1 Introduction
The classical Mannich reaction, in which an aminomethyl group is introduced to the α position of a carbonyl function, provides useful routes for the synthesis of β-amino ketones and esters, which are versatile building blocks for the preparation of many nitrogen-containing biologically important compounds [1–4].
Recently, a three-component approach for this reaction using organocatalyst and other catalysts, with in situ formation of imines, has gained considerable importance as it allows structural variations. Accordingly, several recent reports of catalytic Mannich reaction using known organocatalyses [5–7], proline [8–11], siloxy serine [12], adenine [13], Trypsin [14], p-dodecyl benzenesulphonic acid (DBSA) [15,16], ionic liquid [17–20], Zn(OTf)2 [21], Silica-supported aluminum chloride [22], ZrOCl2·8H2O [23], Bismuth Triflate [24–26], cationic organobismuth complex [27], HCl/SDS [28], boric acid and glycerol [29], HBF4 [30], Zn(proline)2 [31], ceric ammonium nitrate [32], heteropoly acid [33], WOx–ZrO2 [34], polyaniline salts [35], Fe(Cp)2PF6[36], DDQ [37], ZnCl2/SiO2 [38], SO42−/TiO2 [39], TiCl4/PhSiCl3 [40,41], perchloric acid in Triton X10 [42], ZnI2 [43], 2-pyrrolidinecarboxylic acid ionic liquid [44], chiral Brønsted acid [45], are found in the literature.
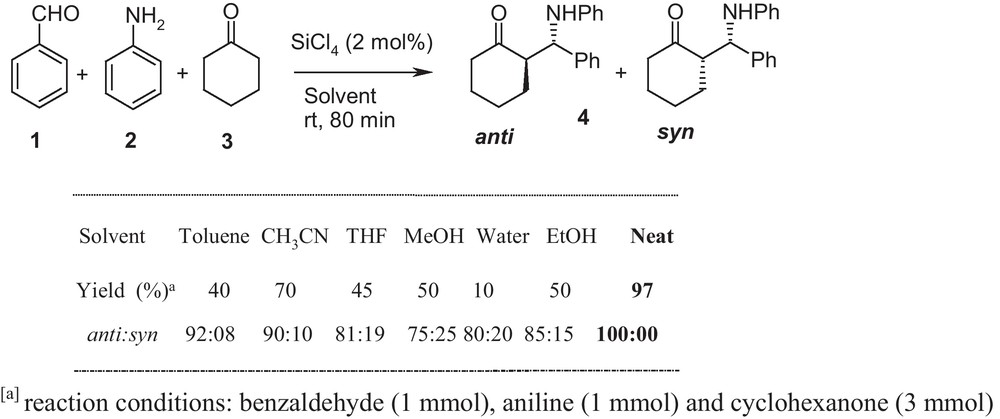
However, the yields obtained were only moderate, even with high catalyst loadings and regardless of the immense importance of this reaction, only a few diastereoselective α-aminomethylation reactions have been developed (Table 1). Thus, expanding the scope of Lewis acid catalysis under solvent-free condition in a one-pot three-component Mannich reaction is a useful and challenging objective. In this context, we wish to report a simple version of an environmentally benign Mannich reaction with different aromatic aldehydes, cyclic ketones and aromatic amines for the synthesis of β-amino carbonyl compounds under solvent-free condition in presence of SiCl4 as catalyst with good yield and excellent selectivity as shown in Scheme 1.
One-pot, three-component Mannich reaction with different catalyst systems.
| Entry | Catalyst | Mol % | Solvent | Yields | Anti:syn | Ref. |
| 1 | Ln(OTf)3 | 2 | Water | 88 | 92:8 | [28] |
| 2 | HCl/SDS | 10 | Water | 83 | 94:6 | [28] |
| 3 | HBF4/SDS | 10 | Water | 91 | 97:3 | [28] |
| 4 | ZnCl2/SiO2 | 1 | Neat | 97 | 85:15 | [38] |
| 5 | Fe(Cp)2PF6 | 5 | Neat | 94 | 76:24 | [36] |
| 6 | WOx–ZrO2 | 50 (mg) | Neat | 90 | 80:20 | [34] |
| 7 | Ionic liqiud | 10 | Water | 83 | 75:25 | [20] |
| 8 | Bismuth triflate | 5 | Water | 84 | 86:14 | [26] |
| 9 | H3MoW12O40 | 0.12 | Water | 84 | 63:37 | [33] |
| 10 | ZrOCl2·8H2O | 15 | Neat | 92 | 99:1 | [23] |
| 11 | DBSA | 1 | Water | 100 | 70:30 | [16] |
| 12 | HClO4/TX10 | 10 | Water | 92 | 76:24 | [42] |
| 13 | ZnI2 | 1 | Neat | 95 | 64:36 | [43] |
| 14 | Boric acid | 10 | Water | 80 | 36:64 | [29] |
| 15 | Adenine/H2O2 | 20 | Water | 95 | 83:17 | [13] |
| 16 | Siloxy serine | 20 | Water | 82 | 81:19 | [12] |
| 17 | Organobismuth | 5 | Water | 98 | 95:5 | [27] |
| 18 | SiCl4 | 2 | Neat | 97 | 100:00 | – |
Optimization of reaction condition.
2 Experimental
2.1 General
All chemicals were obtained from commercial suppliers and used as supplied. NMR spectra were recorded on a Bruker ACF 500 using CDCl3/CCl4 or CDCl3/DMSO-d6 as solvent. Column chromatography was performed on silica gel, Merck grade 60. Melting points were recorded on Buchi 535 melting point apparatus and are uncorrected. Ethyl acetate, hexane, diethyl ether, ethanol and water were distilled prior to use. Reactions were carried out at room temperature and all aldehydes, ketones, amines and catalyst employed are commercially available.
2.2 General procedure
To the mixture of cyclic ketones (cyclohexanone or cyclohepatone, 3 mmol, and tetrahydro-4H-pyran-4-one, 1.5 mmol), aromatic aldehyde (1 mmol) and aromatic amine (1 mmol) in the test tube, SiCl4 (2 mol %) was added. The mixture was stirred at room temperature for appropriate time until the reaction mixture was solidified. To the crude product, diethyl ether or ethyl acetate (10 ml) was added and washed with saturated aqueous sodium chloride (5 mL) and water (10 mL). The organic layer was dried over anhydrous Mg2SO4 and solvent was evaporated under vacuum (rotary evaporator). The oily products were subjected to column chromatography over silica gel (230–400) with ethyl acetate:hexane (2:8) as eluent. The solid products were crystallized directly from ethanol and some cases with diethyl ether. All compounds were known and characterized on the basis of their spectroscopic data (1H NMR, and m.p.) and by comparison with those reported in the literature.
3 Results and discussion
With the increasing interest in developing environmentally benign reactions, the atom-economic catalytic process that employs unmodified ketones, aromatic amine, and aromatic aldehyde would be an ideal Mannich-type reaction, and is attracting more attention. In continuation of our studies on the new variants, of one-pot, three-component Mannich type reaction, and our ongoing program for developing green organic reactions by using water as reaction medium or by performing organic transformations under solvent-free conditions [33,46–48]. Herein, we report, direct three-component anti-selective Mannich reactions promoted by silicon tetrachloride under solvent-free condition. First, the three-component Mannich reaction of benzaldehyde, aniline, and cyclohexanone were selected as a model. As a preliminary study, reaction temperature and solvents were screened in the model reaction. The results of extensive solvent screening and optimization are shown in Scheme 1. SiCl4 catalyzed Mannich reactions in organic solvents such as ether, dichloromethane, methanol, and ethanol led to recovery of starting material and imines in addition to small amounts of the desired products 4 in low yield. Among the screened reaction solvent systems, solvent-free conditions (neat) was the ideal reaction media, since in this condition, the Mannich-type reactions proceed smoothly and afford the desired adducts in excellent yield and excellent anti-selectivity. Solvent-free organic reactions would be faster and more selective, leading to more favorable kinetics. Furthermore, no Mannich base could be detected when a mixture of benzaldehyde, aniline and cyclohexanone was stirred for 12 h in the absence of silicon tetrachloride, which indicated that the catalyst should be absolutely necessary for the Mannich reaction (Scheme 1).
Then, the scope of the SiCl4 catalyzed Mannich-type reaction of other aromatic aldehydes (1), aromatic amines (2) and ketones (3) were investigated under the optimized reaction conditions (Table 2). In general, the reaction proceeded smoothly at room temperature to give the corresponding products in reasonable to good yields ranged from 70 to 97%. At the beginning, we made a search for the aryl-aldehyde substrate scope with aniline and cyclohexanone as model substrates and the results are shown in Table 2. Generally, excellent yields of β-amino ketones were obtained under optimized reaction conditions with aromatic aldehydes as well as heterocyclic aldehydes. This reaction worked well for a variety of aldehydes including those bearing an electron-withdrawing group, and the corresponding β-amino ketones were obtained with excellent yields. Several electron-rich aromatic aldehydes lead to the desired products in good yields, too. The scope of our method was extended to other amines. In the case of amines having an electron-donating group, such as 4-isopropyl aniline, the corresponding amino alcohols was obtained in good yields. Furthermore, amines with electron-withdrawing groups, such as 4-chloroaniline and 3,4-dichloroaniline, gave the desired product in good yields. However, there is not any relationship between electronic effects and stereoselectivity of this reaction.
One-pot, three-component direct Mannich reaction catalyzed by SiCl4.
| Entry | Ar | Ar’ | Z | Syn: Anti | Yielda,b (%) | Ref. |
| 1 | C6H5 | C6H5 | CH2 | 00:100 | 97 | [33] |
| 2 | C6H5 | C6H5 | O | 10:90 | 92 | [45] |
| 3 | 4-ClC6H4 | C6H5 | CH2 | 00:100 | 92 | [19] |
| 4 | 4-ClC6H4 | C6H5 | O | 15:85 | 82 | [39] |
| 5 | 4-BrC6H4 | C6H5 | CH2 | 00:100 | 88 | [33] |
| 6 | 3-ClC6H4 | C6H5 | CH2 | 10:88 | 78 | [19] |
| 7 | C6H5 | 3-CH3C6H4 | CH2 | 00:100 | 86 | [34] |
| 8 | C6H5 | 3-CH3C6H4 | O | 20:80 | 84 | [45] |
| 9 | 4-BrC6H4 | 2-CH3C6H4 | CH2 | 13:87 | 80 | [36] |
| 10 | 4-ClC6H4 | 4-ClC6H4 | CH2 | 25:75 | 70 | [34] |
| 11 | C6H5 | 4-OCH3C6H4 | CH2 | 7:93 | 82 | [34] |
| 12 | C6H5 | 4-(CH3)2CHC6H4 | CH2 | 21:79 | 82 | [33] |
| 13 | 4-BrC6H4 | 4-OCH3C6H4 | CH2 | 13:87 | 85 | [35] |
| 14 | C6H5 | 4-BuC6H4 | CH2 | 23:77 | 83 | [33] |
| 15 | 4-ClC6H4 | 4-BuC6H4 | CH2 | 15:85 | 78 | [44] |
| 16 | 4-CH3C6H4 | C6H5 | O | 8:92 | 95 | [45] |
| 17 | 4-Pyridyl | C6H5 | CH2 | 10:90 | 92 | [33] |
| 18 | 4-Pyridyl | C6H5 | O | 12:88 | 88 | [45] |
| 19 | 3-BrC6H4 | C6H5 | CH2 | 14:86 | 80 | [33] |
| 20 | 3-OCH3C6H4 | C6H5 | O | 15:85 | 80 | [45] |
| 21 | 2-Thienyl | C6H5 | CH2 | 12:88 | 90 | [44] |
| 22 | 2-Thienyl | 3-CH3C6H4 | CH2 | 20:80 | 72 | [19] |
a Isolated yields.
b Reaction conditions: aromatic aldehyde (1 mmol) and aromatic amine (1 mmol) cyclohexanone, (3 mmol), and tetrahydro-4H-pyran-4-one, (1.5 mmol), SiCl4 (2 mol %).
In addition, besides the cyclohexanone, cycloheptanone and tetrahydro-4H-pyran-4-one could also be employed to give good yields. However, in case of cycloheptanone as substrate the anti-selectivity is lower than that of cyclohexanone and, this procedure could not afford the corresponding single Mannich base (Table 3).
Direct Mannich reaction of aldehyde, amines and cycloheptanone under solvent-free condition.
| Entry | Ar’ | Ar | Syn:Anti | Yielda,b (%) | Ref. |
| 1 | C6H5 | C6H5 | 05:95 | 91 | [23] |
| 2 | C6H5 | 3-CH3C6H4 | 24:76 | 82 | [23] |
| 3 | 4-ClC6H4 | C6H5 | 19:81 | 70 | [23] |
| 4 | 4-ClC6H4 | 4-(CH3)2CHC6H4 | 24:76 | 74 | [16] |
| 5 | C6H5 | 4-MeC6H4 | 15:85 | 86 | [39] |
| 6 | 4-CH3C6H4 | 3-ClC6H4 | 10:90 | 90 | [16] |
a Isolated yields.
b Reaction condition: aromatic aldehyde (1 mmol) and aromatic amine (1 mmol), cyclohepatone (3 mmol) and SiCl4 (2 mol%).
In general, the anti-products were invariably formed in a major scale, dependent of the nature of substituents on the aldehyde and amines as depicted in Table 2. It is worth mentioning that the aliphatic aldehydes and aliphatic amines were inactive and failed to furnish the desired products with this protocol (Table 4).
Three component reaction in the presence of HCl.
| Entry | Catalyst | Yield (%)a 3 | Yield (%) 4 | Anti:Syn |
| 1 | 10 μl HCl (32%) | 40 | 50 | 72:18 |
| 2 | 100 μl HCl (32%) | 55 | 35 | 85:15 |
| 3 | 2 mL HCl (32%) | 5 | 80 | – |
| 4 | 1 mL HCl (1 M) | 60 | 35 | 85:15 |
| 5 | HCl (g)b,c | 5 | 20 | – |
a NMR yields.
b Anilinium chloride (70%) and benzaldehyde were recovered.
c HCl gas was prepared by the reaction of concentrated sulfuric acid and sodium chloride.
Differentiation between aromatic aldehyde (cross-aldol condensation) and imine (Mannich reaction) in the competitive reactions is an important task in organic synthesis and in fact, only Mannich reaction product was obtained in this reaction media, which demonstrated that the reaction catalyzed by silicon mentioned above had a good chemoselectivity (Scheme 2) [49–51].

Chemoselectivity of catalyst.
The structures of all the synthesized compounds were established on the basis of their spectroscopic data. The anti- and syn-isomers were identified by the coupling constants (J) of the vicinal protons adjacent to C=O and NH in their 1H NMR spectra. The anti isomer showed a doublet at δ 4.54 due to CH and the coupling constant J = 7.6 Hz in the 1H NMR spectrum.
In summary, silicon tetrachloride was found to be a highly efficient catalyst for one-pot three-component Mannich reaction under solvent-free conditions. The procedure provides several advantages, such as mild conditions, high yields, and good to excellent stereoselectivities, shorter reaction time and clear reaction profile.
Acknowledgment
Financial support of this work by Chemistry and Chemical Research Center of Iran is gratefully appreciated.


