1 Introduction
Hexathiocyanato-rhenium(IV) derivatives are well documented. [Re(NCS)6]2− salts of Cs+ [1], NR4+ [2–5], PPh4+ [6], AsPh4+ [2,7], among others, are reported, and structural characterizations have been performed [3,4,6,7]. Surprisingly, while the potassium derivative is an intermediate in the synthesis of several other salts, a procedure describing its preparation in a pure and crystalline form is not reported. Tackling this point revealed that K2[Re(NCS)6] is difficult to purify from traces of KNCS. Herein we report a procedure leading to K2[Re(NCS)6] as a crystalline solid in good yield. Its crystal structure has been established and the magnetic properties are described. The later revealed that the compound is a weak ferromagnet at low temperature.
2 Results and discussion
2.1 Preparation and crystal structure
The reaction of K2ReCl6 with molten KNCS is a well established procedure for the preparation of [K2Re(NCS)6] [1]. However, the presence of a large excess of KNCS makes the purification tricky and usually the crude [K2Re(NCS)6] is converted into a different salt to circumvent the difficulty. We have found that [K2Re(NCS)6] can be extracted from the crude product with THF to obtain the potassium salt with a good purity and with excellent yields (90%). The compound is then easily crystallized from a H2O solution and isolated as [{K(H2O)2}2{Re(NCS)6}].
The IR spectrum for [{K(H2O)2}2{Re(NCS)6}] is characterized by a strong and broad band at 1996 cm−1 corresponding to the νCN stretching and a much weaker absorption band around 950 cm−1 that is due to the C-S moiety [5,8]. Such a broadening might be related to the bonds with the K+ or hydrogen bonds with the H2O located in the crystal lattice. The THF-extracted product (i.e. before crystallization in H2O) exhibited IR bands characteristic for THF, suggesting this solvent to act as ligand for the K atoms. However, upon standing in air, these bands evolved to lead to the same spectrum as for [{K(H2O)2}2{Re(NCS)6}].
[{K(H2O)2}2{Re(NCS)6}] was crystallized from a H2O solution as deep amber crystals. Its molecular crystal structure has been investigated by X-ray diffraction experiments on a single crystal. Crystallographic data are given in Table 1, further details are provided in the experimental section.
Crystallographic data.
| [{K(H2O)2}2{Re(NCS)6}] | |
| Formula | C6 H8 K2 N6 O4 Re1 S6 |
| FW | 684.96 |
| Crystal system | Monoclinic |
| Crystal color | Brown |
| Space group | P 1 21/c 1 |
| a/Å | 8.29132(8) |
| b/Å | 15.0296(2) |
| c/Å | 8.5249(1) |
| α/° | 90 |
| β/° | 90.885(1) |
| γ/° | 90 |
| V/Å3 | 1062.21(2) |
| Z | 2 |
| T/K | 180 |
| ρcalcd/g.cm-3 | 2.14 |
| μ (Mo-Kα)/mm−1 | 6.724 |
| F(000) | 654.000 |
| Absorption correction | Multiscan |
| Tmin | 0.25 |
| Tmax | 0.71 |
| Index ranges | −12 ≤ h ≤ 12 |
| −22 ≤ k ≤ 21 | |
| −12 ≤ l ≤ 12 | |
| Reflections collected | 118436 |
| Independent reflections (Rint) | 3473 (0.064) |
| R1/wR2 | 0.0230/0.0238 (I > 3 σ [I]) |
| R1/wR2 (all data) | 0.0331/0.0298 |
A view of the molecular structure is given in Fig. 1. Six thiocyanate ligands are bound to the Re ion by their N atom to form an octahedral coordination sphere. The two Re-N(2) distances are shorter than the remaining four (2.002 Å versus 2.011 and 2.013 Å) leading to a slightly compressed octahedra. The Re-NCS linkages are very slightly bent with a mean angle of 174.9°. The charge of the anionic unit is balanced by two K+ cations. Each K+ is eight-coordinated with a coordination sphere containing four O atoms of H2O molecules and four S atoms from four [Re(NCS)6]2− units. The H2O and two of the S atoms act as bridges between two K+ ions leading to chain organizations developing along the c axis (Fig. 2). Related 1-D organizations of vertex-sharing polyhedra have been reported for potassium ions [9,10]. The [Re(NCS)6]2− complexes are connected by their six terminal S atoms to four different K chains, thus developing an overall 3-D coordination polymer. This supramolecular organization favors rather close packing of the [Re(NCS)6]2− with intermolecular S···S separations of less than 4 Å. Actually the six S atoms of a Re unit are involved in such short contacts, hence these propagate in the three directions of space as shown in Fig. 3.

Molecular structure for [{K(H2O)2}2{Re(NCS)6}]. Selected bond lengths (Å): Re-N2, 2.002(2); Re-N1, 2.011(2); Re-N3, 2.013(1); N1-C1, 1.167(2); N2-C2; 1.171(2); N3-C3, 1.171(2); S1-C1, 1.609(2); S2-C2, 1.602(2); S3-C3, 1.611(1); and angles (°): Re-N1-C1, 175.8(1); Re-N2-C2, 175.8(2); Re-N3-C3, 173.2(1).
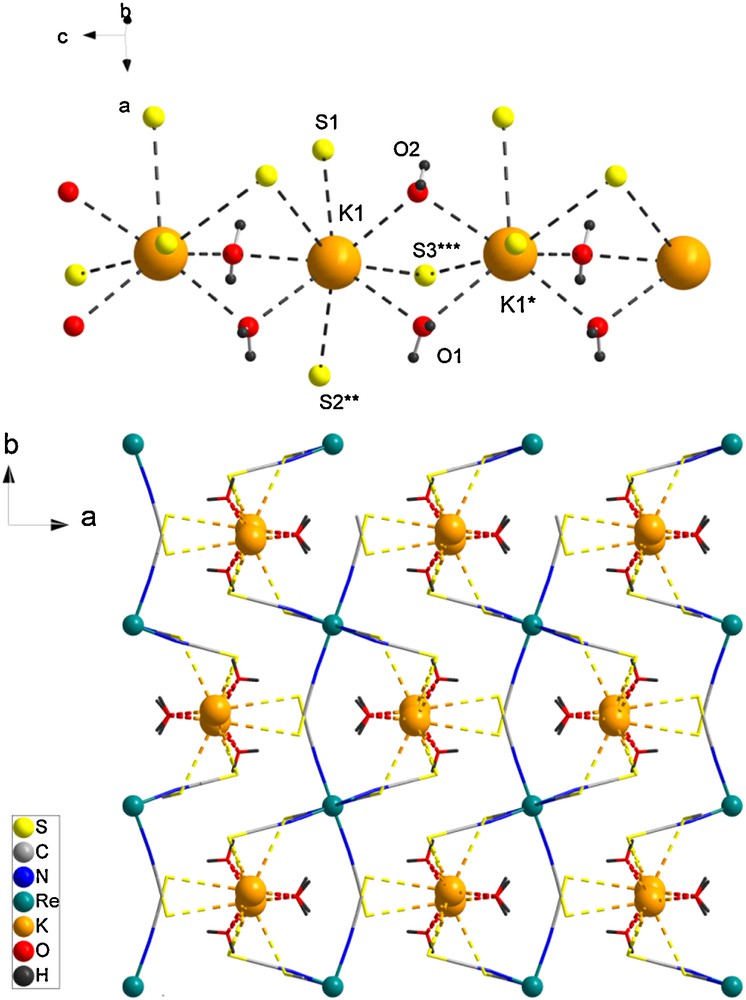
Crystal packing for [{K(H2O)2}2{Re(NCS)6}]. (top) detail of the potassium arrays. (bottom) View of the 3-D network (Re and K atoms are depicted as spheres). Selected bond lengths (Å) K-O1, 2.821(2); K-O2, 2.780(2); K-S1, 3.5297(8); K-S2**, 3.3426(7); K-S3***, 2.3427(6); K*-O1, 2.915(1); K*-O2, 2.839(2); K*-S3***, 3.3453(6); K···K*, 4.2835(6); and angles (°): K-O1-K*, 96.61(5); K-O2-K*, 99.33(5); K-S3***-K*, 78.51(1); S1-K-S2**, 105.73(2) (symmetry operations: *x,0.5-y,z; **1-x,-0.5+y,1.5-z; ***1-x,-0.5+y,0.5+z).
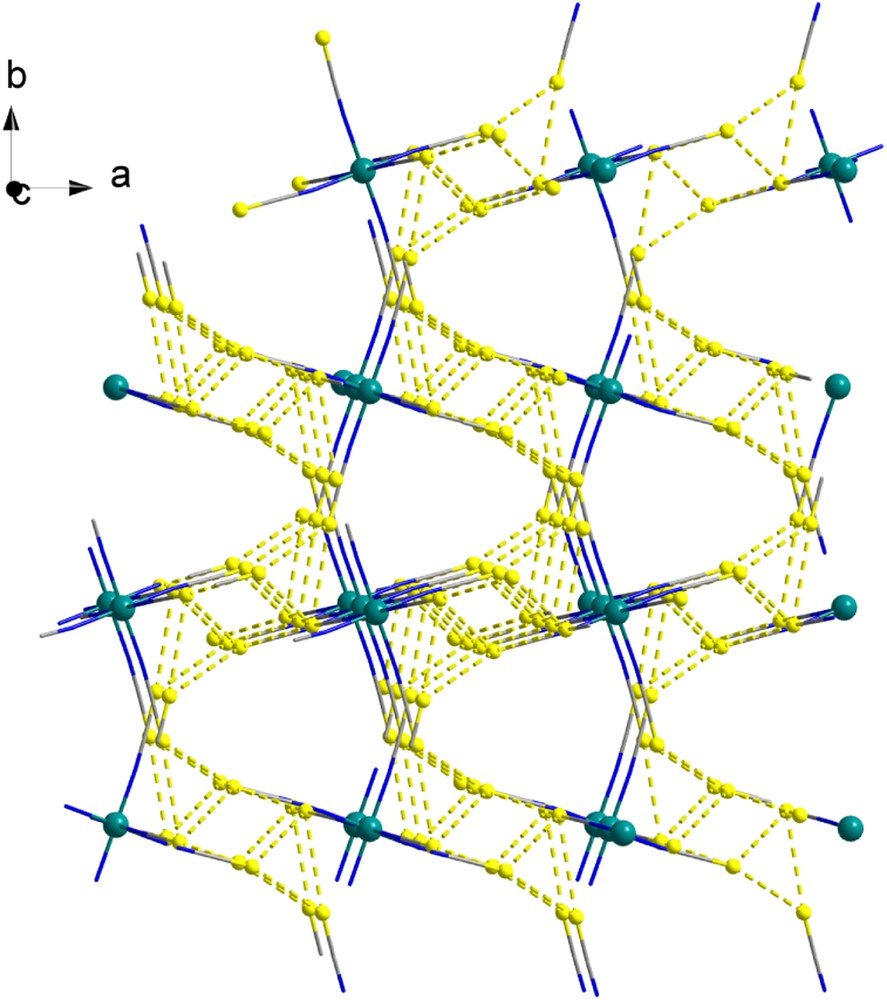
View of the propagation of the S···S contacts (<4 Å, dotted lines) in the crystal of [{K(H2O)2}2{Re(NCS)6}] (the Re and S atoms are drawn as spheres and the K and H2O moieties are not shown for clarity). Depicted contacts (Å): S1···S2(x, 0.5-y, -0.5+z), 3.5470(8); S1···S3(1-x, -0.5+y, 0.5-z), 3.9415(7); S2···S1(x, 0.5-y, 0.5+z), 3.5470(8); S2···S3(x, y, 1+z), 3.6039(6); S2···S3(1-x, 1-y, 1-z), 3.9425(6); S3···S2(x, y, -1+z), 3.6039(6); S3···S1(1-x, 0.5+y, 0.5-z), 3.9415(7), S3···S2(1-x, 1-y, 1-z), 3.9425(6).
2.2 Magnetic properties
The temperature dependence of the molar magnetic susceptibility, χM, has been investigated between 300 and 2 K. The result is given as χMT versus T plots in Fig. 4. The χMT between 300 and 15 K continuously decreases, reaching 0.10 cm3mol−1K at 14 K, followed by a sharp peak with maximum at 10 K. It can be noticed that the value for χMT at 300 K, 1.36 cm3mol−1K, is below the value anticipated of 1.875 cm3mol−1K for an S = 3/2 spin state (with 4A2g ground state). The behavior in the 300–15 K domain is characteristic for substantial antiferromagnetic interactions. Analysis of the linear part of the 1/χM = f(T) plot by the Curie-Weiss equation yielded C = 1.93cm3mol−1 and θ = −171 K, these values are in good agreement with that reported earlier [11]. The low temperature peak value was found to be strongly dependent on the applied field (Fig. 4 insert). Such a behavior is characteristic for a weak ferromagnet. This is supported by field cooled magnetizations (FCM) recorded with an applied field of 10 and 50 Oe showing the appearance of a spontaneous magnetization below 13 K (Fig. 5). Moreover, the in-phase signal (χM′) of the AC susceptibility at different frequencies exhibits a maximum at 12 K (Fig. 5 insert), and the out-of phase component (χM′′), while weak, deviates from zero below 13 K and is frequency independent.
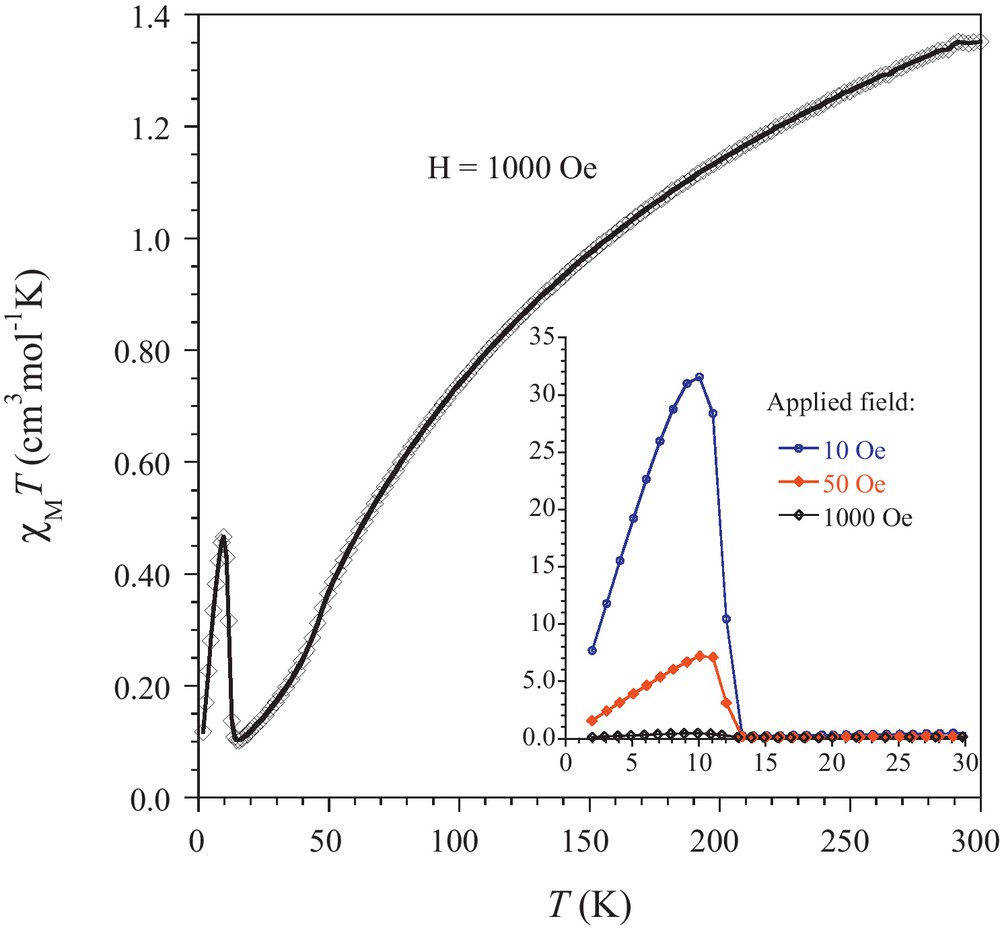
Temperature dependence of χMT for [{K(H2O)2}2{Re(NCS)6}] recorded with an applied field of 1000 Oe; insert: detail of the variation of the low temperature behavior as a function of the applied field. Solid lines are eye-guides.
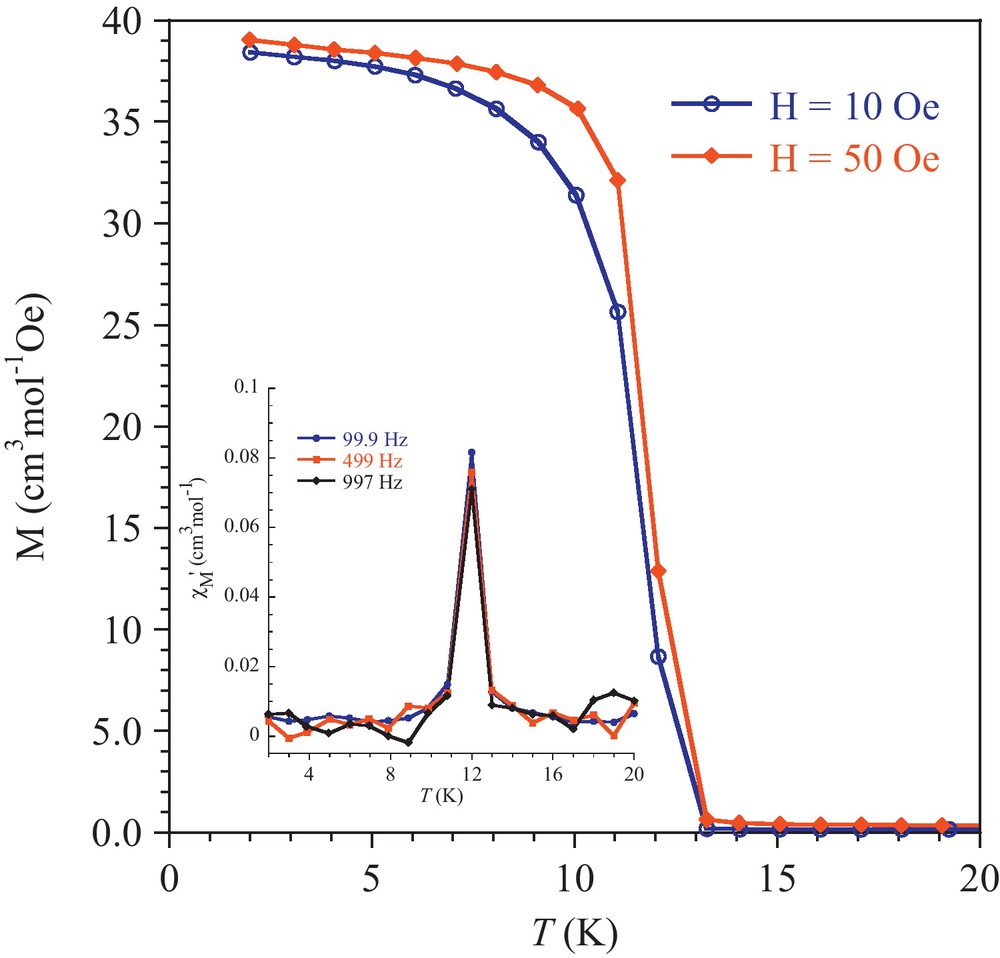
Field cooled magnetizations recorded with applied fields of 10 and 50 Oe; insert: in-phase AC magnetic susceptibility for different frequencies.
The field dependence of the magnetization recorded at 2 K is shown in Fig. 6. The value reached even for an applied field of 7 T is far below the magnetization at saturation expected for an S = 3/2 spin. This is a further support to the substantial antiferromagnetic interactions taking place between the Re centers, and to the weak ferromagnet nature of this compound.
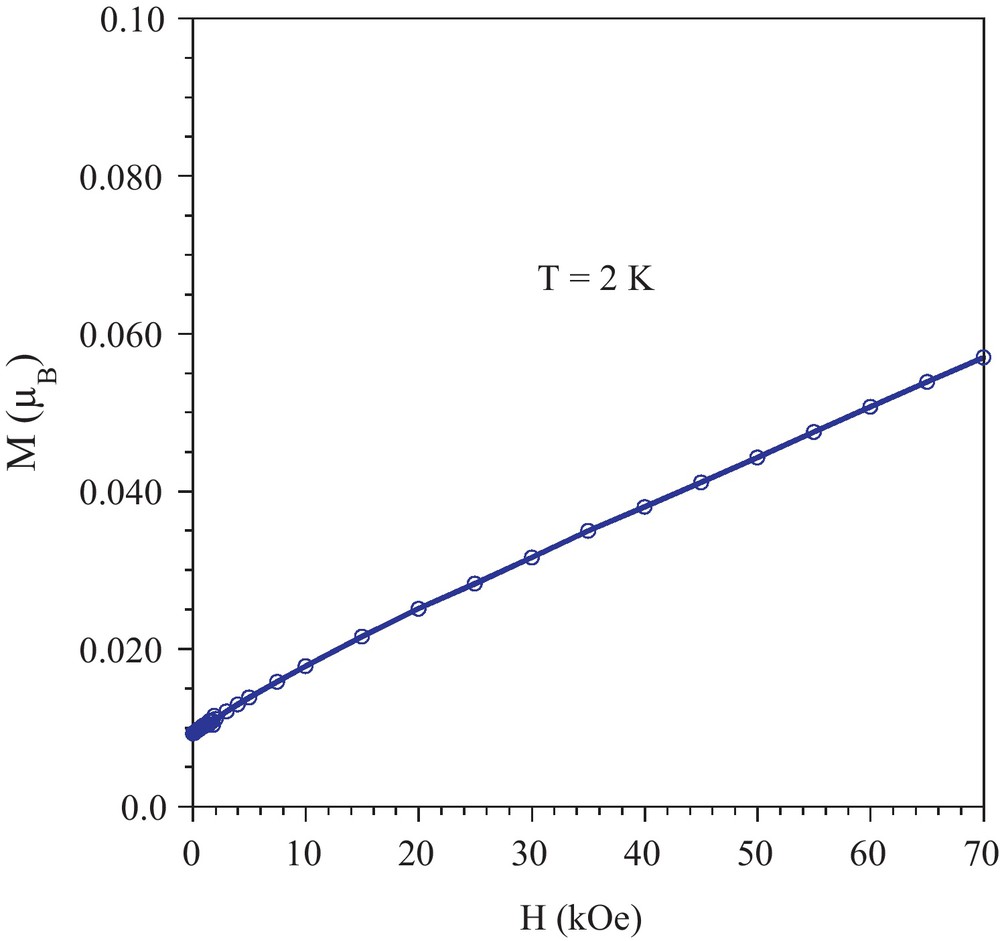
Field dependence of the magnetization recorded at 2 K (the solid line is just an eye-guide).
The weak ferromagnetic state can be attributed to a slight deviation from collinear alignment of the local moments of the exchange coupled Re centers. Such spin canting has been reported for other ReIV salts [12]. This situation results from the substantial magnetic anisotropy of this ion that is the consequence of both the lifting of the degeneracy of the d level by the deviation for ideal Oh symmetry and the spin-orbit coupling that takes place for 5d ions [13]. The evaluation of the canting angle is given by sin(α) = Mi/MS [14] where Mi is the magnetization induced by a very weak field and MS is the magnetization at saturation. Taking Mi = 0.092 μB, the value obtained in the M versus H investigation at 2 K for an applied field of 0 Oe (probably because of a weak remnant field in the magnetometer), and MS = g·S = 2.7 μB (with S = 3/2 and g = 1.8 [7]), a canting angle of α = 1.9° is obtained.
3 Concluding remarks
The rather strong antiferromagnetic interactions that take place between the Re units are unusual for a chemical system with its magnetic centers more than 8 Å away from each other and without bridging ligands. The origin of this intermolecular coupling is actually found in the short S···S intermolecular interactions in the solid state. For the compound reported here, intermolecular distances between sulfur atoms are found below the sum of the van der walls radius of S (2 Å); this is a favorable situation for propagating magnetic interactions between the molecules even if the involved atoms bear only little magnetic information. It has been established that moving from 3d to 5d metal ions leads to a increase of the spin density transferred from the metal ion to its ligand system [15]. This also applies for the thiocyanate complexes for which a significant spin density has been found on the peripheral S atoms for a Mo(III) derivative [16]. For 3d ions complexes the spin at the periphery is usually too small to yield significant intermolecular exchange interactions but obviously this is no longer the case for the 5d compound described here. A study aimed at quantifying the spin density distribution for this Re compound is underway. In a more general point of view, such enlarged magnetic information on the ligand is certainly desirable for more efficient exchange interaction through a bridging ligand but may become a drawback when intermolecular interactions are to be avoided.
Despite the preparation of the potassium salt of [Re(NCS)6]2− was well established the use of this compound was limited because of the difficulty to get rid of remaining free NCS−. The quite simple and straightforward purification procedure we have introduced here opens now the possibility to use this salt for the preparation of heterometallic materials.
4 Experimental
All the syntheses were accomplished under a nitrogen atmosphere. The chemicals were bought from commercial sources and used as received. The deoxygenated solvents were prepared by reflux under a nitrogen atmosphere. K2ReCl6 [17] was prepared as described in the literature. Elemental C, H and N analyses were performed on a Perkin-Elmer 2400 II analyser on freshly prepared and isolated samples. IR spectra were recorded in the 4000–600 cm−1 region with a Perkin-Elmer Spectrum 100 FTIR using the ATR mode. Magnetic measurements were carried out with a Quantum design MPMS 5S SQUID susceptometer in the temperature domain 2–300 K in an applied field of 1000 Oe. The molar susceptibility was corrected for sample holder and for the diamagnetic contribution of all the atoms by using Pascal's tables. The measurements were performed on crushed crystals from freshly isolated samples to avoid solvent loss. The powders were mixed to grease and put in gelatin caps.
4.1 Synthesis of [{K(H2O)2}2{Re(NCS)6}]
The preparation of [{K(H2O)2}2{Re(NCS)6}] was adapted and improved from a published procedure [1].
KSCN (8 g; 82.26 mmol) was melted at 200 °C in an oil bath under nitrogen. Then K2ReCl6 (1 g; 2.1 mmol) was added in the KSCN melt. The mixture was cooled down to room temperature after 50 min of vigorous stirring. After addition of EtOH to the solid mixture obtained after cooling, the KCl formed during the reaction was removed by filtration. The brown filtrate was evaporated to dryness. The crude K2Re(NCS)6 obtained was dissolved in a minimum of THF, the solution filtered to remove the excess of KSCN before evaporation of the solvent under reduced pressure. This purification was repeated until the disappearance of the IR signal from free KNCS (νCN = 2040 cm−1), yielding m = 1.15 g (90% based on K2Re(NCS)6) of a brown solid. Finally crystallization was performed by dissolving 200 mg of this solid in 3–4 mL H2O. Upon cooling this solution to 0 °C in an ice bath, amber crystals formed. These were collected and washed two times with the minimum of cold water and vacuum dried. Crystallization yield: 135 mg. IR (ν, cm−1): 3594 (m), 3544 (w), 3520 (w), 2978 (w), 2922 (w), 2349 (w), 2053 (w), 1996 (s), 1940 (s), 1738 (w), 1657 (w), 1604 (m), 1259 (w), 1096 (w), 1023 (w), 956 (w), 878 (w), 865 (w), 800 (w), 747 (w), 667 (w). Chemical analysis calculated (found) for C6N6K2ReS6.3H2O: C, 10.81 (10.95) %; H, 0.91 (0.79) %; N, 12.60 (12.41) %.
4.2 Crystal structure determination
X-ray diffraction data for the crystal were collected at 180 K on an Oxford Diffraction Gemini diffractometer using a graphite-monochromated Mo-Kα radiation source (λ = 0.71073 Å). The structure was solved by direct methods using SIR92 [18] and refined by means of least-square procedures on F using the programs of the PC version of CRYSTALS [19]. Multiscan absorption corrections were applied. Atomic scattering factors were taken from the International tables for X-ray crystallography [20]. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined with riding constraints. Crystallographic and refinement data are given in Table 1.
5 Supporting Information
CCDC-870553 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request.cif.


