (Table 5). As can be seen, this catalytic system is more efficient than the others. In order to show the advantage of this method, the tetrahydropyranylation of benzyl alcohol was carried out in the presence of some of available catalysts listed in Table 5 under the same conditions described for [VIV(TPP)(OTf)2]. It was observed that the [VIV(TPP)(OTf)2]/DHP catalytic system is much more efficient than the others.
Comparison of the results obtained for the tetrahydropyranylation of benzyl alcohol catalyzed by [VIV(TPP)(Otf)2] with those obtained for the recently reported catalysts.
| Entry | Catalyst | Catalyst (mol%) | Temperature | Time (min) | Yield (%) | Ref. |
| 1 | [VIV(TPP)(Otf)2] | 1 | R.T. | 3 | 100 | – |
| 2 | bis[trimethylsilyl]sulfate | 2 | 0 | 12 | 97 | [22] |
| 3 | CuCl2 | 15 | R.T. | 20 | 83 | [24] |
| 4 | AlCl3.6H2O | 1 | R.T. | 30 | 94 | [28] |
| 5 | In(Otf)3 | 0.5 | 0 | 30 | 85 | [29] |
| 6 | ZrCl4 | 5 | Reflux | 180 | 92 | [30] |
| 7 | K5CoW12O40·3H2O | 1 | R.T. | 5 | 97 | [31] |
| 8 | Ionic liquid/PPh3.HBr | 10 | R.T. | 240 | 96 | [32] |
| 9 | n-Bu4N+Br3− (TBATB) | 2.5 | R.T. | 60 | 85 | [33] |
| 10 | LiOTf | 60 | Reflux | 150 | 96 | [34] |
| 11 | K-10 clay | 25 mg | R.T. | 5 | 92 | [35] |
| 12 | Al2O3/ZnCl2 | – | 5 | 10 | 89 | [36] |
| 13 | Silica chloride | 10 mg | R.T. | 10 | 93 | [37] |
| 14 | AlCl3@PS | 15 | R.T. | 40 | 97 | [38] |
| 15 | SiO2-SO3H | 2 | R.T. | 15 | 92 | [39] |
| 16 | H6P2W18O62 | 1 | 20 | 120 | 98 | [41] |
| 17 | PdCl2(CH3CN)2 | 10 | R.T. | 60 | 72 | [42] |
| 18 | Vanadyl (IV) acetate | 27 | R.T. | 60 | 95 | [43] |
| 19 | [SnIV(TPP)(Otf)2] | 1 | R.T. | 4 | 96 | [44] |
| 20 | [SnIV(TPP)(Otf)2]@CMP | 1 | R.T. | 4 | 97 | [45] |
| 21 | CuCl2 | 1 | R.T. | 3 | 26 | – |
| 22 | AlCl3.6H2O | 1 | R.T. | 3 | 17 | – |
| 24 | ZrCl4 | 1 | R.T. | 3 | 11 | – |
| 25 | K5CoW12O40·3H2O | 1 | R.T. | 3 | 78 | – |
| 26 | LiOTf | 1 | R.T. | 3 | 8 | – |
| 27 | H6P2W18O62 | 1 | R.T. | 3 | 12 | – |
| 28 | Vanadyl (IV) acetate | 1 | R.T. | 3 | 10 | – |
The actual mechanism is not clear at present. However, based on a plausible mechanism, DHP is first activated by V(IV) porphyrin to afford 1. Nucleophilic attack of alcohol to 1 gives 2, which upon a proton transfer step produces the final product 3 and releases the catalyst for the next run (Scheme 3). Comparison of catalytic activity of [VIV(TPP)(OTf)2] with Vanadyl(IV) acetate shows that the presence of OTf−groups increases the interaction of DHP with catalyst. This can be attributed to the high electron-deficiency of V(IV) center in the presence of OTf−groups.
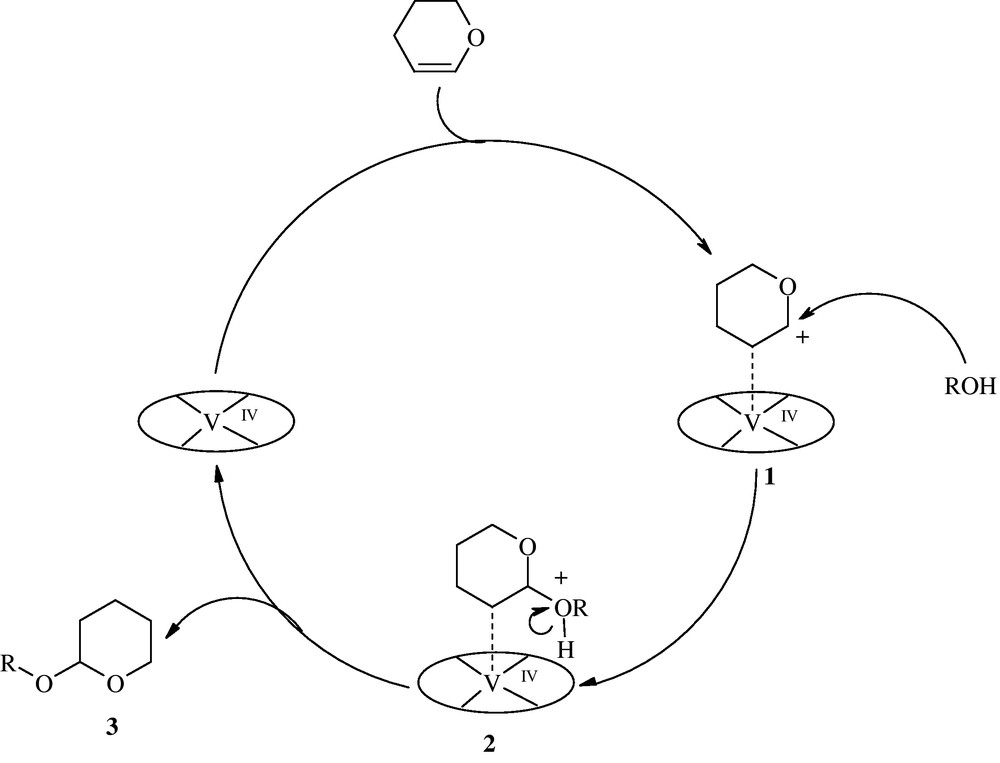
The proposed mechanism for tetrahydropyranylation of alcohols and phenols with DHP catalyzed by [VIV(TPP)(OTf)2].
3.2 Catalyst reusability
The reusability of this catalyst was also investigated in the multiple pyranylation of benzyl alcohol with DHP under the same reaction conditions described in the general procedure. At the end of each reaction, the solvent was evaporated, Et2O was added and the catalyst was filtered. The recovered catalyst was used with fresh benzyl alcohol and DHP. The results showed that after using the catalyst for several consecutive times (five times were checked), no decrease was observed in its catalytic activity. The nature of the recovered catalyst was monitored by its UV-Vis spectrum (Fig. 1B), in which no change was observed in its spectrum and since the catalyst retained its activity in the protection reaction, it seems that the recovered catalyst is porphyrin triflates.

UV-vis spectrum of: A: [VIV(TPP)(OTf)2] in tetrahydrofuran (1.0 × 10−4 M, path length = 1.0 cm) at 25 °C and B: recovered [VIV(TPP)(OTf)2].
4 Conclusion
In conclusion, electron-deficient [VIV(TPP)(OTf)2] can be used as an efficient and reusable catalyst for tetrahydropyranylation of alcohols and phenols with DHP. This catalyst efficiently catalyzed the conversion of primary, secondary and tertiary alcohols as well as phenols to their corresponding THP-ethers. Short reaction times, good to excellent yields and reusability of the catalyst are noteworthy advantages of this catalytic system.
Acknowledgement
We are thankful to the Center of Excellence of Chemistry of University of Isfahan (CECUI)) for financial support of this work.


