1 Introduction
Ever since the pioneering work of Breslow in the field of artificial enzymes [1], modification of cyclodextrins (CDs) with nitrogen-containing groups has been considered of paramount importance in enzyme mimicry. Particular attention was drawn to imidazole-appended CDs, as they proved to act as supramolecular catalysts for the hydrolysis of esters [2] or, when complexed to zinc, for the hydration of CO2 [3]. In order to take full advantage of metallated CDs [4], it appears crucial to bring the metal centre as close as possible to the receptor entrance [5,6]. This may be achieved by chelation with coordinating groups anchored onto the CD scaffold [7,8]. However, such a ring-closure process has hardly been investigated in the case of nitrogen-containing CDs [9] because large chelate complexes can only be efficiently formed if the coordinating arms are sufficiently rigid and preorganised [10]. Herein, we report on the synthesis of permethylated α- and β-CDs disubstituted at the 6A and 6D positions with imidazole groups and their ability to chelate both platinum and ruthenium metal centres.
2 Results and discussion
2.1 Synthesis of ligands
Ligands L1 and L2 were obtained in 76% and 73% yield respectively, by reacting dimesylates 1 [8] and 2 [11] with excess sodium imidazolide in DMF (DMF = N,N-dimethylformamide). Note that undeprotonated imidazole only led to partial substitution of the mesylate groups, even when operating with a large excess of nucleophilic reagent. The ESI-MS spectrum of each ligand showed a major peak corresponding to the [M + H]+ ion (see experimental section). Both the 13C and 1H NMR spectra of L1 are consistent with the C2 symmetry of the ligand. Thus for example, its 1H NMR spectrum displays a single ABX spectrum for the carbon atoms of the two imidazole rings. In contrast, that of L2 displays two distinct ABX patterns reflecting the absence of symmetry of this β-CD-based molecule (Fig. 1 and Scheme 1).
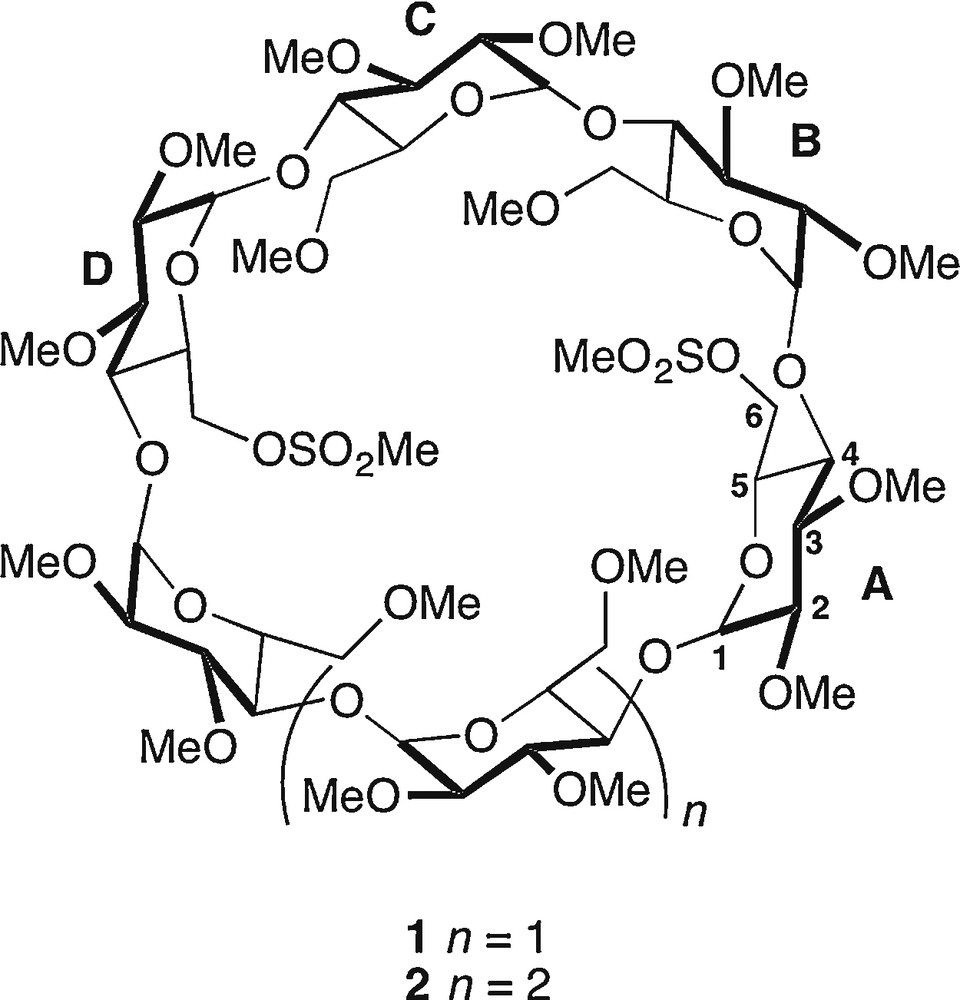
Dimesylates 1 and 2.
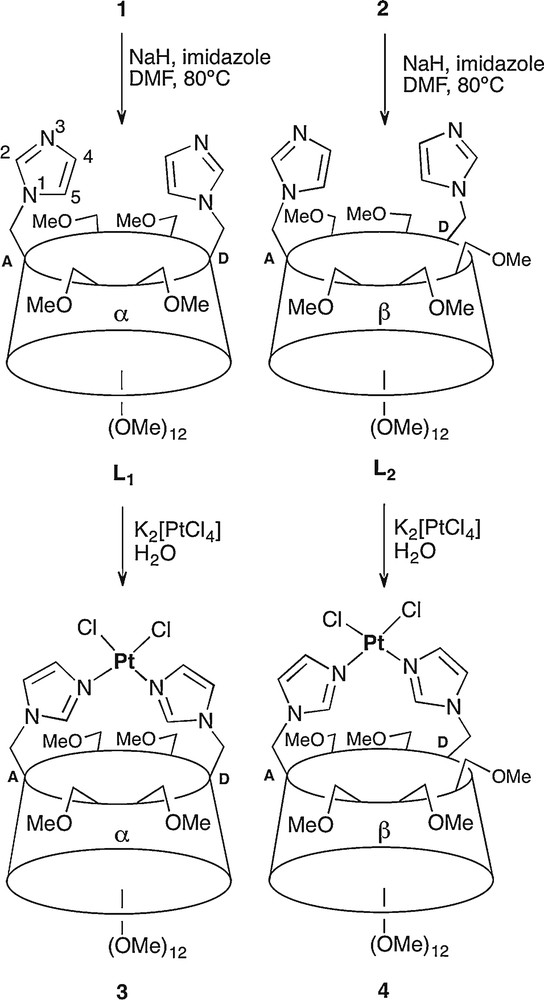
Synthesis of the Pt(II) chelate complexes 3 and 4.
A single crystal X-ray diffraction study carried out on L1 (Table 1) allowed us to locate the imidazole rings with respect to the cavity in the solid state (Fig. 2). The H-4 and H-5 atoms of both imidazoles are pointing towards the CD axis, thus blocking the entrance of the cavity. The interplanar angle between the imidazole rings is 64°; the H-5…H’-5 and H-4…H’-4 separations being 2.49 Å and 2.89 Å, respectively. The N-3 atoms, which are susceptible to coordinate metal centres, have both their lone pair oriented towards the cavity exterior. Despite the large separation between these two nitrogen atoms (N-3…N’-3 6.19 Å) in the solid state, it may be anticipated that rotation of the imidazole moieties by 180° about the corresponding C-6–N axes will make them suitable for chelation. Noteworthy, the CD scaffold has an almost perfect circular shape with all the glucose units adopting the standard 4C1 conformation. Such a conformation is likely to occur also in solution because of the very narrow range of chemical shifts in which the anomeric protons resonate (Δδ = 0.08 ppm). Finally, a pentane molecule is hosted inside the CD cavity, which augurs well for the ability of the cone-shaped hollow molecule to form inclusion complexes in solution.
Crystal data and structure refinement for compound L1.
| Chemical formula | C58H96N4O28•0.5 C5H12 |
| Molar weight (g mol-1) | 1333.46 |
| Calculated density (g cm-3) | 1.205 |
| Crystal system | orthorhombic |
| Space group | P212121 |
| a (Å) | 15.4784(3) |
| b (Å) | 21.5356(6) |
| c (Å) | 22.0470(5) |
| V (Å3) | 7349.1(3) |
| Z | 4 |
| Radiation λ | 0.71073 |
| F(000) | 2868 |
| μ (mm−1) | 0.095 |
| Temperature (K) | 120(2) |
| θ range for data collection | 2.80 to 27.00° |
| Index ranges | –19 ≤ h ≤ 13, –27 ≤ k ≤ 23, |
| –28 ≤ l ≤ 22 | |
| Reflections collected | 21901 |
| Independent reflections | 14608 [R(int) = 0.0396] |
| Refinements method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 14608/0/839 |
| Goodness-of-fit on F2 | 0.615 |
| Final R indices [I > 2σ(I)] | R1a = 0.0495, wR2b = 0.1220 |
| R indices (all data) | R1a = 0.0952, wR2b = 0.1386 |
| Largest diff. peak and hole | 0.615 and -0.221 e.Å−3 |
a R1 = Σ||Fo|–|Fc||/Σ|Fo|.
b wR2 = [Σ[w (Fo2-Fc2)2]/Σ[w (Fo2)2]]1/2.
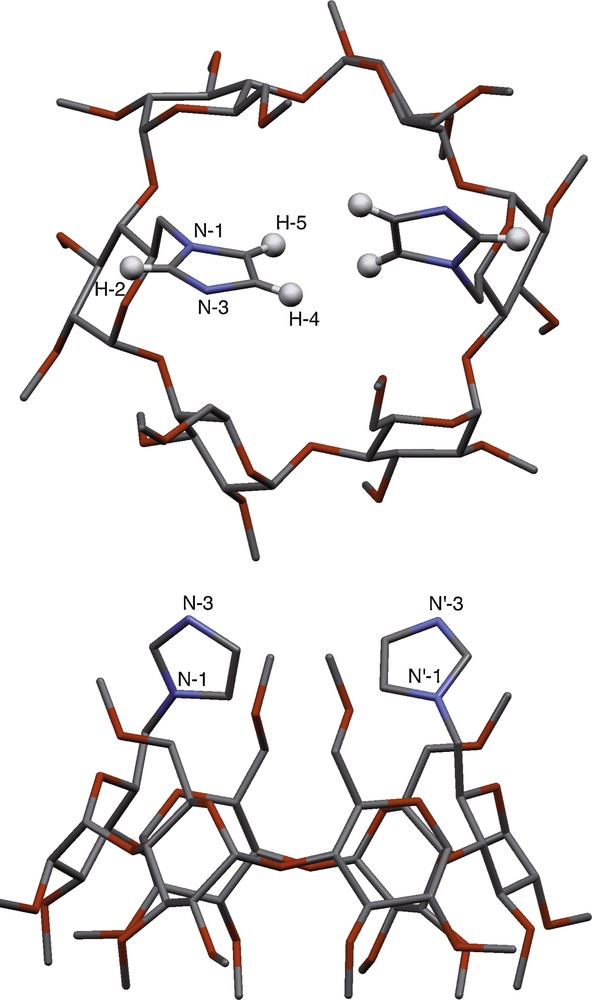
Molecular structure of ligand L1. Top (top) and side (bottom) views show the two imidazole coordinating units sitting at the top of the cavity entrance. The pentane molecule, which is disordered over two positions, has been omitted for clarity.
2.2 Synthesis of chelate complexes
Despite the presence of freely rotating imidazole arms, both ligands turned out to behave as chelators towards platinum(II) ions. Indeed, reaction of L1 and L2 with K2[PtCl4] in water led to the formation of chelate complexes 3 and 4 in 23% and 51% yield, respectively, after column chromatography. Interestingly, the use of water as solvent seems to be critical since much lower yields of 3 and 4 were obtained when the ligands were reacted with [PtCl2(PhCN)2] in organic solvents, the reaction leading then to significant amounts of oligomeric material. Formation of chelate complexes was inferred from the mass spectra, which revealed major peaks at m/z = 1584.5 for 3 and m/z = 1789.7 for 4 corresponding to [M + Na]+ ions. Consistent with a coordinated platinum atom, the H-4 and H-5 imidazole protons of complex 3 no longer resonate at the same frequency (δ = 7.29 and 7.07) as in the free ligand (δ = 7.00 for both protons in L1). The same observation holds for complex 4, as its H-4 and H-5 imidazole protons underwent an even stronger differentiation (up to Δδ = 0.53 ppm vs. 0.11 ppm in the free ligand). For both complexes, the anomeric signals appear in a narrow chemical shift range (Δδ = 0.08 ppm and Δδ = 0.19 ppm, respectively) close to that observed in the free ligands, which means that the CD scaffolds did not undergo major distortions upon complexation. The coordination geometry about the Pt(II) ion was assigned using IR spectroscopy. The observation of two narrow peaks at 336 and 330 cm−1 for complex 3, and at 338 and 330 cm−1 for complex 4 is in accord with a cis-arrangement of the chlorido ligands. Noteworthy, the PtCl2 unit is most likely located outside the cavity since no downfield shift was detected for inner cavity protons upon complexation [6]. The water-soluble complexes 3 and 4 can be regarded as analogues of cis-platinum or similar complexes displaying antitumoral activity [12]. The possibility of having biologically-active metallocyclodextrins capable of hosting coformulating drugs in water [13] prompted us to synthesise an analogue of the promising cytotoxic KP 418 ruthenium complex [14]. Thus, treatment of ligands L1 and L2 with Na[trans-RuCl4(DMSO)2] [15] in an acetone/DMSO mixture afforded modest yields of complexes 5 (5%) and 6 (12%) respectively, together with oligomeric materials that were not recovered. Again, water-soluble complexes were obtained. As for the platinum chelate complexes, the best yield was observed with ligand L2, probably because the larger β-CD is better suited for accommodating the sterically demanding metal fragment. The chelation of a [RuCl3(DMSO)] unit by both ligands was inferred from mass spectrometry. The ESI-MS spectra of 5 and 6 showed major peaks corresponding to [M + Na]+ ions, respectively at m/z = 1606.4 and m/z = 1810.6. In keeping with trans disposed imidazolyl groups, the 1H NMR spectrum of paramagnetic 5 is typically that of a C2-symmetric molecule. In the 1H NMR spectrum of 6, the imidazole signals closely resemble those of their counterparts in 5, so that a trans N–Ru–N arrangement can reasonably be assigned also to this complex. The IR spectra of both complexes display strong bands that are indicative of a S-coordinated DMSO ligand (1086 and 426 cm−1 for complex 5, and 1086 and 428 cm−1 for complex 6) (Scheme 2) [15].
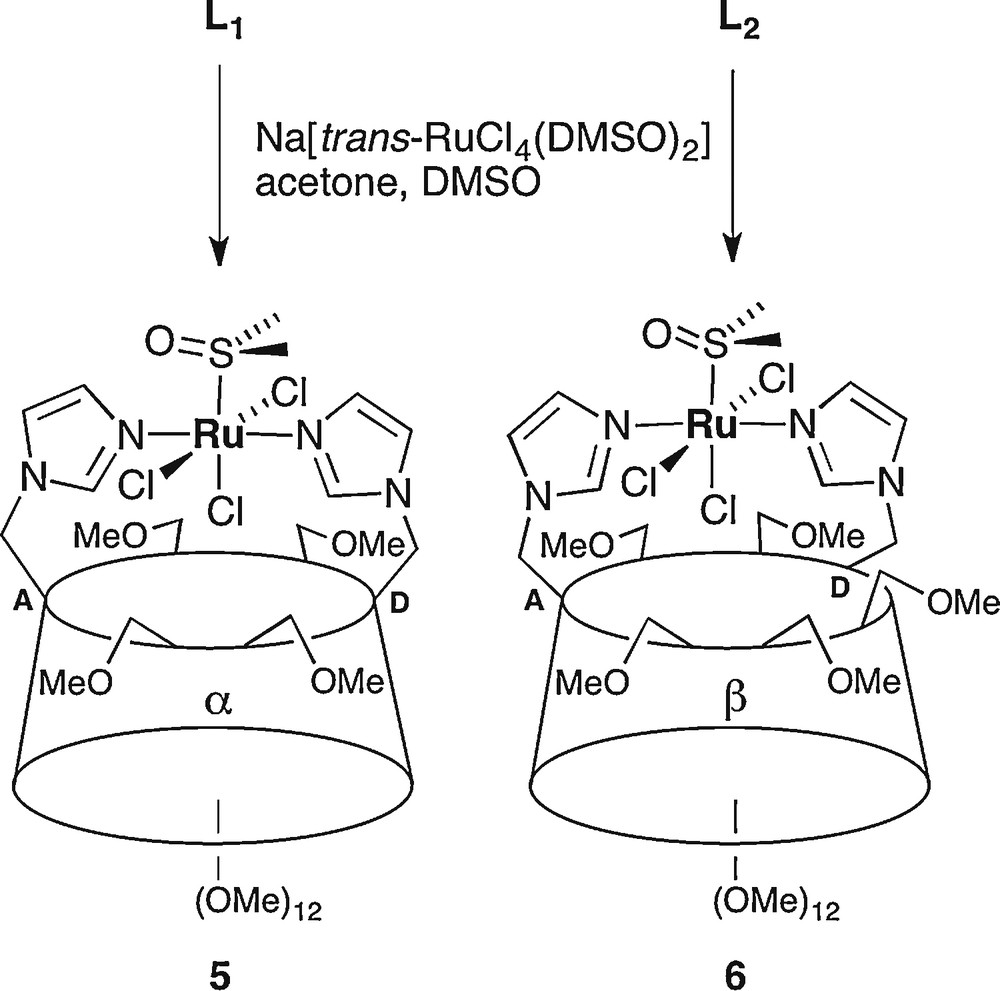
Synthesis of Ru(III) chelate complexes 5 and 6.
3 Conclusion
In the present study, we have shown that imidazole groups can be efficiently grafted onto the primary face of both permethylated α- and β-CD scaffolds. The two bis-imidazole ligands L1 and L2 were found suitable for forming N,N’-chelated complexes with platinum(II) and ruthenium(III) ions. Whereas the nitrogen donors are cis-disposed about the metal centre in the platinum complexes, the more sterically-demanding ruthenium ion forces both ligands L1 and L2 to behave as trans-chelators. The water-soluble complexes that have been prepared can be considered as prototypes for new anticancer drugs that may combine their own cytotoxic properties with those of an included biologically-active guest. Biological assessment and host-guest studies are currently underway.
4 Experimental
4.1 General procedures
All manipulations were performed in Schlenk-type flasks under dry nitrogen. Solvents were dried by conventional methods and distilled immediately prior to use. Deuterated solvents were passed down a 5 cm-thick alumina column and stored under nitrogen over molecular sieves (4 Å). FAR-IR and IR spectra were recorded, respectively, on Nicolet 6700 and Bruker Alpha spectrophotometers. All NMR spectra were recorded on a FT Bruker AVANCE 300 instrument. 1H NMR spectral data were referenced to residual protiated solvents (δ = 7.26 for CDCl3), 13C{1H} chemical shifts are reported relative to deuterated solvents (δ = 77.00 for CDCl3). Mass spectra were recorded on a Bruker MicroTOF spectrometer (ESI) using CH2Cl2, MeCN or MeOH as solvent. Elemental analyses were performed by the Service de Microanalyse, Institut de Chimie UMR 7177 CNRS-UDS, Strasbourg. Melting points were determined with a Büchi 535 capillary melting-point apparatus. All commercial reagents were used as supplied. Dimesylates 1 [8] and 2, [11] and Na[trans-RuCl4(DMSO)2] [15] were prepared according to literature procedures. The numbering of the atoms within a glucose unit is as shown in Fig. 3.

Numbering of the carbon atoms within a glucose unit.
4.2 Synthesis of 6A6D-dideoxy-6A,6D-bis(1-imidazolyl)-2A,2B,2C,2D,2E,2F,3A,3B,3C,3D,3E,3F,6B,6C,6E,6F-hexadeca-O-methyl-α-cyclodextrin (L1)
Imidazole (0.327 g, 4.80 mmol) was treated with NaH (0.211 g, 5.28 mmol, 60% dispersion in oil) in dry DMF (10 mL) at room temperature. After 20 min, a solution of 1 (0.650 g, 0.48 mmol) in dry DMF (10 mL) was added to the imidazole/NaH mixture. The reaction mixture was stirred for 14 h at 80°C. MeOH (10 mL) was then added in order to quench excess NaH before removing the solvents in vacuo. NaOH 2 M (30 mL) was added and the aqueous layer was extracted with CH2Cl2 (3 × 50 mL). The organic layer was dried over MgSO4, filtrated and evaporated to dryness. The residue was submitted to column chromatography (SiO2, CH2Cl2/MeOH/NH4OH (30% w/w NH3 in water), 93:6:1, v/v) to afford ligand L1 (0.470 g, 76%) as a colourless solid. Rf (SiO2, CH2Cl2/MeOH/NH4OH, 93:6:1, v/v) = 0.09. mp dec. > 250 °C. 1H NMR (300.1 MHz, CDCl3, 25°C): δ (assignment by COSY) = 3.05 (3 H, H-2), 3.15 (3 H, H-2), 3.30–3.99 (26 H, H-3, H-4, H-5, H-6B,C,E,F), 3.32 (s, 12 H, OMe-6), 3.42 (s, 6 H, OMe-2), 3.44 (s, 6 H, OMe-2), 3.46 (s, 6 H, OMe-2), 3.55 (s, 6 H, OMe-3), 3.60 (s, 12 H, OMe-3), 4.39 (d, 4 H, 3JH-6,H-5 = 3.8 Hz, H-6A,D), 4.92 (d, 2 H, 3JH-1,H-2 = 3.4 Hz, H-1), 4.97 (d, 2 H, 3JH-1,H-2 = 3.4 Hz, H-1), 5.00 (d, 2 H, 3JH-1,H-2 = 3.1 Hz, H-1), 7.01 (4 H, A and B parts of ABX system, H-4Im and H-5Im), 7.59 (2 H, X part of ABX system, H-2Im) ppm. 13C{1H} NMR (75.5 MHz, CDCl3, 25°C): δ (assignment by HMQC) = 47.6 [×2] (C-6A,D), 57.9 [×2], 58.0 [×4], 59.2 [×4], 61.7 [×2], 61.8 [×2], 61.9 [×2] (OMe), 70.6 [×2] (C-6), 71.1 [×2], 71.3 [×2], 71.7 [×2] (C-5), 72.1 [×2] (C-6), 80.9 [×2], 81.0 [×2], 81.2 [×2], 81.9 [×4], 82.1 [×2], 82.2 [×2], 83.4 [×4] (C-2, C-3, C-4), 99.5 [×2], 100.0 [×2], 100.4 [×2] (C-1), 119.7 [×2], 129.1 [×2], 138.5 [×2] (C-imidazole) ppm. MS, m/z (%): 1297.6 (100) [M + H]+. Anal. Calcd for C58H96N4O28: C, 53.69; H, 7.46; N, 4.32. Found C, 53.98; H, 7.55; N, 3.60.
4.3 Synthesis of 6A,6D-dideoxy-6A,6D-bis(1-imidazolyl)-2A,2B,2C,2D,2E,2F,2G,3A,3B,3C,3D,3E,3F,3G,6B,6C,6E,6F,6G-nonadeca-O-methyl-β-cyclodextrin (L2)
Ligand L2 (0.351 g, 73%) was synthesised as described above from 2 (0.5 g, 0.32 mmol), imidazole (0.22 g, 3.2 mmol) and NaH (0.14 g, 3,5 mmol, 60% dispersion in oil). Rf (SiO2, CH2Cl2/MeOH/NH4OH (30% w/w NH3 in water), 93:6:1, v/v) = 0.09. mp dec. > 250°C. 1H NMR (300.1 MHz, CDCl3, 25°C): δ (assignment by COSY) = 3.05–3.23 (14 H, H-2, H-4), 3.35 (s, 9 H, OMe-6), 3.37 (s, 3 H, OMe-6), 3.39 (s, 3 H, OMe-6), 3.46 (s, 3 H, OMe-2), 3.47 (s, 3 H, OMe-2), 3.48 (s, 3 H, OMe-2), 3.50 (s, 3 H, OMe-2), 3.51 (s, 3 H, OMe-2), 3.52 (s, 3 H, OMe-2), 3.53 (s, 3 H, OMe-2), 3.60 (s, 3 H, OMe-3), 3.61 (s, 3 H, OMe-3), 3.62 (s, 3 H, OMe-3), 3.63 (s, 3 H, OMe-3), 3.64 (s, 3 H, OMe-3), 3.65 (s, 3 H, OMe-3), 3.66 (s, 3 H, OMe-3), 3.35–4.02 (24 H, H-3, H-5, H-6B,C,E,F,G), 4.27–4.35 (2 H, H-6A or D), 4.44–4.51 (2 H, H-6D or A), 4.96 (d, 1 H, 3JH-1,H-2 = 3.7 Hz, H-1), 5.00 (d, 1 H, 3JH-1,H-2 = 3.8 Hz, H-1), 5.01 (d, 2 H, 3JH-1,H-2 = 3.31 Hz, H-1), 5.10 (d, 2 H, 3JH-1,H-2 = 4.1 Hz, H-1), 5.11 (d, 1 H, 3JH-1,H-2 = 4.4 Hz, H-1), 6.98 and 7.01 (two pseudo t, 1H [x2], A parts of ABX systems, H-4Im or H-5Im), 7.07 and 7.11 (two pseudo t, 1H [x2], B parts of ABX systems, H-5Im or H-4Im), 7.59 and 7.62 (two pseudo t, 1 H [x2], X parts of ABX systems, H-2Im) ppm. 13C{1H} NMR (75.5 MHz, CDCl3, 25°C): δ (assignment by HMQC) = 47.72 [×2] (C-6A,D), 58.21, 58.29, 58.38, 58.44, 58.69, 58.92, 58.93, 59.10 [×4], 59.18, 61.02, 61.14, 61.23, 61.42, 61.58 [×3] (OMe), 70.35, 70.53 (C-6), 70.74, 70.86, 70.92, 70.99 [×2], 71.07, 71.20 (C-5), 72.22, 72.30 [×2] (C-6), 80.13, 80.26, 80.32, 80.68, 80.71, 81.11 [×2], 81.49 [×4], 81.68 [×3], 81.87, 81.99, 82.06 [×2], 82.14, 82.68, 83.20 (C-2, C-3, C-4), 98.16, 98.31, 99.26, 99.60 [×3], 99.79 (C-1), 119.91, 120.02, 128.78, 128.84, 138.36, 138.44 (C-imidazole) ppm. MS, m/z (%): 1500.7 (100) [M + Na]+. Anal. Calcd for C67H112N4O33: C, 53.59; H, 7.52; N, 3.73. Found C, 53.41; H, 7.71; N 3.52.
4.4 Synthesis of cis-dichlorido[6A,6D-dideoxy-6A,6D-bis(1-imidazolyl-κN3)-2A,2B,2C,2D,2E,2F,3A,3B,3C,3D,3E,3F,6B,6C,6E,6F-hexadeca-O-methyl-α-cyclodextrin]platinum(II) (3)
A solution of L1 (0.150 g, 0.12 mmol) in water (5 mL) was added to a solution of K2[PtCl4] (0.50 g, 0.12 mmol) in water (5 mL). After stirring for 14 h, the orange solution turned pale yellow and water (50 mL) was added. The organic layer was extracted with CH2Cl2 (3 × 50 mL) before being dried over MgSO4 and evaporated to dryness. The crude product was purified by column chromatography (SiO2, CH2Cl2/MeOH, 92:8, v/v) and afforded analytically pure 3 (0.040 g, yield 23%) as a beige solid. Rf (SiO2, CH2Cl2/MeOH/NH4OH, 93:6:1, v/v) = 0.17. mp dec. > 250°C. FAR-IR ν(Pt-Cl) 336 (m) cm−1, ν(Pt-Cl) 330 (s) cm−1. 1H NMR (300.1 MHz, CDCl3, 25°C): δ (assignment by COSY) = 3.10–3.21 (12 H, H-2, H-4), 3.47 (s, 6 H, OMe), 3.48 (s, 6 H, OMe), 3.49 (s, 6 H, OMe), 3.51 (s, 6 H, OMe), 3.54 (s, 6 H, OMe), 3.58 (s, 6 H, OMe), 3.61 (s, 6 H, OMe), 3.62 (s, 6 H, OMe), 3.47–4.07 (22 H, H-3, H-5, H-6B,C,E,F, H-6aA,D), 4.88 (d, 2 H, 2JH-6b-H6a = 14.1 Hz, H-6bA,D), 4.96 (d, 2 H, 3JH-1,H-2 = 3.4 Hz, H-1), 4.99 (d, 2 H, 3JH-1,H-2 = 3.2 Hz, H-1), 5.04 (d, 2 H, 3JH-1,H-2 = 2.9 Hz, H-1), 7.07 (br signal, 2 H, A or B part of ABX system, H-4Im or H-5Im), 7.29 (br signal, 2 H, B or A part of ABX system, H-5Im or H-4Im), 8.01 (br signal, 2 H, X part of ABX system, H-2Im) ppm. 13C{1H} NMR (75.7 MHz, CDCl3, 25°C): δ (assignment by HMQC) = 50.05 [×2] (C-6A,D), 58.0 [×2], 58.16 [×4], 59.32 [×2], 59.78 [×2], 61.65 [×2], 61.74 [×2], 61.99 [×2] (OMe), 70.41 [×2] (C-6), 71.47 [×2], 72.01 [×2], 72.26 [×2] (C-5), 73.39 [×2] (C-6), 80.53 [×2], 80.83 [×2], 81.14 [×2], 81.26 [×2], 81.68 [×2], 82.01 [×2], 82.26 [×2], 83.81 [×2], 85.04 [×2] (C-2, C-3, C-4), 99.23 [×2], 100.13 [×2], 100.50 [×2] (C-1), 121.82 [×2], 129.9 [×2], 139.06 [×2] (C-imidazole) ppm. MS, m/z (%): 1584.5 (100) [M + Na]+. Anal. Calcd for C58H96Cl2N4O28Pt: C, 44.56; H, 6.19; N, 3.58. Found C, 45.19; H, 6.42; N, 3.178.
4.5 Synthesis of cis-dichlorido[6A,6D-dideoxy-6A,6D-bis(1-imidazolyl-κN3)-2A,2B,2C,2D,2E,2F,2G,3A,3B,3C,3D,3E,3F,3G,6B,6C,6E,6F,6G-nonadeca-O-methyl-β-cyclodextrin]platinum(II) (4)
Complex 4 (0.070 g, 51%) was synthesized as described above from L2 (0.115 g, 0.077 mmol) and K2[PtCl4] (0.032 g, 0.077 mmol). Rf (SiO2, CH2Cl2/MeOH/NH4OH, 93:6:1, v/v) = 0.14. mp dec. >250°C. FAR-IR ν(Pt-Cl) 338 (m) cm−1, ν(Pt-Cl) 330 (s) cm−1. 1H NMR (300.1 MHz, CDCl3, 25°C): δ (assignment by COSY) = 3.12–4.10 (40 H, H-2, H-3, H-4, H-5, H-6B,C,E,F,G, H-6aA,D) 3.24 (s, 3 H, OMe), 3.32 (s, 3 H, OMe), 3.40 (s, 3 H, OMe), 3.44 (s, 3 H, OMe), 3.47 (s, 3 H, OMe), 3.50 (s, 3 H, OMe), 3.51 (s, 6 H, OMe), 3.52 (s, 6 H, OMe), 3.54 (s, 3 H, OMe), 3.55 (s, 3 H, OMe), 3.59 (s, 3 H, OMe), 3.60 (s, 3 H, OMe), 3.61 (s, 9 H, OMe), 3.63 (s, 3 H, OMe), 3.67 (s, 3 H, OMe), 4.58 (d, 1 H, 2JH-6b,H-6a = 12.8 Hz, H-6bA or D), 4.69 (d, 1 H, 2JH-6b,H-6a = 14.3 Hz, H-6bD or A), 4.95 (d, 1 H, 3JH-1,H-2 = 3.3 Hz, H-1), 5.07–5.10 (5 H, H-1), 5.26 (d, 1 H, 3JH-1,H-2 = 2.4 Hz, H-1), 6.34 and 6.87 (br. signals, 1 H [×2], A parts of ABX systems, H-4Im or H-5Im), 7.21 and 7.34 (br. signals, 1 H [×2], B parts of ABX systems, H-5Im or H-4Im), 7.93 and 8.47 (br. signals, 1 H [×2], X parts of ABX systems, H-2Im) ppm. 13C{1H} NMR (75.7 MHz, CDCl3, 25°C): δ (assignment by HMQC) = 50.47 (C-6A or D), 50.66 (C-6D or A), 58.13, 58.20, 58.50, 58.54, 58.90, 59.16 [×3], 59.27, 59.36 [×3], 60.24, 60.92, 60.97, 61.02, 61.15 [×2], 61.41 (OMe), 69.97 (C-5), 70.21 [×2] (C-6), 71.15, 71.28, 71.33 (C-5), 71.57 (C-6), 71.79, 72.22, 72.48 (C-5), 72.63, 72.82 (C-6), 75.88, 77.25, 78.26, 78.51, 80.12, 80.58, 80.70, 81.06 [×2], 81.36, 81.50 [×3], 81.57, 81.67, 81.85, 82.08, 82.43, 82.80, 82.90 [×2] (C-2, C-3, C-4), 96.32, 97.13, 97.29, 99.17, 99.61, 99.94, 100.10 (C-1), 119.92, 122.41, 127.80, 129.72, 138.38, 139.79 (C-imidazole) ppm. MS, m/z (%): 1789.7 (100) [M + Na]+. Anal. Calcd for C67H112Cl2N4O33Pt: C, 45.53; H, 6.39; N, 3.17. Found C, 44.85; H, 6.39; N, 2.79.
4.6 Synthesis of trans,mer-trichlorido(dimethyl sulfoxide-κS)[6A,6D-dideoxy-6A,6D-bis(1-imidazoyl-κN3)-2A,2B,2C,2D,2E,2F,3A,3B,3C,3D,3E,3F,6B,6C,6E,6F-hexadeca-O-methyl-α-cyclodextrin]ruthenium(III) (5)
A solution of L1 (0.150 g, 0.12 mmol) in acetone (6.3 mL) was added to a solution of Na[trans-RuCl4(DMSO)2] (0.049 g, 0.12 mmol) in a mixture of acetone (4.1 mL) and DMSO (0.8 mL). After stirring for 14 h at room temperature, the red-orange solution turned yellow-orange. The solution was evaporated to dryness and the crude product was purified by column chromatography (SiO2, CH2Cl2/MeOH, 93:7, v/v), affording analytically pure 5 (0.010 g, 5%). Rf (SiO2, CH2Cl2/MeOH, 90:10, v/v) = 0.33. mp dec. >250°C. IR ν(S = O) 1086 (s) cm−1, ν(Ru-S) 426 (s) cm−1. 1H NMR (300.1 MHz, CDCl3, 25°C, paramagnetic): δ = –26.00 (s, 2 H, H-2Im or H-4Im or H5Im),–11.00 (s, 2 H, H-5Im or H-2Im or H-4Im),–5.00 (s, 2 H, H-4Im or H-5Im or H-2Im), 2.80–5.55 (42 H, H-1, H-2, H-3, H-4, H-5, H-6), 2.96 (s, 6 H, OMe), 3.07 (s, 6 H, OMe), 3.12 (s, 6 H, OMe), 3.24 (s, 6 H, OMe), 3.46 (s, 6 H, OMe), 3.55 (s, 6 H, OMe), 3.63 (s, 12 H, OMe) ppm. The DMSO ligand was not detected in the 1H NMR spectrum. MS, m/z (%): 1606.4 (100) [M + Na]+. Anal. Calcd for C61H104Cl3N4O20RuS: C, 45.88; H, 6.56; N, 3.51. Found C, 46.01; H, 6.72; N, 3.39.
4.7 Synthesis of trans,mer-trichlorido(dimethyl sulfoxide-κS)[6A,6D-dideoxy-6A,6D-bis(1-imidazolyl-κN3)-2A,2B,2C,2D,2E,2F,2G,3A,3B,3C,3D,3E,3F,3G,6B,6C,6E,6F,6G-nonadeca-O-methyl-β-cyclodextrin]ruthenium(III) (6)
Complex 6 (0.020 g, 12%) was synthesized as described above from L2 (0,140 g, 0.09 mmol) and Na[trans-RuCl4(DMSO)2] (0.039 g, 0.09 mmol). Rf (SiO2, CH2Cl2/MeOH, 90:10, v/v) = 0.41. mp dec. > 250°C. IR ν(S = O) 1086 (s) cm−1, ν(Ru-S) 428 (s) cm−1. 1H NMR (300.1 MHz, CDCl3, 25°C, paramagnetic): δ = –26.00 (2 H, H-2Im or H-4Im or H-5Im),–11.00 (2 H, H-5Im or H-2Im or H-4Im),–5.00 (2 H, H-4Im or H-5Im or H-2Im), 2.80–5.55 (49 H, H-1, H-2, H-3, H-4, H-5, H-6), 2.89 (s, 3 H, OMe), 2.96 (s, 3 H, OMe), 2.99 (s, 3 H, OMe), 3.03 (s, 3 H, OMe), 3.14 (s, 3 H, OMe), 3.21 (s, 6 H, OMe), 3.27 (s, 3 H, OMe), 3.47 (s, 3 H, OMe), 3.40 (s, 3 H, OMe), 3.46 (s, 3 H, OMe), 3.51 (s, 3 H, OMe), 3.57 (s, 3 H, OMe), 3.62 (s, 3 H, OMe), 3.70 (s, 3 H, OMe), 3.74 (s, 3 H, OMe), 3.82 (s, 3 H, OMe), 3.87 (s, 12 H, OMe) ppm. The DMSO ligand was not detected. MS, m/z (%): 1810.6 (100) [M + Na]+. Anal. Calcd for C69H118Cl3N4O34RuS: C, 46.37; H, 6.66; N, 3.13. Found C, 46.11; H, 7.08; N, 2.92.
4.8 Crystal structure of L1·0.5 C5H12
Single crystals of L1 were obtained by slow diffusion of pentane into a dichloromethane solution of the compound. The sample was studied with an Oxford Diffraction Xcalibur Saphir 3 CCD with graphite monochromatised MoKα radiation (for crystal data and structure refinement, see Table 1). The structure was solved with SIR-97 [16], which revealed the non hydrogen atoms of the molecule. After anisotropic refinement, many hydrogen atoms were found with a Fourier Difference. The whole structure was refined with SHELX-97 [17] by the full-matrix least-square techniques (use of F square magnitude; x, y, z, βij for N, C and O atoms, x, y, z in riding mode for H atoms). The methoxy carbon atom C18 is subject to large thermal motion. CCDC 727143 contains the supplementary crystallographic data for this report. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgements
We gratefully acknowledge financial support from the International Center for Frontier Research in Chemistry (ICFRC), the CNRS and the Région Alsace (grants to R. G.-D. and M. J.)


