1 Introduction
Nitrosoarenes are an important class of chemical compounds which are used for the preparation of some organic intermediates. These compounds also find applications in analysis as reagents [1,2]. Because of the low electrophilicity of nitrosating agents, there are fewer reports about the nitrosation reaction in comparison with nitration reaction. One of the most common procedures for the synthesis of nitrosoarenes is the electrophilic aromatic nitrosation reaction using HNO2 as nitrosating agent [3]. A major problem in this reaction is the instability of the aromatic nitroso-compounds formed. These compounds can decompose by oxidation by NO2 [4], or by nitration with the nitric acid which was produced from the aerobic oxidation of nitrous acid [5]. Zyk et al. reported the synthesis of nitrosoarenes via the nitrosation of O-alkylphenols and N,N-dialkylanilines using nitrosonium ethyl sulfate as nitrosonium reagent [2]. Zolfigol et al. used inorganic acidic salts such as aluminum hydrogen sulfate for in situ generation of HNO2 for the nitrosation of secondary amines [6]. Ionic liquids have interesting physical properties and are used as solvents in organic chemistry [7]. They are usually an ion pair of weakly coordinated organic cations such as 1-butyl-3-methylimidazolium, N-alkylpyridinium or tetraalkylammonium with inorganic anions such as Cl−, BF4− or HSO4− and a classical example is the readily available 1-butyl-3-methylimidazolium tetrafluoroborate [bmim][BF4] [8].
Ionic liquids containing chemically active functional groups on their cations or anions are known as task-specific ionic liquids (TSILs). Functional groups are covalently bonded to cation or anion in these compounds. TSILs have been increasingly used as solvents and reagents or catalysts due to their specific properties [9–13]. In continuation of our interest to use ionic liquids (IL's), water or solventless systems as green reaction media [14–23], in this report, we wish to highlight our results on using new nitrite ionic liquid (IL-ONO) and new immobilized ionic liquid as nitrosonium source reagents in nitrosation reaction to produce nitrosoarenes in good to excellent yields at 0–5 °C in aqueous media (Scheme 1).
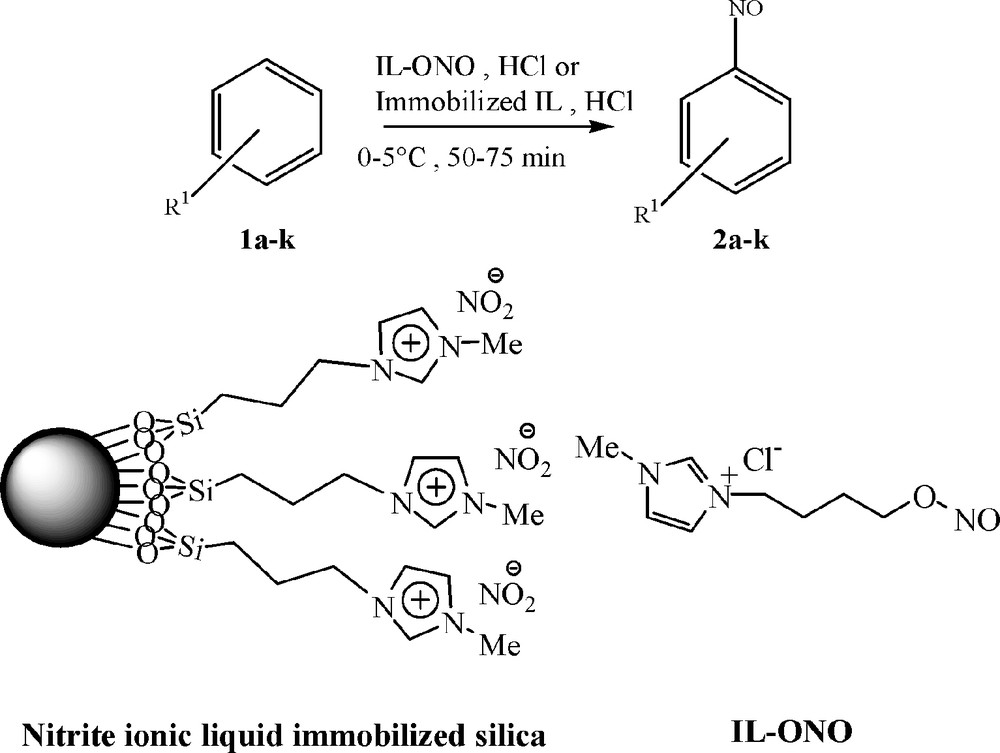
Electrophilic aromatic nitrosation reaction using immobilized ionic liquid and nitrite IL-ONO.
Nanoparticles and ionic liquids are very important in materials chemistry. The combination of properties of nanoparticles and ionic liquids has already been studied by some researchers [24]. The ionic liquid 1-methyl-3-(20-mercaptoacetoxyethyl) imidazolium chloride was used to stabilize palladium nanowires [25] and organosilanes containing covalently bonded catalyst were used as a linker or coupling agent to an oxide support [26]. Catalytic properties of the ionic liquids were transferred to a solid catalyst in immobilization process [27]. Immobilized ionic liquids should be more widely applicable than their neat precursors because of their miscibility with the organic solvent. Ionic liquids immobilized on silica can successfully be studied by optimized multinuclear one- and two-dimensional 1H and 13C HRMAS NMR spectroscopy of their suspensions in a suitable solvent, such as DMSO. In comparison to the pure ionic liquids, immobilized ionic liquids offer the additional features that they facilitate recovery of the catalyst and can be used in gas phase reactions [28].
2 Results and discussion
N-methyl-3-(3-trimethoxysilylpropyl) imidaz- olium chloride 3, [MTMSPIM]Cl−, was prepared from the reaction of N-methyl imidazole with (3-chloropropyl) trimethoxysilane at 80 °C. The solution of ionic liquid 3 in CH2Cl2 was then added to the suspension of freshly prepared silica nanoparticles in CH2Cl2. After stirring the mixture for 3 days at 40–50 °C, the ionic liquid immobilized on silica 4 was prepared. Immobilized nitrite ionic liquid (Im-IL) 5 was obtained in 98% yield as a white powder by stirring of 4 with excess amount of NaNO2 in deionized water for 24 h at room temperature (Scheme 2).
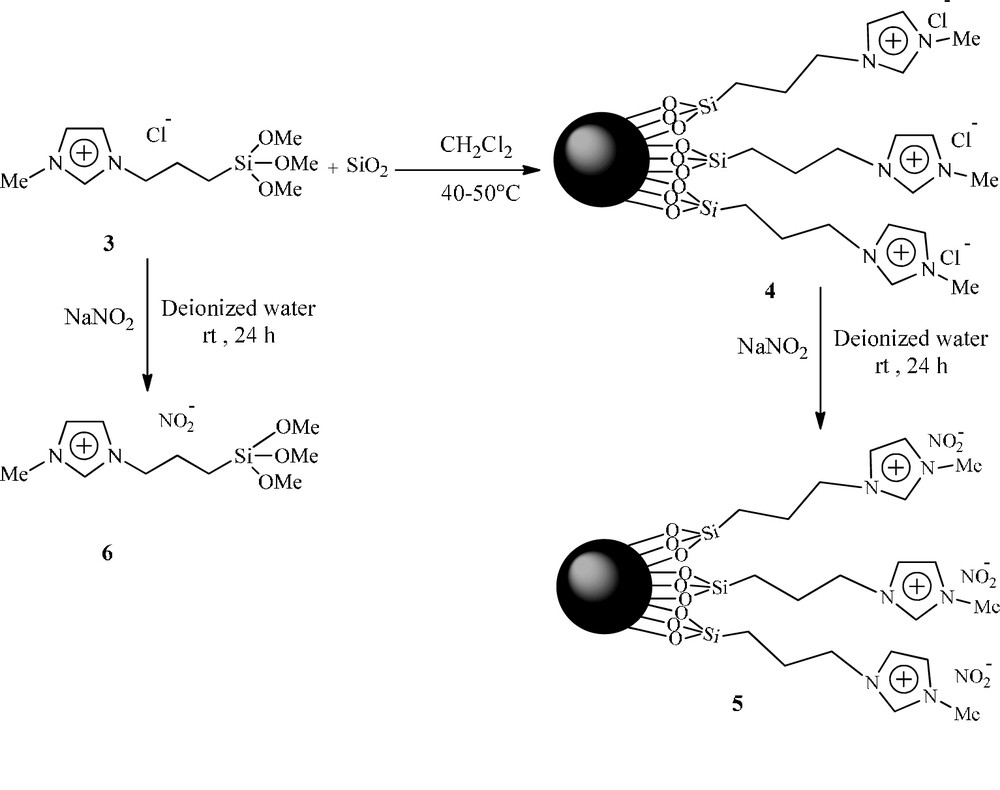
Preparation of immobilized nitrite ionic liquid 5.
The SEM image of silica nanoparticles was obtained and compared with the SEM images of immobilized nitrite ionic liquid 5 (Figs. 1 and 2). Comparing the two SEM images showed that the size of the silica nanoparticles is larger than immobilized nitrite ionic liquid 5.
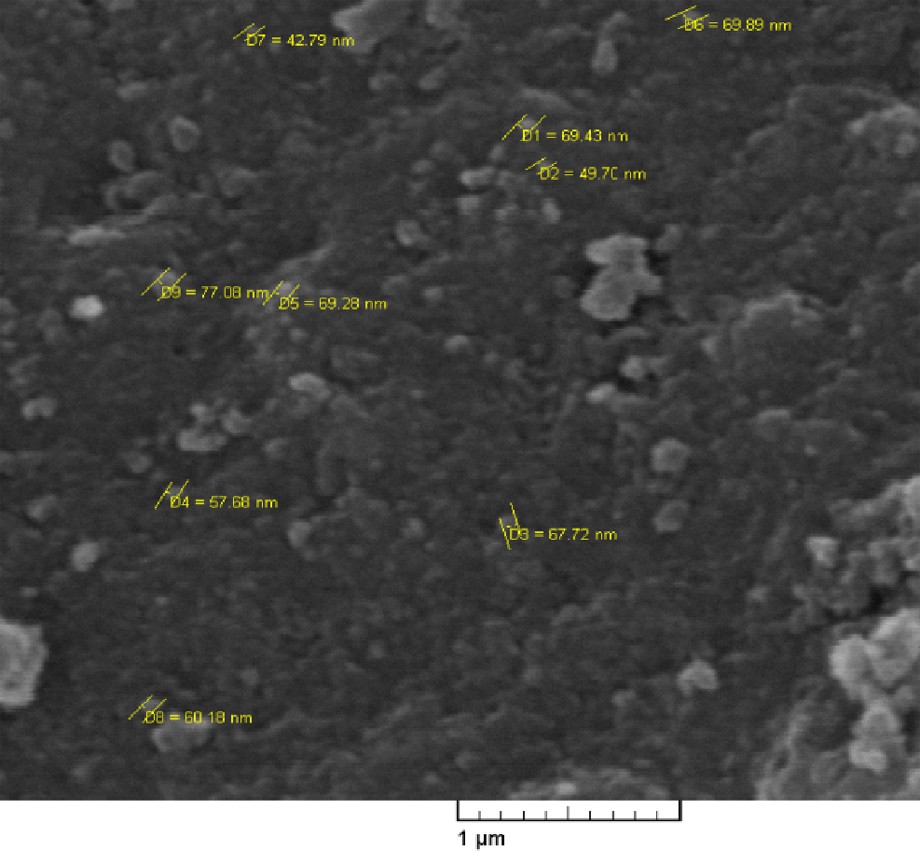
The SEM image of immobilized nitrite ionic liquid 5.

The SEM image of silica nanoparticles.
Ionic liquid, 1-(4-nitritobutyl)-3- methylimidazolium chloride was synthesized according our very recently published article [29].
To examine its activity as nitrosonium source, this ionic liquid (IL-ONO) was used for the nitrosation of N,N-dimethylaniline in the presence of HCl 37%, at 0–5 °C. The experimental results revealed that nitrite-functionalized IL- ONO, exhibits a very high activity and 4-nitroso-N,N-dimethylaniline 2a was prepared in excellent yield. Optimization of the reaction conditions was studied with different molar ratios of the ionic liquid, N,N-dimethylaniline and HCl. The best ratio was found to be 1:1:1.5. Increasing the amount of ionic liquid led to the mixture of products. To examine the versatility of this procedure, IL-ONO was then used for nitrosation of other aromatic compounds.
As it can be seen from Table 1, active aromatic compounds, containing electron releasing groups react faster and afforded the related nitroso products in higher yields in comparison with toluene. Nitrobenzene and benzoic acid were not nitrosated under these conditions. Phenol, phenethol, N,N-dialkylaniline and anisole nitrosated regioselectively at 4 position as showed in Table 1. β-Naphtol nitrosated regioselectively at α position. α-Naphtol was nitrosated at 2 and 4 positions with 56:35 ratio (Table 1, entry 4). Despite previous reports, the nitroso products were stable during the reaction and no oxidated nitro product was observed under these conditions [5]. Dealkylation is very often observed in the nitrosation of alkyl phenyl ethers [35]. However, using the nitrite IL-ONO for the nitrosation of anisole and phenethol in virtually afforded to related pure nitroso products and the reactions were not accompanied by dealkylation. All of products were characterized by comparing their (1H, 13C NMR and IR) spectroscopic data and melting points with literature values.
Electrophilic aromatic nitrosation using new nitrite ionic liquid (IL-ONO) and new immobilized ionic liquid (Im-IL).
| Entry | Ar-H | Product | Time (minute) | M.P (°C) | Yielda (%) | |||
| IL (ONO) | Im-IL | Found | Reported (reference) | IL (ONO) | Im-IL | |||
| 1 | 65 | 60 | 92–94 | 92 [2] | 91 | 93 | ||
| 2 | 60 | 57 | 103–106 | 109 [30] | 92 | 92 | ||
| 3 | 75 | 72 | 135–138 | 133 [31] | 89 | 90 | ||
| 4 | 55 | 55 | 155–157 | 142–144 [32] | 56b | 56b | ||
| 5 | 60 | 61 | 82–84 | 84–84 [2] | 91 | 90 | ||
| 6 | 62 | 58 | 70–73 | 71–72 [2] | 90 | 92 | ||
| 7 | 65 | 60 | 35–36 | 30–32 [2] | 94 | 95 | ||
| 8 | 65 | 60 | 23 | 23–24 [2] | 88 | 87 | ||
| 9 | 50 | 42 | 156–159 | – | 32 | 35 | ||
| 10 | 68 | 63 | 156–158 | 160–162 [33] | 63c | 63c | ||
| 11 | 75 | 72 | Liquid | Liquid [34] | 31 | 37 |
a Isolated yield.
b Total yield of reaction was 91% and 4-nitroso-1-naphtol was also isolated in 35%, yield in this reaction.
c Total yield of reaction was 93% and 2-nitrosocresol was also isolated in 30% yield in this reaction.
The plausible mechanism for acid-catalyzed nitrosation of 2-naphtol in IL-ONO was depicted in Scheme 3.
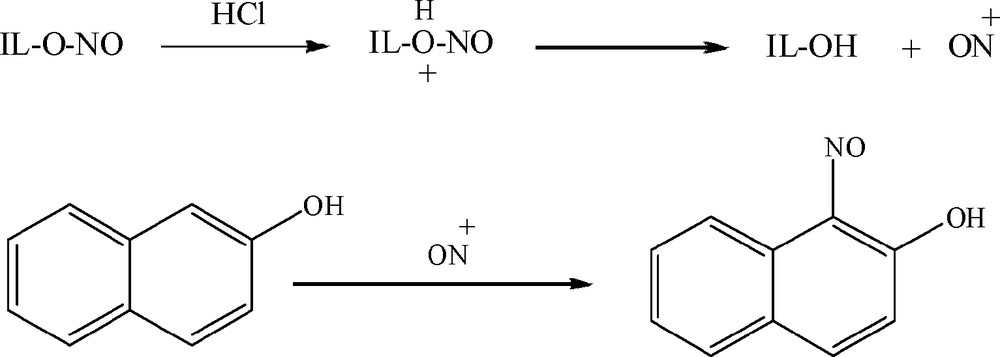
Plausible mechanism for nitrosation of 2-naphtol.
Initially, the nitrosonium ion was formed from protonated IL-ONO. Electrophilic substitution of nitrosonium ion at α-position of 2-naphtol was accrued to afford 1-nitroso-2-naphtol. In the present study, we investigated that IL-OH can be recycled. After removal of the mixture of product, possible impurities and unreacted materials, the remaining IL-OH was reused for the preparation of IL-ONO and consecutive three cycles without significant loss in its efficiency (Table 2).
The comparison of efficiency of recycled IL-OH for the preparation of new nitrite ionic liquid (IL-ONO) and consecutive electrophilic aromatic nitrosation.
| Run | Yielda (%) | |||
| 2a | 2b | 2g | 2j | |
| 1 | 91 | 92 | 94 | 93 |
| 2 | 89 | 91 | 92 | 91 |
| 3 | 90 | 90 | 93 | 92 |
a Yields of recrystallized product.
For comparison, Im-IL 5 was used in place of IL-ONO in this procedure at the same optimized conditions as described above. The results were shown in Table 1. After removal of the mixture of product, possible impurities and unreacted materials, the immobilized ionic liquid in the feed was washed tree times with ethanol and reused after exchanging of the chlorid anion with nitrite ionic liquid. No significant decrease in yield after three cycles of the immobilized ionic liquid.
3 Conclusions
In conclusion, it was found that IL-ONO and immobilized nitrite ionic liquids are convenient reagents for electrophilic aromatic nitrosation of aromatic compounds. Reactions of these reagents with arenes are not accompanied by side processes such as oxidation or dealkylation. Regioselectivity, easy and clean work-up procedure and high yields make this method attractive for organic chemists. Thus, it provides a more practical alternative to the existing methodologies.
4 Experimental
4.1 General information
All reagents were purchased from Merck Company and used without further purification. Infrared spectra were recorded in KBr and were determined on a Perkin Elmer FT-IR spectrometer. 1H NMR spectra were recorded on a Bruker Avance AC-400 MHz using DMSO-d6 or CDCl3 as the deuterated solvents and TMS as internal standard. All melting points measured in open glass-capillaries using Stuart melting point apparatus.
4.1.1 Synthesis of 1-methyl-3-(3-trimethoxysilylpropyl) imidazolium chloride 1-Methylimidazole
N-Methylimidazole (20 mL, 0.25 mol) and (3-chloropropyl) trimethoxysilane (6.04 g, 0.25 mmol) were refluxed at 80 °C for three days in the absence of any catalyst and solvent. The unreacted materials were washed by diethyl ether (3 × 8 mL). The diethyl ether was removed under reduced pressure at room temperature, followed by heating under high vacuum, to yield a yellowish viscous liquid. Isolated yield was 98%. IR (KBr): 1656, 1612, 1584/cm. 1H NMR (400 MHz, CDCl3): δ = 10.22 (1H, broad, Ar-H), 7.59 (1H, dd, Ar-H), 7.26 (1H, dd, Ar-H), 4.06 (2H, t, -NCH2), 3.86 (3H, s, -NCH3), 3.30 (9H, s, -OCH3), 1.74 (2H, tt, CH2), 0.37 (2H, t, SiCH2).
4.1.2 Synthesis of silica nanoparticles
Ammonia solution 25% (750 μL, 10 mmol) and water (1.98 mL) were added into a 250 mL round bottom flask containing absolute methanol (100 mL). The solution is stirred for 10 min at room temparature. While stirring of the solution, tetraethoxysilane, TEOS (10.41 g, 500 mmol) was added dropwise. The final solution is stirred continously for three days at ambient temperature. The particle size was examined under scanning electron microscopy.
4.1.3 Procedure for the immobilization of chloride ionic liquid onto silica nanoparticles
The silica nanoparticles suspension are precipitated with n-hexane and extracted through centrifugation (twice at 6000 rpm) before being re-suspended in dichloromethane. Silica (1.016 g) was suspended in CH2Cl2 (5 mL) and the solution of 1-methyl-3-(3-trimethoxy silylpropyl) imidazolium chloride (300 mg, 0.929 mmol) in CH2Cl2 was then added. The mixture was stirred for 3 days at 40–50 °C. In the following step, the solvent and the methanol created in the grafting step were distilled off and the remaining solid dried under high vacuum and the excess of 1-methyl-3-(3-trimethoxy silylpropyl) imidazolium chloride removed by extraction with boiling dichloromethane. After drying of residue under vacuum at room temperature, the nanoparticles of immobilized chloride ionic liquid on to silica was prepared.
4.1.4 Anion exchange in the immobilized ionic liquid
Immobilized chloride ionic liquid 4 and an excess amount of NaNO2 were added into the deionized water and stirred for 24 h at room temperature. NaCl which was prepared during the exchange of chloride with nitrite anions, was removed by washing the mixture with deionized water (3 × 30 mL). Immobilized nitrite ionic liquid was obtained typically in 98% yield as a white powder. Immobilized nitrite ionic liquid nanoparticles were examined under scanning electron microscopy.
4.2 General procedure
4.2.1 Electrophilic aromatic nitrosation
Aromatic compound (50 mmol) and freshly prepared IL-ONO or immobilized nitrite ionic liquid (50 mmol) were added to water (5 mL) and mixed thoroughly at 0–5 °C. Then HCl 37% (75 mmol, 2.3 mL) was added to the mixture continuously and stirred for a time as shown in Table 1. The participated products were filtered and washed three times with cold water to afford the crude nitrosoarenes. The crude products were purified by recrystallization from ethyl acetate/n-hexane. Product 2h was purified by flash chromatography on silica gel.
4.3 Selected spectroscopic data
4.3.1 1-Nitroso-2-naphthol 2b
m.p. 103-106 °C. IR (KBr): 3405, 1625, 1530–1600/cm. 1H NMR (400 MHz, CDCl3): δ = 14.08 (1H, s, OH), 8.83 (1H, d), 7.66–7.64 (1H, d), 7.6–7.49 (3H, m), 6.40–6.38 (1H, d). Anal. Calc. for C10H7NO2: C 69.36; H 4.07; N 8.09%. Found: C 70.05; H 4.10; N 8.00%.
4.3.2 2-Nitroso-1-naphthol 2d
m.p. 155-157 °C. IR (KBr): 3201, 1669, 1594–1627/cm. 1H NMR (400 MHz, DMSO-d6): δ = 13.49 (1H, s, OH) 7.81–7.61 (4H, m), 7.15–7.12 (1H, d), 7.06–7.04 (1H, d). Anal. Calc. for C10H7NO2: C 69.36; H 4.07; N 8.09%. Found: C 70.12; H 4.01; N 8.12%.
4.3.3 2-Nitroso hydroquinone 2i
m.p. 156-159 °C. IR (KBr): 3221, 1631, 1500–1600/cm. 1H NMR (400 MHz, DMSO-d6): δ = 13.62 (1H, s, OH), 8.63 (1H, s, OH), 6.88 (1H, s), 6.56 (2H, s). Anal. Calc. for C6H5NO3: C 51.80; H 3.62; N 10.07%. Found: C 52.05; H 3.64; N 9.99%.
4.3.4 4-Nitroso-m-cresol 2j
m.p. 156-158 °C. IR (KBr): 3120, 1633, 1570–1594/cm. 1H NMR (400 MHz, DMSO-d6): δ = 13.54 (1H, s, OH), 7.66–7.63 (1H, d), 6.39–6.36 (1H, dd), 6.32 (1H, s), 2.09 (3H, s, CH3). Anal. Calc. for C7H7NO2: C 61.31; H 5.14; N 10.21%. Found: C 61.51; H 5.16; N 10.18%.
Acknowledgements
The partial financial assistance from the Research Vice Chancellor of Azarbaijan University of Tarbiat-Moallem is gratefully acknowledged.


