1 Introduction
Transition metal-, especially palladium-catalyzed cross-coupling reactions have become a very important protocol since its development in the 1970s with versatile applications in organic synthesis [1–3]. Among them, the Suzuki–Miyaura and Mizoroki–Heck coupling reactions have been used extensively for the synthesis of natural products, pharmaceutical intermediates, conducting polymers, pesticides, and liquid crystals [4–18].
Improvement of these reactions greatly relies on the reactivity of the palladium catalyst by using increasingly efficient supporting ligands. To-date, many efforts have been made to search for more efficient ligands. During the past decades, the most common ligands used for these two coupling reactions are the phosphine-based ones [19–21]. Since most phosphine ligands are air- and moisture-sensitive, P–C bond degradation sometimes occurs at elevated temperatures, which poisons the metal leading to decomposition of the catalyst, and this strongly affects the conversion and selectivity [22,23].
In recent years, phosphine-free ligands include the N-heterocyclic carbene class of compounds (NHCs) [24]; thioureas [25], tetrazoles [26], phenanthroline [27], bisimidazole [28], bispyridines [29–31], amino acids [32,33], hydroxyquinolines [34], hydrazones [35,36], N-phenylurea [37] and Schiff bases [38–40] have also been employed.
In the past few years, the development of ligands has provided highly active homogeneous palladium catalysts for the Heck [41–47] and Suzuki [48–51] coupling reactions. However, homogeneous processes suffer from problems concerning separation of the expensive palladium catalysts from reaction mixture, and their reuse. In addition, most of the ligands used for the Heck and Suzuki reactions are undesirable in industrial chemistry because of their toxicity, high price, and air-sensitivity. In principle, the use of supported palladium catalysts could address some of the problems mentioned above. Therefore, practical applications of the Heck [52–57] and Suzuki [58–64] reactions have greatly driven the need for the development of recyclable and efficient heterogeneous palladium catalysts.
Furthermore, in most of the catalytic processes, organic solvents are employed as the reaction media, often creating safety, health, and environmental problems due to their flammability, toxicity, and volatility. From an economic and environmental standpoint, it is desirable to avoid the use of hazardous and expensive organic solvents. The use of water or aqueous solutions represents economically and environmentally viable alternatives to organic solvents for metal-catalyzed reactions [65–69]. Several examples of Pd-catalyzed Heck [70–73] and Suzuki [74–77] reactions in aqueous media have been reported.
As part of our continuing interest in heterogeneous palladium-catalyzed carbon–carbon cross-coupling reactions [78–83], we have recently reported a mild protocol for the copper- and solvent-free Sonogashira coupling reactions catalyzed by 1-phenyl-1,2-propanedione-2-oxime thiosemi-carbazone-functionalized polystyrene resin supported Pd(II) [PS-ppdot-Pd(II)] complex under aerobic conditions [84].
In this article, we wish to report the development of a mild protocol for the Suzuki–Miyaura coupling reactions of aryl halides, including iodides and bromides, and Mizoroki–Heck reactions of aryl iodides and bromides catalyzed by PS-ppdot-Pd(II) as catalyst (Fig. 1).
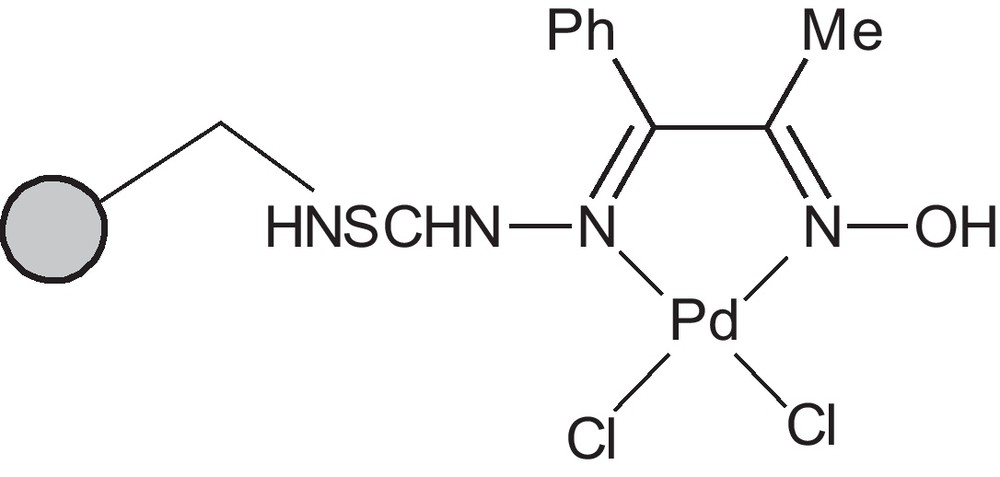
Heterogeneous catalyst Ps-ppdot-Pd (II).
2 Results and discussion
The PS-ppdot-Pd(II) complex was synthesized according to the procedure previously reported [85]. Successful synthesis of PS-ppdot-Pd(II) complex was characterized by FT-IR, SEM, and ICP.
The IR spectrum of PS-ppdot-Pd(II) shows a band around 3420 cm−1 due to the N–H vibrations. The sharp CCl peak (due to CH2Cl groups) at 1264 cm−1 in the starting polymer was practically omitted or was seen as a weak band after introduction of the 1-phenyl-1,2-propanedione-2-oxime thiosemi-carbazone ligand on the polymer. The N-content of the resin was obtained to be 4.2% (0.72 mmol/g), which indicates that only 57% of the total chlorines was substituted by ligand. The amount of palladium incorporated into the PS-ppdot-Pd(II) complex was also determined by inductively coupled plasma (ICP), which showed a value of 0.30 mmol/g for the heterogenized catalyst.
In order to show the merit of the application of heterogeneous catalysts in organic synthesis, we applied the PS-ppdot-Pd(II) complex as the catalysts in the Heck and Suzuki reactions.
In our initial investigations, the catalytic activities of PS-ppdot-Pd(II) complex (1.0 mol%) were studied in the Heck reaction using iodobenzene and methyl acrylate as substrates, in the presence of K2CO3 as base, in water at 70 °C for 10 h (Table 1). As it can be seen in Table 1, K2CO3 showed the best result, and the corresponding coupling product was obtained in 97% yield (Table 1, entry 7). The effect of temperature on the activity of PS-ppdot-Pd(II) complex was also studied. As the temperature decreased from 70 to 25 °C, the yield of product decreased from 98% to 40% (entry 8). A low palladium concentration gave a decreased yield (entry 9).
Optimization of the conditions for Heck reaction of iodobenzene with methyl acrylate.a
| Entry | Base | Cat (mol %) | Yield (%)b |
| 1 | Et3N | 1.0 | 95 |
| 2 | DIEA | 1.0 | 90 |
| 3 | Pyridine | 1.0 | 86 |
| 4 | Piperidine | 1.0 | 94 |
| 5 | Pyrrolidine | 1.0 | 90 |
| 6 | KOH | 1.0 | 93 |
| 7 | K2CO3 | 1.0 | 97 |
| 8c | K2CO3 | 1.0 | 40 |
| 9 | K2CO3 | 0.5 | 82 |
a Conditions: iodobenzene (1.0 mmol), methyl acrylate (1.5 mmol), base (2.0 mmol), H2O (5 mL), 70 °C.
b GC yield.
c Reaction at 25 °C.
Using the optimized reaction conditions, we explored the general applicability of PS-ppdot-Pd(II) complex with methyl acrylate and aryl halides containing electron withdrawing or donating substituents, and the results are tabulated in Table 2. Among the various substituted aryl iodides, both deactivated (electron-rich) and activated (electron-poor) examples were converted efficiently to the desired products in good to excellent yields (entries 1–4). As expected, aryl iodides were more reactive than aryl bromides, and the substituent effects in the aryl iodides appeared to be less significant than in the aryl bromides. As shown in Table 2, activated aryl bromides such as 4-nitrobromobenzene underwent the Heck reaction with methyl acrylate under similar conditions to afford the corresponding product in 85% yield (entry 6) whereas, unactivated aryl bromides such as 4-methoxybromobenzene gave 60% yield (entry 7).
Heck reactions of aryl halides with methyl acrylate using PS-ppdot-Pd(II) complex.a
| Entry | R | X | Product | Yield(%)b |
| 1 | H | I | 3a | 97 |
| 2 | Cl | I | 3b | 98 |
| 3 | NO2 | I | 3c | 98 |
| 4 | MeO | I | 3d | 95 |
| 5 | H | Br | 3a | 72 |
| 6 | NO2 | Br | 3c | 85 |
| 7 | MeO | Br | 3d | 60 |
a Conditions: aryl halide (1.0 mmol), methyl acrylate (1.5 mmol), PS-ppdot-Pd(II) (1.0 mol %), K2CO3 (2.0 mmol), H2O (5 mL), 70 °C.
b GC yield.
Furthermore, under these optimized conditions, we investigated the usefulness of PS-ppdot-Pd(II) complex in the Suzuki reaction involving cross-coupling of an aryl halide with phenylboronic acid. Table 3 illustrates that the reaction was effective in the presence of a wide variety of functional groups on the aryl iodides and aryl bromides giving good to excellent conversions to the corresponding products.
Suzuki reaction of aryl halides with phenyl boronic acid using PS-ppdot-Pd(II) complex.a
| Entry | R | X | Product | Yield(%)b |
| 1 | H | I | 4a | 98 |
| 2 | 4-Cl | I | 4b | 97 |
| 3 | 4-Br | I | 4c | 98 |
| 4 | 4-MeCO | I | 4d | 97 |
| 5 | 4-NO2 | I | 4e | 100 |
| 6 | 3-NO2 | I | 4f | 100 |
| 7 | MeO | I | 4g | 96 |
| 8 | H | Br | 4a | 68 |
| 9 | 4-Cl | Br | 4b | 80 |
| 10 | 4-CN | Br | 4h | 98 |
| 11 | 4-NO2 | Br | 4e | 97 |
| 12 | 3-NO2 | Br | 4f | 90 |
| 13 | MeO | Br | 4g | 80 |
a Reaction conditions: aryl halide (1.0 mmol), phenyl boronic acid (1.2 mmol), K2CO3 (2.0 mmol), PS-ppdot-Pd(II) (1.0 mol%), H2O (5 ml), 70 °C.
b GC yield.
The Suzuki reactions of electron-rich aryl iodides with phenyl boronic acid proceeded smoothly to give the corresponding coupling products in high yields (entry 7). Unsurprisingly, 4-nitroiodobenzene and 3-nitroiodobenzene were found to be the most reactive among the aryl iodides studied (entries 5 and 6).
To extend the scope of our work, we next investigated the coupling reaction of various aryl bromides with phenyl boronic acid. The less reactive bromobenzene showed low yield (entry 8). However, the activated aryl bromides, 4-bromobenzonitrile and 4-nitrobromobenzene, gave the corresponding product in excellent yield (entries 10 and 11).
One of the purposes for designing this heterogeneous catalyst is to enable recycling of the catalyst for use in subsequent reactions. For the recycle experiment, we used iodobenzene with methyl acrylate (Heck reaction) and iodobenzene with phenyl boronic acid (Suzuki reaction) as the representative reactants and in the presence of 1.0 mol% of PS-ppdot-Pd(II) to study the recyclability of this heterogeneous catalyst. After the completion of the reaction, the mixture from the first-run reaction was centrifuged, and the solid obtained was washed alternately with ethanol and CH3CN. After drying, the recovered catalyst was then reused in the same reaction under identical conditions. We found that the product yield decreased slightly over four recycling runs (Table 4).
The Heck and Suzuki reactions catalyzed by the recycled catalyst.a
| Entry | Cycle | Heck Yield (%)b | Suzuki Yield (%) |
| 1 | 1 | 97 | 98 |
| 2 | 2 | 95 | 96 |
| 3 | 3 | 90 | 93 |
| 4 | 4 | 85 | 90 |
a Reaction conditions: methyl acrylate (1.5 mmol), or phenyl boronic acid (1.2 mmol), iodobenzene (1.0 mmol), Catalyst (1.0 mol%), K2CO3 (2.0 mmol), H2O (5 ml), 70 °C
b GC yield.
3 Conclusion
In conclusion, we showed that the PS-ppdot-Pd(II) complex efficiently catalyzes the Heck reaction of methyl acrylate with various aryl iodides and bromides and the Suzuki cross- coupling of aryl iodides and bromides, with phenyl boronic acid in water. The catalyst used is easily separated, and can be reused for several times without a noticeable change in activity.
4 Experimental
4.1 Typical experimental procedure for the Heck reaction
A round-bottomed flask was charged with aryl halide (1.0 mmol), methyl acrylate (1.5 mmol), K2CO3 (2.0 mmol), PS-ppdot-Pd(II) complex (1.0 mol%), and H2O (5 mL). The reaction mixture was heated at 70 °C for 10 h.
4.2 Typical experimental procedure for the Suzuki reaction
A round-bottomed flask was charged with aryl halide (1.0 mmol), phenylboronic acid (1.2 mmol), K2CO3 (2.0 mmol), PS-ppdot-Pd(II) complex (1.0 mol %), and H2O (5 mL). The reaction mixture was heated at 70 °C for 8 h.
Both reactions were monitored by gas chromatography. Upon completion of the reaction, the reaction mixture was dissolved in acetonitrile (10 mL). The palladium catalyst was separated from the mixture by filtration, washed with water (10 mL) and acetonitrile (10 mL), and reused in the next run. The solution was concentrated in vacuo, and the crude product was subjected to silica gel column chromatography using CHCl3–CH3OH (98:2) as eluent to afford the pure product.
Acknowledgements
We gratefully acknowledge the financial support of the Research Council of Shahrood University of Technology.


