1 Introduction
Nanomaterials have attracted a great deal of attention in organic synthesis owing to their high surface-to-volume ratio, which are responsible for the higher catalytic activity [1].
The catalytic activity of nano CuO and its green chemistry exhibits good reusability, simple workup procedure and a straightforward approach to use in organic synthesis [2–4].
Phenazines are present in natural and synthetic products showing a variety of biological functions, including antimalarial, trypanocidal, fungicidal, antitumor, and antiplatelet activities [5–9]. Chromenes have been showed remarkable effects as pharmaceuticals [10,11], including antifungal [12,13] and antimicrobial activities [14]. Molecules with phenazines [15] and chromenes [16] moieties have attracted great attention in drug discovery.
The designs of multi-component reactions (MCRs) for the synthesis of diverse groups of compounds, especially the ones that are biologically active, have commanded great attention in green organic synthesis [17–19]. In continuation of our research on multi-component reactions [20–22], herein we wish to describe the preparation of functionalized benzo[a]pyrano[2,3-c]phenazine derivatives with phenazines and chromenes moieties from a four-component reaction of 2-hydroxynaphthalene-1,4-dione, o-phenylenediamine, aldehydes, and malononitrile in the presence of nano copper(II) oxide as the catalyst under thermal solvent-free conditions (Scheme 1).
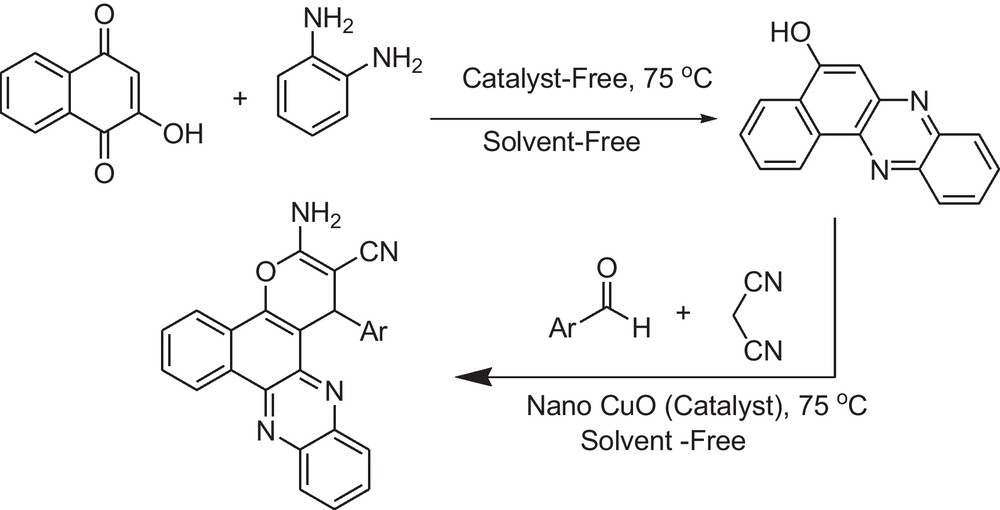
Synthesis of 3-amino-2-cyano-1-aryl-1H-benzo[a]pyrano[2,3-c]phenazines derivatives.
2 Results and discussion
First, to find optimization conditions, the one-pot four-component reaction of 2-hydroxynaphthalene-1,4-dione, o-phenylenediamine, 4-chlorobenzaldehyde and malononitrile in the presence of the nano copper(II) oxide as catalyst was selected as model. 2-Hydroxynaphthalene-1,4-dione (1 mmol) and o-phenylenediamine (1 mmol) were mixed at 75 °C until a orange solid of benzo[a]phenazine was formed (< 5 min) without using any catalyst under solvent-free conditions. Then, 4-chlorobenzaldehyde (1 mmol), malononitrile (1 mmol), and nano copper(II) oxide as catalyst was added and stirred under thermal conditions. The reaction was carried out with different amount of nano copper(II) oxide as catalyst (5, 10, 15, 25 mol%) at various temperatures (25, 50, 75 °C) (Table 1). As it was shown from Table 1, 10 mol% of nano copper(II) oxide as catalyst at 75 °C afforded 3-amino-2-cyano-1-(4-chlorophenyl)-1H-benzo[a]pyrano[2,3-c]phenazine in 7 min with 94% of yield.
Optimization conditions for preparation 3-amino-2-cyano-1-(4-chlorophenyl)-1H-benzo[a]pyrano[2,3-c]phenazine from 2-hydroxynaphthalene-1,4-dione, o-phenylenediamine, 4-chlorobenzaldehyde, and malononitrile in the presence of different amount of nano copper(II) oxide as catalyst in varieties temperature.
| Entry | Catalyst (mol%) | Temperature ( °C) | Time (min) | Yield (%)a |
| 1 | 25 | 25 | 30 | Trace |
| 2 | 15 | 50 | 30 | 35 |
| 3 | 25 | 50 | 28 | 50 |
| 4 | 5 | 75 | 12 | 83 |
| 5 | 10 | 75 | 7 | 94 |
| 6 | 15 | 75 | 7 | 95 |
a Yields refer to isolated pure product.
Next, four-component condensation reaction of 2-hydroxynaphthalene-1,4-dione, o-phenylenediamine, aromatic aldehydes, and malononitrile under solvent-free conditions for preparation of 3-amino-2-cyano-1-aryl-1H-benzo[a]pyrano[2,3-c]phenazines was investigated (Table 2). The wide ranges of substituted and structurally diverse aldehydes afforded the corresponding products in high to excellent yields using the nano copper(II) oxide as catalyst (Table 2). Aliphatic aldehyde such as heptanal was intact in this reaction (Table 2, Entry 13).
Four-component synthesis of 3-amino-2-cyano-1-aryl-1H-benzo[a]pyrano[2,3-c]phenazine derivatives from the reaction of 2-hydroxynaphthalene-1,4-dione, o-phenylenediamine, malononitrile and aldehydes in the presence of nano copper(II) oxide as catalyst.
| Entry | Aldehydes | Time (min) | Yield (%)a | Melting Point m.p. ( °C)/Lit. m.p. ( °C) [Ref] |
| 1 | Ph | 8 | 92 | 301–302/(298–300) [23] |
| 2 | 4-NO2C6H4 | 8 | 90 | 282–283/(281–283) [23] |
| 3 | 4-FC6H4 | 7 | 93 | 276–277/(274–276) [23] |
| 4 | 4-BrC6H4 | 7 | 91 | 285–286/(283–285) [23] |
| 5 | 4-ClC6H4 | 7 | 94 | 290–291/(288–291) [23] |
| 6 | 3-NO2C6H4 | 8 | 89 | 280/(278–279) [23] |
| 7 | 4-OH-3-CH3OC6H3 | 7 | 89 | 248–249/(247–248) [23] |
| 8 | 4-OH-3-NO2C6H3 | 8 | 90 | 292/(290–291) [23] |
| 9 | 3,4,5-(CH3O)3C6H2 | 7 | 91 | 253–254/(252–254) [23] |
| 10 | 4-CH3C6H4 | 7 | 90 | 293–295/(293–294) [23] |
| 11 | 2-CH3OC6H4 | 7 | 90 | 273/(270–272) [23] |
| 12 | 2,3-(CH3O)2C6H3 | 8 | 91 | 295/(292–294) [23] |
| 13 | n-Heptanal | 24 h | – | – |
a Yields refer to the isolated pure products. The desired known pure products were characterized by comparison of their physical data (melting points, IR, 1H and 13C NMR) with those of known compounds [23]. The same products are made in the presence of acetic acid as catalyst (2.0 mL, 3500% mol) under microwave heating (120 °C) [23].
The mechanism for the formation of the products has been suggested in Scheme 2 according to the literature [23]. First, 2-hydroxynaphthalene-1,4-dione (1) tautomrizes to intermediate (2). The initial condensation of (2) with o-phenylenediamine (3) afford 6H-benzo[a]phenazin-5-one (4), which in tautomerism equilibrium causes to prepare benzo[a]phenazin-5-ol (5). In addition, standard Knoevenagel condensation of malononitrile (6) and aryl aldehydes (7) in the presence of nano CuO as the catalyst was afforded benzylidenemalononitrile (8). The Michael addition of 6H-benzo[a]phenazin-5-ol (5) with benzylidenemalononitrile (8) formed intermediate (9), which in subsequent cyclization and tautomerism affords the corresponding products (10).
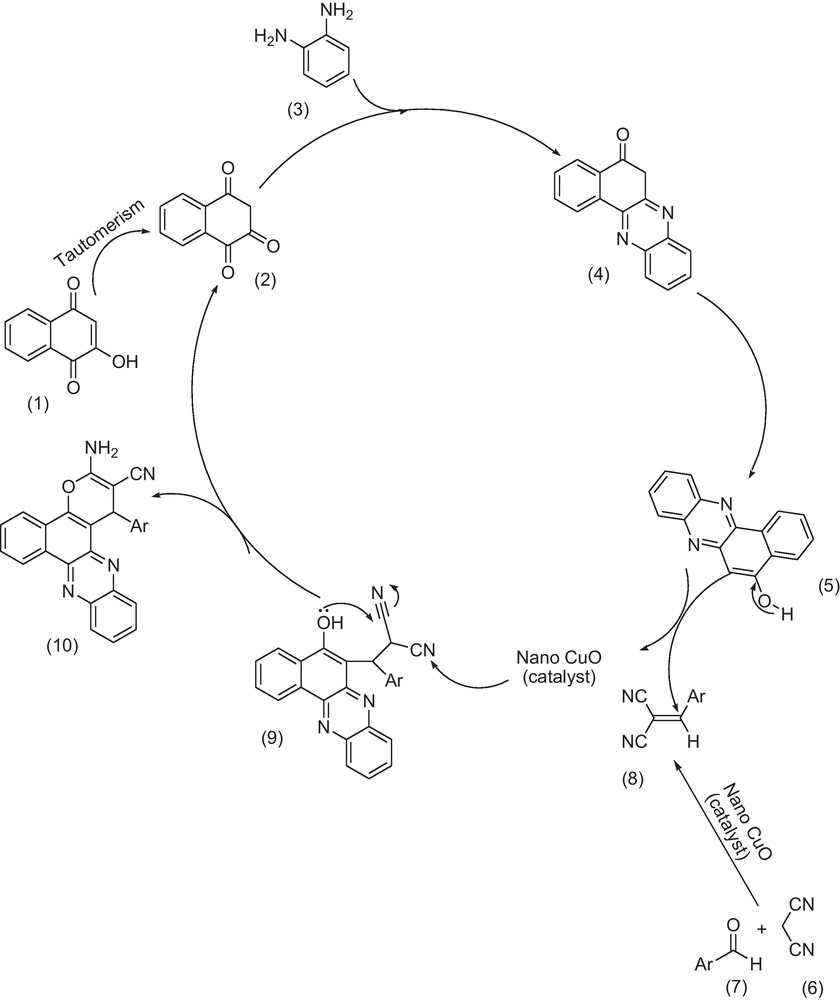
Suggested mechanism for the formation of 3-amino-2-cyano-1-aryl-1H-benzo[a]pyrano[2,3-c]phenazines.
We also investigated the recycling of the nano CuO as the catalyst under solvent-free conditions using the model reaction of 2-hydroxynaphthalene-1,4-dione, o-phenylenediamine, 4-chlorobenzaldehyde, and malononitrile (Table 2, Entry 5). After completion of the reaction, the reaction mixture was cooled to room temperature and dissolved in dichloromethane. Then, nano CuO as the catalyst was separated by centrifugation and washed with CH2Cl2 for subsequent experiments to check its reusability under similar reaction conditions. The results showed us that nano CuO is a stable catalyst in reaction media and reused several times without significant loss of its catalytic activity (Fig. 1).
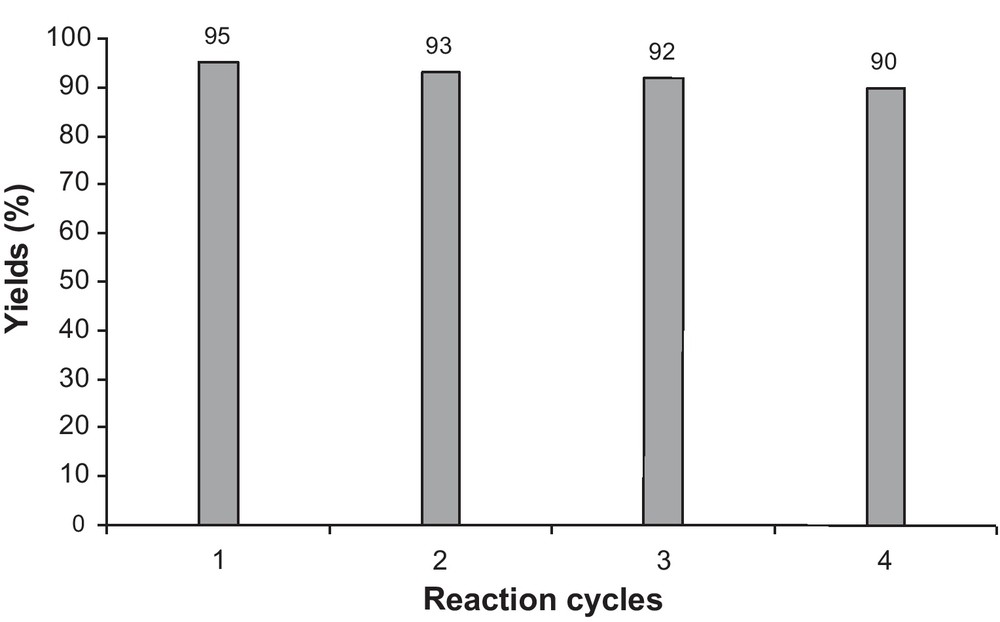
The recycling of the nano CuO as catalyst.
In order to show the accessibility of the present work in comparison with only one reported result in the literature, we summarized some of the results for the preparation of 3-amino-2-cyano-1-(4-chlorophenyl)-1H-benzo[a]pyrano[2,3-c]phenazine in Table 3. The results show that the nano CuO (10 mol%) under solvent-free conditions relative to acetic acid as catalyst (2.0 mL, 3500 % mol) under microwave heating (120 °C) [23], are the most efficient catalysts with respect to the reaction time and exhibits broad applicability in terms of yields.
Comparison the results of nano CuO with HOAc in the synthesis 3-amino-2-cyano-1-(4-chlorophenyl)-1H-benzo[a]pyrano[2,3-c]phenazine.
| Entry | Catalyst (mol%) | Conditions | Time (min) | Yield (%)a [Ref] |
| 1 | HOAC (2.0 mL 3500% mol)b | Microwave heating (120 °C) | 10 | 89 [23] |
| 2 | Nano CuO (10 mol%)c | Solvent-free, 75 °C | 7 | 94 (Present work) |
a Yields refer to isolated pure products and based on the reaction of 2-hydroxynaphthalene-1,4-dione, o-phenylenediamine, 4-chlorobenzaldehyde, and malononitrile.
b Acetic acid as catalyst is not reusable.
c The Nano CuO is recyclable catalyst.
3 Conclusion
In this research, nano CuO was used for preparation of 3-amino-2-cyano-1-aryl-1H-benzo[a]pyrano[2,3-c]phenazine derivatives under solvent-free conditions for the first time. The attractive features of this protocol are simple procedure, cleaner reaction, use of reusable nano catalyst. Satisfactory yields of products, as well as a simple experimental, isolation and purification of the products make it a useful protocol for the green synthesis.
4 Experimental
4.1 General
All reagents were purchased from Merck and Aldrich and used without further purification. Copper(II) oxide nanopowder, less than 50 nm particle size was purchased from Sigma-Aldrich chemical company, CAS NO: 1317-38-0. All yields refer to isolated products after purification. The NMR spectra were recorded on a Bruker Avance DPX 300 MHz instrument. The spectra were measured in DMSO-d6 relative to TMS (0.00 ppm). Melting points were determined in open capillaries with a BUCHI 510 melting point apparatus. TLC was performed on silica-gel Poly Gram SIL G/UV 254 plates.
4.2 General procedure for the synthesis of functionalized benzo[a]pyrano[2,3-c]phenazine derivatives under solvent-free conditions
2-Hydroxynaphthalene-1,4-dione (10 mmol) and o-phenylenediamine (10 mmol) were mixed at 75 °C until a orange solid of benzo[a]phenazine was formed (< 5 min). Then, aryl aldehydes (10 mmol), malononitrile (10 mmol), and nano copper(II) oxide (10 mol%) was added and stirred under thermal conditions at 75 °C for the specific time. After completion of the reaction, monitored by TLC, the reaction mixture was cooled to room temperature. Then, the mixture was diluted with dichloromethane and the catalyst was separated by centrifugation and washed with CH2Cl2 (2 × 5 mL) for checking the reusability. The decanted solution containing the product was evaporated to give the yellow solid. The solid was recrystallized with acetone to give the pure yellow solid. All of the desired product(s) were characterized by comparison of their physical data with those of known compounds.
Selected spectra for three known products are given below:
- • 3-Amino-2-cyano-1-phenyl-1H-benzo[a]pyrano[2,3-c]phenazine (Table 2, entry 1): yellow solid, m.p.: 301–302 °C; IR (KBr): νmax = 3445, 3312, 3175, 2187, 1659, 1621, 1592, 1543, 1495, 1477, 1409, 1386, 1355, 1329, 1261, 1162, 1126, 1051, 1021, 765, 733, 703 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 9.16 (d, 1H, J = 7.6 Hz, Ar-H), 8.43 (d, 1H, J = 8.0 Hz, Ar-H), 8.25–8.21 (m, 1H, Ar-H), 8.12–8.07 (m, 1H, Ar-H), 8.02–7.89 (m, 4H, Ar-H), 7.42–7.33 (m, 4H, Ar-H), 7.24 (t, 2H, J = 7.6 Hz, NH2), 7.12–7.07 (m, 1H, Ar-H), 5.46 (s, 1H, CH) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 159.9, 146.3, 145.1, 141.2, 140.6, 140.2, 139.6, 130.6, 130.2, 130.3, 130.0, 129.2, 129.0, 128.7, 128.3, 127.6, 126.5, 125.6, 124.8, 122.1, 120.2, 113.9, 58.1, 37.4 ppm;
- • 3-Amino-2-cyano-1-(4-chlorophenyl)-1H-benzo[a]pyrano[2,3-c]phenazine (Table 2, entry 5): yellow solid, m.p.: 290–291 °C; IR (KBr): νmax = 3455, 3310, 3172, 2190, 1665, 1622, 1591, 1487, 1474, 1401, 1386, 1351, 1330, 1292, 1267, 1162, 1101, 1082, 1051, 1017, 848, 754, 744 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 9.18–9.15 (m, 1H, Ar-H), 8.43–8.45 (m, 1H, Ar-H), 8.23–8.22 (m, 1H, Ar-H), 8.11–8.01 (m, 1H, Ar-H), 8.02–7.89 (m, 4H, Ar-H), 7.43–7.41 (m, 4H, Ar-H), 7.29–7.27 (m, 2H, NH2), 5.44 (s, 1H, CH) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 159.9, 152.2, 144.8, 140.8, 139.6, 131.1, 130.4, 130.2, 130.2, 130.1, 129.6, 129.2, 128.7, 128.3, 125.6, 124.9, 122.2, 120.0, 113.3, 57.6 ppm;
- • 3-Amino-2-cyano-1-p-tolyl -1H-benzo[a]pyrano[2,3-c]phenazine (Table 2, entry 10): yellow solid, m.p.: 293–295 °C; IR (KBr): νmax = 3445, 3315, 3177, 2188, 1664, 1623, 1592, 1491, 1472, 1392, 1382, 1352, 1328, 1291, 1267, 1222, 1161, 1102, 1053, 1022, 830, 759, 744 cm−1; 1H NMR (300 MHz, DMSO-d6): δ = 9.22 (d, 1H, J = 8.0 Hz, Ar-H), 8.46–8.44 (m, 1H, Ar-H), 8.28–8.26 (m, 1H, Ar-H), 8.19–8.15 (m, 1H, Ar-H), 8.03–7.91 (m, 4H, Ar-H), 7.34–7.28 (m, 4H, Ar-H), 7.02 (d, 2H, J = 8.0 Hz, NH2), 5.47 (s, 1H, CH), 2.15 (s, 3H, CH3) ppm; 13C NMR (75 MHz, DMSO-d6): δ = 168.1, 164.4, 159.8, 150.3, 146.5, 141.6, 140.1, 135.6, 130.8, 130.0, 129.5, 129.1, 128.9, 127.5, 125.7, 123.6, 122.1, 118.0, 58.2, 37.0, 20.50 ppm.
Acknowledgments
We are thankful to the University of Sistan and Baluchestan Research Council for the partial support of this research.


