1 Introduction
The development of simple routes toward making widely used organic compounds from readily available reagents and compounds is one of the major tasks in organic synthesis [1]. Multi-component condensation reactions (MCRs) occupy an advantageous position among other reactions, because of high atom economy, high yields, convergent character and simplicity of procedures [2–4]. Therefore, the discovery and development of novel MCRs is one of the goals of chemistry research groups.
Pyrazolo[1,5-a]pyrimidines are attractive heterocyclic compounds for drug discovery since many of these scaffolds exhibit wide range of biological, medicinal and pharmaceutical activities such as antimicrobial [5,6], anti-tumor [7], antischistosomal [8], antirypanosomal [9], hepatotoxic [10], antianxiety agents [11], Peripheral Benzodiazpine receptor (PBR) [12], CRF1 receptor antagonist (R121919) [13], estrogen receptor antagonist [14], inhibitors of phosphodiesterase [15], KDR kinase [16], selective cyclooxygenase-2 (COX-2) [17], HMG-CoA reductase [18], tyrosine kinase [19], and BMP signaling [20]. Therefore the development of a simple synthetic method for synthesis of these derivatives is important in organic synthesis.
A careful literature survey reveals that a wide range of methods have been reported for the synthesis of pyrazolo[1,5-a]pyrimidine [21–45]. Some of the methods among these reports are based on MCRs protocols; condensation of arylhydrazone derivatives with 3-aminopyrazole [43], Wittig reaction [44] using orthoesters and 1,3-diketons in a three-component reactions with MW irradiation [45].
Some reviews on the chemistry of aminopyrazoles have been written by several groups [46–48]. Undoubtedly, one of the most important compounds for synthesis of pyrazolo[1,5-a]pyrimidine [21–43] and pyrazolo[3,4-b]pyridine [49–51] is 3(5)-aminopyrazole. 3(5)-amino-5(3)-methylpyrazole 1 with hydrogen atom on nitrogen of ring can be produce pyrazolo[1,5-a]pyrimidines (4,5) or pyrazolo[3,4-b]pyridines (6,7) and/or both of them (4,5 and 6,7) while with alkyl substitutes on nitrogen (2) produce pyrazolo[3,4-b]pyridines (8 or 9) (Scheme 1). Although, reports from α,β-unsaturated nitrile compounds are a few, Elnaghdi [40], Wendt and Anwar [41,42] groups separately carefully show that a pyrazole ring with hydrogen atom on nitrogen of pyrazole ring generates only product 4. Also, Quiroga et al. demonstrated that pyrazole ring with aryl substitutes on nitrogen produces pyrazolo[3,4-b]pyridines 8 between isomers 8 and 9 (Scheme 1) [52]. Recently, Zhang et al. generated pyrazolo[3,4-b]pyridines 8 using a three-component reaction [53].
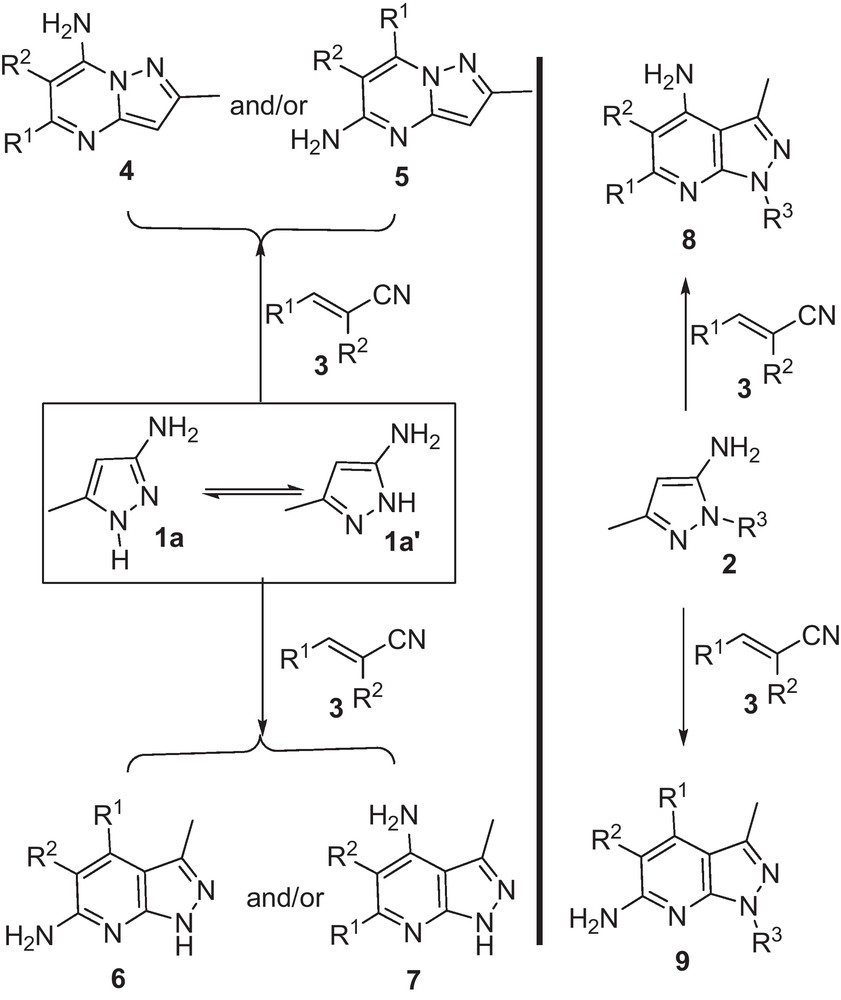
Possible pyrazolopyri(mi)dines from reactions of 3(5)-amino-5(3)-methylpyrazole and/or 1-alkyl-5-aminopyrazole with α,β-unsaturated nitrile compounds.
In continuing our interest in aminoazole based MCRs [54,55], here we want to report the synthesis of 2-alkyl-7-amino-5-aryl-pyrazolo[1,5-a]pyrimidine-6-carbonitrile 4 via the one-pot three-component condensation reaction of an aldehyde 10 and 3(5)-amino-5(3)-methylpyrazole 1 in the presence of malononitrile 11 under reflux conditions in ethanol without using any catalyst (Scheme 2). To the best of our knowledge, there is no report for synthesis of these compounds via three-component condensation reaction.
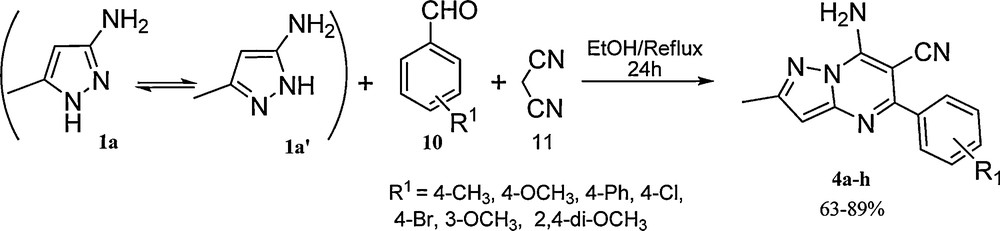
Synthesis of 2-alkyl-7-amino-5-aryl-pyrazolo[1,5-a]pyrimidine-6-carbonitrile 4.
2 Results and discussion
In a typical experiment, the reaction of p-methylbenzaldehydes with 3(5)-amino-5(3)-methylpyrazole and malononitrile afforded 7-amino-2-methyl-5-p-tolylpyrazolo[1,5-a]pyrimidine-6-carbonitrile in EtOH as solvent in relatively high yields. At first, to achieve suitable conditions for the above transformation, a series of solvents was applied. We found that the best solvent for this reaction was ethanol at reflux.
To explore the scope and limitations of this reaction, we have extended it to various substituted benzaldehydes with 3(5)-amino-5(3)-methylpyrazole in the presence of malononitrile. As indicated in Table 1, the reaction proceeds efficiently with various substituted benzaldehydes.
Synthesized substituted 2-alkyl-7-amino-5-aryl-pyrazolo[1,5-a]pyrimidines 4a–h.
| Entry | R1 | Product | Yielda (%) |
| 1 | 4-CH3- | 4a | 82 |
| 2 | 4-CH3O- | 4b | 86 |
| 3 | 4-Cl- | 4c | 80 |
| 4 | 4-Br- | 4d | 80 |
| 5 | 4-Ph- | 4e | 73 |
| 6 | 3-CH3O- | 4f | 81 |
| 7 | 2,4-di-CH3O- | 4g | 89 |
| 8 | 3,4-di-CH3O- | 4h | 85 |
a Isolated yield.
Scheme 1 shows that products 4, 5, 6 and 7 could be generated during the reaction due to the presence of three various active nucleophilic sites on 3(5)-amino-5(3)-methylpyrazole and two different electerophilic centers on benzylidene malononitrile. Monitoring of reaction solution by TLC showed that only one product was generated. After purification, IR and 1H NMR spectra of product indicated that all of starting materials participated in structure of product. In addition, 1H NMR spectrum of this product straightforwardly specified that this product could be 4 or 5 because of the presence of CH and absence of NH signals of pyrazole ring. Although between structures 4 and 5, structure 4 was selected as final product based on Elnaghdi [40], Wendt and Anwar [41,42] report, but (1H,15N) HMBC spectra were also achieved from product. Correlation of hydrogens of NH2 group with nodal nitrogen confirmed that only isomer 4 was produced during the present reaction.
The IR spectrum of 4c showed absorptions at 3449 and 3309 for NH2, 3165 (CH) of pyrazole, 2217 (CN), 1650 and 1603 for (CN) and 1181 (CCl) cm−1 indicating the presence of this functional groups. The mass spectrum of 4c displayed the molecular ion peak at m/z = 285 (37Cl) and 283 (35Cl), which were consistent with the 1:1:1 adduct of starting materials losing of water and H2. The 1H NMR spectrum of 4c exhibited a singlet for CH3 group at δ = 2.42 ppm, other singlet at δ = 6.43 ppm for CH of pyrazole ring. Two doublet for aromatic hydrogens appeared at δ = 7.58 and 7. 79 ppm with 3JHH = 6.99 Hz. A broad singlet line is readily recognized as arising from NH2 group at δ = 8.81 ppm. The 1H decoupled 13C NMR spectrum of 4c showed 12 distinct resonances in agreement with the suggested structures. Finally, correlation of hydrogens of NH2 group with nodal nitrogen in (1H,15N) HMBC spectra exactly confirmed structure 4c. The 1H and 13C NMR spectrum of other structures of 4 are similar to those of 4c except for the X groups, which exhibit characteristic signals with appropriate chemical shifts.
It is important to note that two types of selectivity could happen during this reaction; regioselectivity (when 4 and 5 were generated) and chemoselectivity (when 6 and 7 were generated). However, this reaction proceeds as regioselective. Regioselectivity in this reaction was investigated via variations in structure and conditions. Both of the variations could have an effect on reactivity of reaction centers. Regarding the effect of structure, different aminoazoles such as 3(5)-amino-4-cyanopyrazole and 3(5)-amino-5(3)-carbonitrilpyrazole have been used instead of 3(5)-amino-5(3)-methylpyrazole, but the reaction did not proceed. Then, reaction conditions have been changed and it is concluded that in different solvents, the reaction was performed but yields of reactions were different. Then, acidic and basic media were tested in this reaction and it is showed that in an acidic medium, the reaction did not take place but in basic conditions the reaction was performed. Finally, by these variations no new product achieved except of 4. To some extent, according to obtained results, the initial reaction of amine group can be confirmed; because when the NH2 group was deactivated, it could not react. This confirmed Went's description about the initial exo amine attack [41]. On the other hand, by considering the resonance forms in each tautomer, it can be seen that in most of the structures, the amine group is free. Therefore, amine group could react more than the others.
We have not established a mechanism for the formation of 2-alkyl-7-amino-5-aryl-pyrazolo[1,5-a]pyrimidine-6-carbonitrile, but possible mechanisms are indicated in Scheme 3. The reaction presumably proceeds in several steps: condensation of aldehydes 10 and malononitrile 11 by a Knoevenagel reaction to produce 3-benzylidene compounds 12, followed by a Michael addition of this product with 3-amino-5-methylpyrazole 1a to give intermediates 13, after this intermediate by cyclization give ring systems 14. Compound 14 with proton transfer produced compound 15; at next step, this compound by tautomerization produce compound 16 and after this step by oxidation of compound 16 2-alkyl-7-amino-5-aryl-pyrazolo[1,5-a]pyrimidine-6-carbonitrile 4 was produced (Scheme 3).

Mechanism for the formation of 2-alkyl-7-amino-5-aryl-pyrazolo[1,5-a]pyrimidine-6-carbonitrile 4.
In order to compare the ability of this three-component reaction with previous reports [41,42], first p-methylbenzylidene malononitrile (17) was prepared [56] and was reacted with 3(5)-amino-5(3)-methylpyrazole (Scheme 4). Although the yield of the two-component reaction [42] was close to the three-component reaction, it was performed in two steps and its total yield was low (72%). In this process, 3(5)-amino-5(3)-methylpyrazole acts as a base and accelerates the synthesis of intermediate 17 and followed by Michael addition and cyclization produces product 4b without any base, while in a previous report, some bases such as NEt3 and NaOEt in ethanol solvent and NaOAc in toxic pyridine solvent have been used [41]. It should be pointed out that this reaction has the advantages of multi-component reactions.

Competitive reactions.
In conclusion, we have developed a novel and efficient three-component condensation reaction of an aldehyde, 3(5)-amino-5(3)-methylpyrazole and malononitrile for synthesis of 2-alkyl-7-amino-5-aryl-pyrazolo[1,5-a]pyrimidine-6-carbonitrile. The present method carries the advantage of being performed under neutral conditions and requires no activation or modification of the educts. The simplicity of this method makes it an interesting alternative to the previous approaches.
3 Experimental
3.1 Typical procedure for the synthesis of 7-amino-2-methyl-5-p-tolylpyrazolo[1,5-a]pyrimidine-6-carbonitrile (4a)
A solution of malononitrile (1 mmol), p-methyl benzaldehyde (1 mmol) and 3(5)-amino-5(3)-methylpyrazole (1 mmol) in ethanol (10 mL) was refluxed for 24 h. After completion of the reactions, the solvent was removed by evaporation under reduced pressure, and the residue was purified by column chromatography using n-hexane- EtOAc (6:1) as eluent. Then, the solvent was removed and the product was obtained.
3.1.1 Data for 7-amino-2-methyl-5-p-tolylpyrazolo[1,5-a]pyrimidine-6-carbonitrile (4a)
White powder (82%). IR (KBr) (vmax, cm−1): 3449, 3307, 2949, 2212, 1650, 1604 cm−1. MS (EI, 70 eV) m/z (%): 263 (M+, 40). Anal. Calcd for C15H13N5: C, 68.42; H, 4.98; N, 26.60. Found: C, 68.31; H, 4.93; N, 26.73. 1H NMR (300 MHz, DMSO-d6) δ 2.38 (s, 3H, CH3), 2.42 (s, 3H, CH3), 6.40 (s, 1H, CH(pyrazole)), 7.31 (d, 3J = 7.80 Hz, 2H, CH (arom)), 7.69 (d, 3J = 7.80 Hz, 2H, CH (arom)), 7.73 (bs, 2H, NH2) ppm. 13C NMR (75 MHz, DMSO-d6) δ 14.82, 21.39, 71.81, 91.72, 117.13, 128.96, 129.22, 135.18, 140.15, 148.82, 150.90, 156.30, 158.80 ppm.
3.1.2 Data for 7-amino-5-(4-methoxyphenyl)-2-methyl pyrazolo[1,5-a]pyrimidine-6-carbonitrile (4b)
White powder (86%). IR (KBr) (vmax, cm−1): 3364, 3311, 3211, 2212, 1661, 1643, 1251. MS (EI, 70 eV) m/z (%): 279 (M+, 30). Anal. Calcd for C15H13N5O: C, 64.51; H, 4.69; N, 25.07. Found: C, 64.38; H, 4.76; N, 25.32. 1H NMR (300 MHz, DMSO-d6) δ: 2.41 (s, 3H, CH3), 3.83 (s, 3H, CH3), 6.38 (s, 1H, CH(pyrazole)), 7.06 (d, 3J = 8.40 Hz, 2H, CH (arom)), 7.78 (d, 3J = 8.40 Hz, 2H, CH (arom)), 8.70 (bs, 2H, NH2) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 14.81, 55.78, 71.55, 97.54, 114.04, 117.28, 130.23, 130.60, 148.84, 150.94, 156.27, 158.31, 161.12 ppm.
3.1.3 Data for 7-amino-5-(4-chlorophenyl)-2-methyl pyrazolo[1,5-a]pyrimidine-6-carbonitrile (4c)
White powder (79%); IR (KBr) (vmax, cm−1): 3449, 3309, 3165, 2217, 1650, 1603, 428; MS (EI, 70 eV): m/z (%):285 (M++2, 5), 283 (M+, 22), 282 (M+–1, 10). Anal. Calcd for C14H10ClN5: C, 59.27; H, 3.55; N, 24.68. Found: C, 59.03; H, 3.48; N, 24.73. 1H NMR (300 MHz, DMSO-d6) δ: 2.42 (s, 3H, CH3), 6.43 (s, 1H, CH(pyrazole)), 7.58 (d, 3J = 6.99 Hz, 2H, CH (arom)), 7.79 (d, 3J = 6.99 Hz, 2H, CH (arom)), 8.81 (bs, 2H, NH2) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 14.78, 71.89, 98.02, 116.91, 128.78, 130.85, 135.25, 136.71, 148.65, 150.81, 156.50, 157.68 ppm.
3.1.4 Data for 7-amino-5-(4-bromophenyl)-2-methyl pyrazolo[1,5-a]pyrimidine-6-carbonitrile (4d)
White powder (80%); IR (KBr) (vmax, cm−1): 3427, 3295, 3222, 3085, 2215, 1651, 1600, 1408, 820, 470; MS (EI, 70 eV): m/z (%): 329 (M++2, 35), 327 (M+, 35). Anal. Calcd for C14H10BrN5: C, 51.24; H, 3.07; N, 21.34. Found: C, 50.37; H, 3.05; N, 21.53. 1H NMR (300 MHz, DMSO-d6) δ: 2.42 (s, 3H, CH3), 6.43 (s, 1H, CH(pyrazole)), 7.73 (bs, 4H, CH (arom)), 8.85 (bs, 2H, NH2) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 14.83, 71.84, 98.04, 116.93, 124.04, 131.10, 131.72, 137.13, 148.69, 150.83, 156.43, 157.75 ppm.
3.1.5 Data for 7-amino-5-(biphenyl-4-yl)-2-methyl pyrazolo [1,5-a]pyrimidine-6-carbonitrile (4e)
White powder (78%). IR (KBr) (vmax, cm−1): 3438, 3320, 3237, 3108, 2211, 1644, 1602, 1348; MS (EI, 70 eV): m/z (%): 325 (M+, 60). Anal. Calcd for C20H15N5: C, 73.83; H, 4.65; N, 21.52. Found: C, 74.02; H, 4.50; N, 21.38. 1H NMR (300 MHz, DMSO-d6) δ: 2.44 (s, 3H, CH3), 6.44 (s, 1H, CH(pyrazole)), 7.38-7.92 (m, 9H, CH (arom)), 8.82 (bs, 2H, NH2) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 14.84, 71.84, 97.93, 117.16, 127.27, 127.39, 128.42, 129.52, 129.69, 136.94, 139.71, 142.00, 148.84, 150.95, 156.39, 158.36 ppm.
3.1.6 Data for 7-amino-5-(3-methoxyphenyl)-2-methyl pyrazolo[1,5-a]pyrimidine-6-carbonitrile (4f)
White powder (81%). IR (KBr) (vmax, cm−1): 3432, 3318, 3117, 2235, 1639, 1600, 1521. MS (EI, 70 eV): m/z (%): 280 (M++1, 40), 279 (M+, 25). Anal. Calcd for C15H13N5O: C, 64.51; H, 4.69; N, 25.07. Found: C, 64.34; H, 4.60; N, 25.29. 1H NMR (300 MHz, DMSO-d6) δ: 2.42 (s, 3H, CH3), 3.81 (s, 3H, OCH3), 6.42 (s, 1H, CH(pyrazole)), 7.23–7.45 (m, 4H, CH (arom)), 8.75 (bs, 2H, NH2) ppm. 13C NMR (75 MHz, DMSO-d6) δ: 14.80, 55.69, 72.03, 79.91, 114.31, 116.12, 117.00, 121.30, 129.83, 139.25, 148.69, 150.86, 156.39, 158.64, 159.35 ppm.
3.1.7 Data for 7-amino-5-(2,4-dimethoxyphenyl)-2-methyl pyrazolo[1,5-a]pyrimidine-6-carbonitrile (4g)
White powder (89%). IR (KBr) (vmax, cm−1): 3357, 2938, 2214, 1599, 1210, 1161, 1119. MS (EI, 70 eV): m/z (%): 309 (M+, 30), 309 (M+–1, 100), 309 (M+–2, 60). Anal. Calcd for C16H15N5O2: C, 62.13; H, 4.89; N, 22.64. Found: C, 61.96; H, 4.97; N, 22.51. 1H NMR (300 MHz, DMSO-d6) δ: 2.49 (s, 3H, CH3), 3.87 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 6.40 (s, 1H, CH(pyrazole)), 6.47 (bs, 2H, NH2), 6.58 (d, 4J = 2.22 Hz, 1H, CH (arom)), 6.62 (d, 3J = 8.45 Hz, 4J = 2.29 Hz, 1H, CH (arom)),7.43 (d, 3J = 8.38 Hz, 1H, CH (arom)), ppm. 13C NMR (75 MHz, DMSO-d6) δ: 14.66, 55.46, 55.51, 75.92, 98.16, 98.67, 105.31, 116.11, 119.69, 131.44, 148.90, 149.23, 157.03, 157.38, 158.05, 162.61 ppm.
3.1.8 Data for 7-amino-5-(3,4-dimethoxyphenyl)-2-methyl pyrazolo[1,5-a]pyrimidine-6-carbonitrile (4h)
White powder (85%). IR (KBr) (vmax, cm−1): 3348, 2939, 2215, 1589, 1213, 1168, 1127. MS (EI, 70 eV): m/z (%): 309 (M+, 25). Anal. Calcd for C16H15N5O2: C, 62.13; H, 4.89; N, 22.64. Found: C, 62.25; H, 4.92; N, 22.51. 1H NMR (300 MHz, DMSO-d6) δ: 2.04 (s, 3H, CH3), 3.82 (s, 6H, 2 × OCH3), 6.39 (s, 1H, CH(pyrazole)), 7.07 (d, 3J = 8.1 Hz, 1H, CH (arom)), 7.40 (s, 1H, CH (arom)), 7.41 (d, 3J = 8.1, 2H, CH (arom)), 8.71 (bs, 2H, NH2), ppm. 13C NMR (DMSO-d6, 75 MHz) δC: 14.81, 55.98, 56.02, 71.65, 97.57, 111.43, 112.42, 117.36, 122.08, 130.21, 148.63, 148.76, 150.79, 150.95, 156.28, 158.32.
Acknowledgements
We gratefully acknowledge financial support from the Iran National Science Foundation (INSF) (research project 90001941) and Research Council of the University of Isfahan.


