1 Introduction
Organic compounds with versatile functional groups such as α-amino nitriles are extremely useful synthetic intermediates. The nitrile functionality can be hydrolyzed easily to produce α-amino acids, and nucleophilic additions to the nitrile group provide access to α-amino aldehydes, α-amino ketones, α-amino alcohols, 1,2- diamines and many nitrogen containing heterocycles [1]. The Strecker reaction [2], which proceeded via the addition of alkaline metal cyanides or hydrogen cyanide to imine proved to be an effective one for the preparation of α-amino nitriles on a laboratory, as well as on an industrial scale. The classical Strecker reaction is usually carried out in aqueous solution, which has some limitations, and the work-up procedure is too tedious leading to generation of large amount of toxic waste. In order to partially avoid these inconveniences, several modifications of the Strecker reaction via a three-component condensation of aldehyde, amine and trimethylsilyl cyanide (a promising alternative to alkali cyanide and HCN due to its nature as a simplest, safe, easily-handled, most soluble in organic solvents and more effective cyanide ion source for the nucleophilic addition reaction) catalyzed by Lewis or protic acids in conventional organic solvents has been described [3–14]. In some cases, the protocols involve the use of strong and expensive or unrecoverable catalysts, harsh acidic conditions, toxic organic solvent, tedious work-up procedure leading to the generation of large amount of metal-containing waste and longer reaction times. Furthermore, many of these catalysts are deactivated or sometimes decomposed by amines and water that exist during imine formation. There are also a few reports using no catalyst [15], but their reaction time lies in the range of hours. Therefore, it is still desirable to develop an efficient, recyclable catalyst and practical method for this reaction under mild conditions.
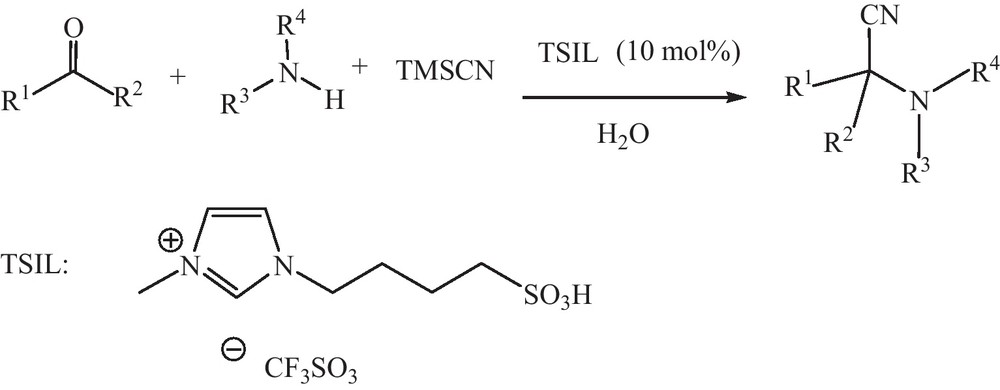
In recent years, the use of ionic liquids (ILs) has received more attention as eco-friendly, reusable and alternative reaction media in organic synthesis because of their unique properties, such as high thermal and chemical stability, negligible vapor pressure, no flammability, high loading capacity, immiscibility with a number of organic solvents and excellent electrical conductivity [16–20]. Moreover, ionic liquids are simple and easy to recycle and their properties can be fine-tuned by changing the anion or the alkyl group attached to cation. Most recently, much more attention has been focused on the preparation of functionalized ionic liquids (FILs), through incorporation of different functional groups as a part of the cation and/or anion, so-called task-specific ionic liquids (TSILs), and their applications in chemical transformations. The incorporation of functional groups offers flexibility to the chemist to devise the most idealized solvent and catalyst, catering for the need of any particular process. Moreover, specific functional groups, such as acid can be incorporated into the ionic liquids for task-specific purposes. For example, BrØnsted acidic ionic liquids, which can take dual role in organic reactions as solvents and catalysts. Many acid-catalyzed organic reactions have been carried out successfully catalyzed by this ionic liquids [21–26]. They have shown great promise not only as alternative green solvents, but also as reagents or catalysts in organic transformations [27–30]. Herein we wish to report the first study on application of inexpensive and easily accessible functionalized ionic liquid as catalyst for Strecker reaction of aldehydes and ketones in mild condition (Scheme 1). [BMIm][CLO4] and [BMIm][BF4] have been previously used as reaction medium for Strecker reaction [31,32]. The present paper could represent an advance since the functionalized ionic liquid is catalytically used in water.
2 Results and discussion
Initially we studied the Strecker reaction with benzaldehyde and aniline as model substrate with TMSCN using TSIL for optimization. The rate of reaction was moderate and 35% yield of α-amino nitrile obtained. The reaction was performed at room temperature using TSIL in water as solvent. The rate of the reaction was fast in H2O and 98% yield of α-amino nitrile obtained. The Strecker-type reaction of benzaldehyde aniline and TMSCN is examined in the presence of catalytic amount of TSIL at room temperature in water. 10 mol % of catalyst give α-amino nitrile with 98% yield in 10 min in H2O, while decreasing the catalyst to 5 mol % gives lower yield of 70% in 10 min, with 20 mol % catalyst the yield is increased that affords the corresponding α-amino nitrile in 98% yield within 10 min. Accordingly, 10 mol % TSIL catalyst loading in H2O as a solvent is considered optimal for the synthesis of the synthesis of α-amino nitriles.
A series of α-amino nitriles were synthesized by using different aldehydes, amines and TMSCN in the presence of TSIL (10 mol %) in water. The catalytic system worked well with acid sensitive aldehydes, α, β-unsaturated aldehyde to generate the corresponding products without observing the formation of other side products. Encouraged by the above results, we continued our task to explore the reactivity of different ketones with aniline and TMSCN under similar reaction conditions. As shown in Table 1, ketones could efficiently undergo reaction with aniline and TMSCN to give the products in excellent yields. However, the Strecker-type reaction using ketones and aromatic amines has been repeatedly cited in the literature as a challenge [33]. This catalytic effect of TSIL could be ascribable to the presence of the solfonic group present on the TSIL. Indeed, it has been previously shown that the water content certainly has an effect on the activity of the TSIL [34].
Strecker reaction of aldehydes/ketones with amines catalyzed by task-specific ionic liquid (TSIL).
| Entry R1 | R2 | R3 | R4 | Time (h) | Yield (%) |
| a Phenyl | H | Phenyl | H | 10 min | 98, 98, 98,98, 97, 96a |
| b 4-Cl-Phenyl | H | Phenyl | H | 15 min | 98 |
| c 4-O2N-Phenyl | H | Phenyl | H | 15 min | 96 |
| d 2-Furyl | H | Phenyl | H | 1 | 95 |
| e tert-Butyl | H | Phenyl | H | 1 | 94 |
| f Cinnamyl | H | Phenyl | H | 30 min | 95 |
| g Cyclohexyl | H | Phenyl | H | 3 | 92 |
| h Phenyl | Me | Phenyl | H | 15 min | 98 |
| i Phenyl | H | Ethyl | Ethyl | 1.5 | 94 |
| j Phenyl | H | Benzyl | Benzyl | 1.5 | 96 |
a The range of the yields of subsequent runs using the same recovered catalyst.
The recycling performance of TSIL was investigated by the Strecker reaction of benzaldehyde with aniline under the identical conditions (Table 1, entry a). As the data listed show that this ionic liquid could be reused for at least five times without noticeable decrease of the catalytic activity. The reaction still provided a reasonable yield and the same selectivity as initially. It should be noted that it is very easy to separate the catalyst from the reaction system after reaction by simple extraction with water.
3 Conclusion
In summary, we have developed a clean and environmentally friendly protocol for the one-pot synthesis of α-amino nitriles in good to excellent yields, from ketones/aldehydes in combination with an amine using BrØnsted acidic ionic liquid as homogeneous and recyclable catalyst and TMSCN as cyanide source. The simple experimental procedure, fast reaction, easy product separation and reuse of ionic liquids makes the use of the above ionic liquid as greener and economically viable catalyst for the Strecker reaction compared with the traditional protocols. Moreover, the TSIL is stable in water in spite of other nonfunctionalized one.
4 Experimental
4.1 Synthesis of sulfonic acid functionalized ionic liquids
The synthesis of this ionic liquid was carried out using a similar method reported in the literature [35].
General procedure for Strecker reaction: TSIL (0.018 g, 0.1 mmol) was added to a mixture of aldehyde or ketone (2 mmol) and amine (2 mmol) in water (1 mL) at r. t. The mixture was stirred for 5 min and the TMSCN (2 mmol) was added. After completion of the reaction, as indicated by TLC, H2O (3 mL) was added. The mixture was extracted with AcOEt (3 × 4 mL) and the organic layer separated, dried over Na2SO4 and concentrated under vacuum. The residue was purified by column chromatoghraphy (SiO2; AcOEt/hexane 1/2) to afford the corresponding α-amino nitrile. The combined aqueous layers were concentrated under vacuum to afford the TSIL. The recovered catalyst was washed with diethyl ether, dried and reused for subsequent runs. The physical data (mp, IR, NMR) of known compounds were found to be identical with those reported in the literature [3,6,9,12–14].
Acknowledgments
This research was supported by Islamic Azad University, Buinzahra Branch. Buinzahra, Iran.
Appendix A
The spectral data (1H, 13C NMR) of known compounds were found to be identical with those reported in the literature [3,6,9,12–14]. Spectral data for selected products: Compound a: 1H NMR (500 MHz, CDCl3): δ = 4.1 (br s, 1H, NH), 5.4 (s, 1H), 6.5–6.8 (m, 5H), 7.1–7.8 (m, 5H); 13C NMR (125 MHz, CDCl3): δ = 48.2, 114.3, 117.8, 128.2, 128.3, 129.2, 131.4, 145.5. Compound d: 1H NMR (90 MHz, CDCl3): δ = 4.1 (br s, 1H, NH), 5.4 (s, 1H), 6.3–6.5 (m, 3H), 7.1–7.8 (m, 5H). 13C NMR (125 MHz, CDCl3): δ = 47.3, 102.3, 113.8, 114.6, 116.1, 118.0, 130.0, 133.4, 139.0, 147.8.


