1 Introduction
The coumarin (benzopyranone or chromenone) ring system, present in natural products that display interesting pharmacological properties, has intrigued chemists and medicinal chemists for decades to explore the natural coumarins, semi-synthetic or synthetic analogs for their applicability as drugs. Many molecules based on the coumarin ring system have been synthesized utilizing innovative synthetic techniques. The diversity oriented synthetic routes have led to interesting coumarins condensed with aromatic, heteroaromatic and alicyclic systems, which have been reported to possess antiallergic, anticoagulant, antidiabetic, antitumor, antibacterial, anti-inflammatory, anti-HIV therapy and analgesic activities [1–4].
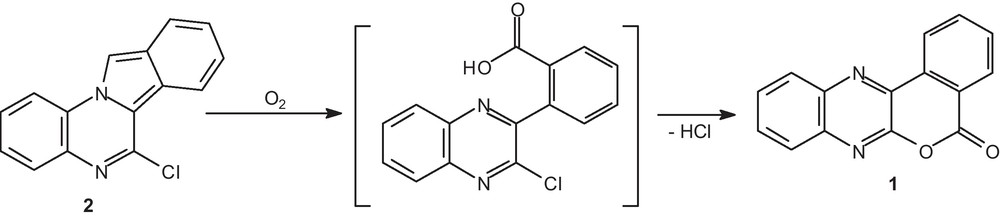
In the present work, we reported herein the structural characterization of [2]benzopyrano[3,4-b]quinoxalin-5-one 1 [5] (Scheme 1), also named isochromeno[3,4-b]quinoxalin-5-one, which was serendipity formed by recrystallization of 6-chloro-isoindolo[2,1-a]quinoxaline 2 [6] through an autoxidation and a rearrangement.
Possible formation of [2]benzopyrano[3,4-b]quinoxalin-5-one 1.
2 Results and discussion
As a part of our program on crystal structure analysis, the crystal structure of [2]benzopyrano[3,4-b]quinoxalin-5-one 1 has been studied. Hence, after crystallization in a mixture of dichloromethane and methanol (4/1–v/v) at room temperature, isochromeno[3,4-b]quinoxalin-5-one 1 was surprisingly isolated as yellow-green needles having a different melting point in comparison with the starting material 6-chloro-isoindolo[2,1-a]quinoxaline 2 (m.p. = 204 °C for 1 versus 183 °C for 2). The title compound was then subjected to spectroscopic analysis to confirm its structure in comparison with its previously described analytical data [5].
The mechanism of formation of 1 from 2 could be probably explained by the described pathway through an autoxidation followed by a rearrangement (Scheme 2).

Hypothetical mechanism for the formation of [2]benzopyrano[3,4-b]quinoxalin-5-one 1.
Isoindoles are well known to be air sensitive and give autoxidation products [7–12]. Oxidation is demonstrated to be free radical chain process. Thus, 6-chloro-isoindolo[2,1-a]quinoxaline 2 reacted with oxygen to give the cyclic peroxide I through a [4 + 2] cycloaddition. The mechanistic step involving O–O bond homolysis led to compound II, then hydrogen atom abstraction from solvent of this diradical II gave the dihydroxy intermediate III. Oxidation proceeds readily in solvents, which can be considered hydrogen donors such as methanol. The ensuing deshydratation of III gave the imine IV. Alternatively a peroxo diradical intermediate V arising via the cyclic peroxide I could be the precursor of the imino-carboxaldehyde IV by peroxide decomposition [10,13,14]. Oxidation of the carboxaldehyde function of IV led to the non isolated carboxylic acid VI. Such a similar autoxidation and reactivity was previously described in polysubstituted isoindoles [10–12]. Finally, an intramolecular aromatic nucleophilic substitution of the chlorine atom with the carboxylic acid function of this adduct furnished the [2]benzopyrano[3,4-b]quinoxalin-5-one 1.
The title compound 1 crystallized in the triclinic system, space group Pī with unit cell parameters: a = 7.173 (1), b = 11.668 (2), c = 13.430 (2) Å, α = 85.56 (1)°, β = 83.26 (1)°, γ = 81.32 (1)°, V = 1101.4 (3) Å3, Z = 4, C15H8N2O2, Dc = 1.497 g/cm3, μ (MoKα) = 1.5418 Å, S = 1.017, F (000) = 512.00, T = 213 (2) K (Table 1). In the unit cell, there are two independent molecules (molecules A and B). The molecular structure of [2]benzopyrano[3,4-b]quinoxalin-5-one 1 is depicted in Fig. 1.
Crystallographic data and structure refinement details.
| CCDC deposit number | 761528 |
| Chemical formula | C15H8N2O2 |
| Formula weight | 248.23 |
| Temperature (K) | 213 (2) |
| Wavelength (Å) | 1.54180 |
| Crystal size (mm) | 0.10 × 0.02 × 0.02 |
| Crystal system | Triclinic |
| Space group | Pī |
| a (Å) | 7.173 (1) |
| b (Å) | 11.668 (2) |
| c (Å) | 13.430 (2) |
| α (°) | 85.56 (1) |
| β (°) | 83.26 (1) |
| γ (°) | 81.32 (1) |
| V (Å3) | 1101.4 (3) |
| Z | 4 |
| Dc (g/cm3) | 1.497 |
| F (000) | 512 |
| Absorption coeff. (mm−1) | 0.838 |
| θ range (°) | 6.78–71.76 |
| Index ranges | −8 ≤ h ≤ 8; −14 ≤ k ≤ 14; −15 ≤ l ≤ 16 |
| Reflection collected | 18,226 |
| Independent reflections | 4,009 [Rint = 0.0673] |
| Observed reflections | 2,075 |
| Data/restraints/parameters | 4,009/0/343 |
| Goodness-of-fit on F2 | 1.017 |
| R, wR indices [I > 2σ(°)] | 0.0758, 01840 |
| R, wR indices (all data) | 0.1018, 0.1921 |
| Largest diff. peak and hole (e Å−3) | 0.366, −0.347 |
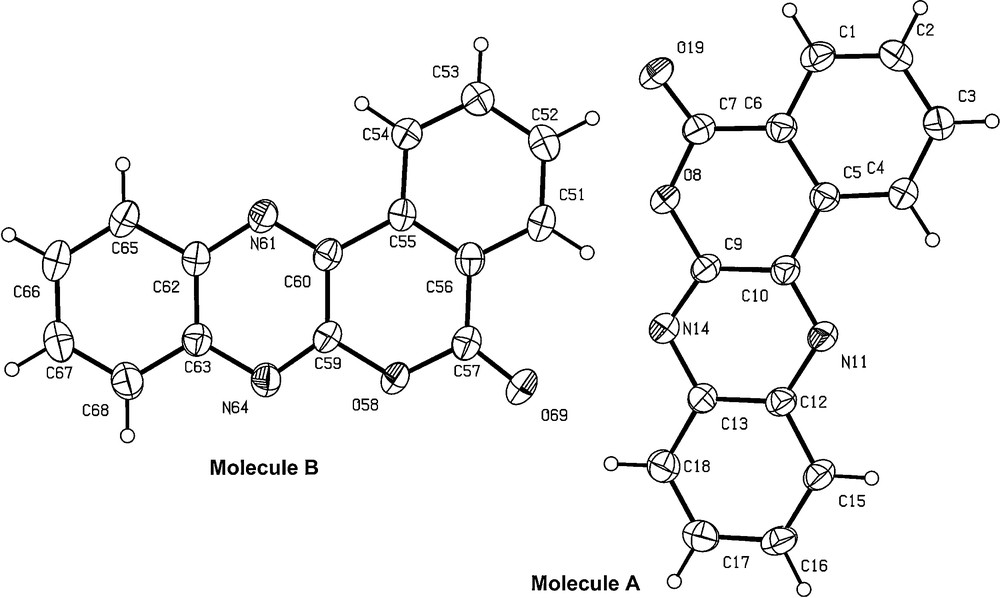
ORTEP drawing of 1 showing the atom numbering scheme of the asymmetric unit containing two independent molecules. Displacement ellipsoids are drawn at the 30% probability level.
The double bonds C7O19 and C57O69 are confirmed by their respective lengths of 1.206 (4) and 1.216 (4) Å. The values of the four C–O bonds (C7–O8 = 1.378 (4) Å, C57–O58 = 1.380 (4) Å, C9–O8 = 1.377 (4) Å, and C59–O58 = 1.373 (4) Å) in the pyrone rings were in agreement with the C(sp2)–O distance [15]. The bond angles O8–C9–N14 and C5–C10–N11, then O58–C59–N64 and C55–C60–N61, at the junction of the pyrone and the quinoxaline rings are, respectively, smaller and greater than 120° (Table 2). This phenomenon has also been observed in some azacoumarins [16–18].
Selected bond lengths (Å) and angles (°).
| Bond lengths | |||
| C(7)–O(19) | 1.206 (4) | C(57)–O(69) | 1.216 (4) |
| C(7)–O(8) | 1.378 (4) | C(57)–O(58) | 1.380 (4) |
| C(9)–N(14) | 1.303 (4) | C(59)–N(64) | 1.298 (4) |
| C(9)–O(8) | 1.377 (4) | C(59)–O(58) | 1.373 (4) |
| C(10)–N(11) | 1.308 (4) | C(60)–N(61) | 1.303 (4) |
| Bond angles | |||
| O(19)–C(7)–C(6) | 125.6 (3) | O(69)–C(57)–C(56) | 125.7 (4) |
| N(14)–C(9)–O(8) | 113.7 (3) | N(64)–C(59)–O(58) | 113.9 (3) |
| N(11)–C(10)–C(5) | 121.0 (3) | N(61)–C(60)–C(55) | 121.3 (3) |
| C(9)–O(8)–C(7) | 122.6 (3) | C(59)–O(58)–C(57) | 122.6 (3) |
The six C–C bond lengths in the phenyl ring of the isochromenone skeleton lie in the range 1.370 (5)–1.406 (4) Å.
The benzopyranoquinoxaline moiety is almost planar; a derivation of the C18 atom (molecule A) was noticed at 0.0790 (3) Å from the plane defined by the tetracyclic system. In molecule B, the derivation of the C67 atom was observed at 0.0790 (3) Å from this latter plane.
The crystal structure cohesion is partially ensured by the formation of π-stacked polymeric units in the crystal packing. Hence, the distances of these intermolecular π-π interactions (rind A1 … ring D2, ring D2 … ring A3, and ring A3 … ring D4; then rind D1 … ring A2, ring A2 … ring D3, and ring D3 … ring A4) were observed with values ranging from 3.35 to 3.73 Å (Fig. 2).

Ring-stacking interactions in the crystal of isochromeno[3,4-b]quinoxalin-5-one 1.
For the depicted interactions, rings A1–D1 belong to the molecule at x, y, z, rings A2–D2 to the molecule at x + 1, y + 1, z, rings A3–D3 to the molecule at x + 1, y, z, and rings A4–D4 to the one at x + 2, y + 1, z. Moreover, the inter-isochromeno[3,4-b]quinoxalinone contacts are of the van der Waals variety.
3 Experimental
3.1 Preparation
The single crystals of compound 1 suitable for determination were obtained by very slow evaporation (12 days) of the solution of 6-chloro-isoindolo[2,1-a]quinoxaline 2 in a mixture dichloromethane: methanol = 4:1 at room temperature.
3.2 X-ray crystallography
A single crystal of the title compound with dimensions 0.10 × 0.02 × 0.02 mm was chosen for X-ray diffraction study. The data were collected on a Rigaku R-axis rapid diffractometer equipped with micro-focus rotating anode Cu-Kα radiation (λ = 1.5418 Å) mode at 213(2) K. In the range of 6.78° < θ < 71.76°, a total of 18,226 reflections were collected, of which 4009 were independent (Rint = 0.0673) and 2075 were observed with I > 2σ(I). The structure was solved by direct methods with SHELXS-97 [19]. Non-hydrogen atoms were refined by full-matrix least-squares techniques on F2 with anisotropic thermal parameters, using SHELXL-97 [20]. All H atoms were located in a difference Fourier map and allowed to ride on their parent atoms at distances of 0.93 Å (C–H aromatic) and 0.96 Å (C–H methyl), with Uiso(H) values of 1.2–1.5 times Ueq of the parent atoms. The final full-matrix least-squares refinement gave R = 0.0758, wR = 0.1840 for 2,075 reflections with I > 2σ(I). The maximum and minimum difference peaks and holes are 0.366 and −0.347 e Å−3, respectively. S = 1.017 and (Δ/σ)max = 0.000. The crystal data and refinement details are listed in Table 1. The selected bond lengths and bond angles are listed in Table 2.


