1 Introduction
Conventional Lewis acids are very effective catalysts for a wide variety of organic reactions [1], but one of the major problems associated with these homogeneous catalysts is the recovery of catalyst from the reaction medium. In recent years, there have been intense efforts to develop methods recovering and reusing the homogeneous catalysts [2–6]. Immobilization of catalysts on a solid support improves the available active sites, stability, hygroscopic properties, handling, and reusability of catalysts which factors are important in industry [7]. Therefore, use of supported and reusable catalysts in organic transformations has economical and environmental benefits. A large number of polymer-supported Lewis acid catalysts have been prepared by immobilization of the catalysts on polymer via coordination or covalent bonds [8]. Such polymeric catalysts are usually as active and selective as their homogeneous or solution-phase counterparts while having the distinguishing characteristics of being easily separable from the reaction mixture, recyclability, easier handling, non-toxicity, enhanced stability, and improved selectivity in various organic reactions. Polystyrene is one of the most widely studied heterogeneous and polymeric supports due to its environmental stability and hydrophobic nature which protects water-sensitive Lewis acids from hydrolysis by atmospheric moisture until it is suspended in an appropriate solvent where it can be used in a chemical reaction [4]. It is well known that gallium trichloride is a strong Lewis acid and an important catalyst in organic transformations. However, it easily hydrolyzes in air, so that its use, storage, and separation from the reaction mixture are inconvenient and difficult. Polystyrene-supported gallium chloride, PS/GaCl3, which is a tightly bound and stable complex between anhydrous GaCl3 and polystyrene-divinylbenzene copolymer beads, has been described for the first time by Ruicheng et al. [9]. The use of PS/GaCl3 complex catalyst has several advantages over conventional Lewis acid catalyst such as its cost-effectiveness, ease of handling, recyclability, and tunable Lewis acidity.
Acylation of alcohols and phenols is of enormous interest in organic synthesis as it provides a useful and efficient protection protocol in a multistep synthetic process [10]. Moreover, this reaction has biological significance because of the presence of alcoholic and phenolic hydroxyl groups in a variety of biologically active compounds that necessitates the manipulation of the chemical reactivity of these functional groups during the synthesis of multifunctional synthetic targets possessing one or more of these groups. Acylation is usually carried out by treatment of an alcohol or phenol with acetyl chloride or acetic anhydride in the presence of an acid or a base catalyst in a suitable organic solvent, although acetic anhydride is the most commonly used, as it is less toxic. However, the catalysts traditionally used are bases or acids, which have many disadvantages in the acetylation process. For example, some of the base catalysts are toxic, flammable, and possess offensive odors [11,12]. In addition, some of the traditional protic acid catalysts are not entirely satisfactory in terms of the stability of reactants or products under the reaction conditions and the time-consuming work-up procedures. A variety of procedures using homogeneous or heterogeneous catalysts such as p-TSA [13], CoCl2 [14], alumina [15], montmorillonie K-10 and KSF [16], zeolite HSZ-360 [17], MgBr2 [18], Bi(TFA)3 [19], TaCl5 [20], NH2SO3H [21], La(NO3)3.6H2O [22], 3-nitrobenzene boronic acid [23], TMSOTf [24], Cp2Ti(OSO2C8F17)2 [25], Cp2ZrCl2 [26], ZrOCl2.8H2O [27], Mg(ClO4)2 [28], Zn(ClO4)2.6H2O [29], HBF4-SiO2 [30], Cu(BF4)2 [31], HClO4-SiO2 [32], electron-deficient tin (IV) porphyrins [33–35], alumina-supported MoO3 [36], zirconium sulfophenyl phosphonate [37], o-benzene disulfonimide [38], saccharinsulfonic acid [39], cerium polyoxometallate [40], BiO(ClO4)2 [41], cobalt(II) and Mn(III) salen complexes [42], Gd(OTf)3 [43], InCl3 [44], Ph3P+CH2COCH3Br− [45], [Mn(III)(haacac)Cl] complex [46], and LiOTf [47] have been reported for acetylation of alcohols and phenols with acetic anhydride. However, most of the catalysts suffer from limitations such as long reaction times, harsh reaction condition or tedious work-up, use of expensive, moisture sensitive and toxic catalysts, regulatory constraints, the occurrence of side reactions, intolerance to other functional groups, poor selectivity, and high catalyst to substrate ratio. Apart from these difficulties, most above methods do not satisfy the requirements of green synthesis due to the inability to recovery and reuse of the catalyst. As a result, new kinds of catalysts, especially using recyclable heterogeneous catalysts or mild, selective and environmentally benign methodologies for these transformations are still in demand.
In continuation of our recent works on the use of polymeric Lewis acid catalysts in organic transformations [48,49], herein we report the preparation and investigation of catalytic activity of polystyrene-supported gallium chloride as a stable, highly active and reusable heterogeneous catalyst in the acetylation and benzoylation of alcohols and phenols with acetic and benzoic anhydride, (Ac2O), (PhCO)2O, respectively (Scheme 1).
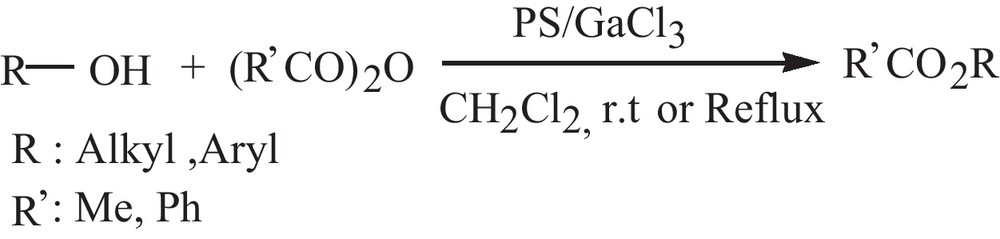
Acetylation and benzoylation of alcohols and phenols with Ac2O and (PhCO)2O catalyzed by PS/GaCl3.
2 Experimental
All chemical reagents were obtained from Fluka or Merck chemical companies and were used without further purification. Cross-linked polystyrene (5–7% divinylbenzene, mesh size: 20–75 mm) was prepared via suspension polymerization as reported in the literature [48]. PS/AlCl3 was also prepared as previously reported [49]. 1H and 13C NMR spectra were recorded on a Bruker DPX-250 Avance spectrometer at 250.13 MHz. FT-IR spectra of the samples were recorded from 400 to 4000 cm−1 on a Unicam Matteson 1000 spectrophotometer. UV spectra were taken using a Pharmacia Biotech Ultraspec 3000 model 80-2106-20 spectrometer. Gas chromatography experiments (GC) were performed with a shimadzu GC-16A instrument using a 2 m column packed with silicon DC-200 or carbowax 20 M. The capacity of the catalyst was determined by the gravimetric method (Mohr titration method) and atomic absorption technique using a Philips atomic absorption instrument. Reaction monitoring and purity determination of the products were accomplished by TLC on silica gel polygram SILG/UV254 plates.
2.1 Preparation of polystyrene-supported gallium trichloride (PS/GaCl3)
Anhydrous GaCl3 (4 g) was added to polystyrene (8% divinylbenzene, mesh size: 20–75 mm, 8 g) in carbon disulfide (30 mL) as the reaction medium. The mixture was stirred using a magnetic stirrer under reflux condition for 1 h, cooled, and then water (50 mL) was cautiously added to hydrolyze the excess GaCl3. The mixture was stirred until the bright red color disappeared, and the polymer became yellow. The polymer beads were collected by filtration and washed with water (300 mL) and then with ether (30 mL) and chloroform (30 mL). The catalyst was dried in a vacuum oven overnight at 50 °C before use. The chlorine content of PS/AlCl3 was 4.17% analyzed by the Mohr titration method [50] and the loading capacity of GaCl3 on the polymeric catalyst or the amount of GaCl3 complexed with polystyrene was calculated to be 0.391 mmol/g.
2.2 General experimental procedure for acetylation of alcohols and phenols with Ac2O catalyzed by PS/GaCl3 complex
To a solution of alcohol or phenol (1 mmol) and Ac2O (1.1–1.3 mmol) in methylene chloride (5 mL) was added PS/GaCl3 (0.1 mmol) and the resulting mixture was stirred at room temperature. The progress of the reaction was monitored by TLC and GLC. After completion of the reaction, the catalyst was filtered off and washed with methylene chloride (2 × 10 mL) and the filtrate concentrated on a rotary evaporator under reduced pressure to afford the crude acetate product. Further purification was achieved by column chromatography on silica gel (Merck, 100–200 mesh, hexane-EtOAC, 90/10, v/v). The spent polymeric catalyst from different experiments was combined, washed with ether and dried overnight in a vacuum oven and reused.
2.3 General experimental procedure for benzoylation of alcohols and phenols with (PhCO)2O catalyzed by PS/GaCl3 complex
To a solution of alcohol or phenol (1 mmol) and (PhCO)2O (1.2–1.5 mmol) in methylene chloride (10 mL) was added PS/GaCl3 (0.1 mmol) and the resulting mixture was stirred under reflux conditions for the appropriate time according to Table 2. The progress of the reaction followed by GLC or TLC. After completion of the reaction, the catalyst was filtered off and washed with methylene chloride (2 × 10 mL) and the filtrate concentrated on a rotary evaporator under reduced pressure to afford the crude benzoate product which was passed through a short pad of silica gel (Merck, 100–200 mesh, hexane-EtOAC, 95/5, v/v) to provide the pure benzoylated product.
Acetylationa and benzoylationb of alcohols and phenols with Ac2O and (PhCO)2O catalyzed by PS/GaCl3.
| Entry | Substrate | Ratioc | Productd | Time (min) | Yielde (%) |
| C6H5CH2OH | 1:1.1 | C6H5CH2OAc | 25 | 97 | |
| 1 | C6H5CH2OH | 1:1.2 | C6H5CH2OCOPh | 45 | 90 |
| 2 | p-(Cl)C6H4CH2OH | 1:1.1 | p-(Cl)C6H4CH2OAc | 30 | 93 |
| p-(Cl)C6H4CH2OH | 1:1.2 | p-(Cl)C6H4CH2OCOPh | 50 | 89 | |
| 3 | p-(NO2)C6H4CH2OH | 1:1.1 | p-(NO2)C6H4CH2OAc | 30 | 91 |
| p-(NO2)C6H4CH2OH | 1:1.2 | p-(NO2)C6H4CH2OCOPh | 65 | 88 | |
| 4 | o-(NO2)C6H4CH2OH | 1:1.1 | o-(NO2)C6H4CH2OAc | 70 | 91 |
| o-(NO2)C6H4CH2OH | 1:1.2 | o-(NO2)C6H4CH2OCOPh | 100 | 84 | |
| 5 | p-(CH3)C6H4CH2OH | 1:1.1 | p-(CH3)C6H4CH2OAc | 28 | 96 |
| p-(CH3)C6H4CH2OH | 1:1.2 | p-(CH3)C6H4CH2OCOPh | 45 | 89 | |
| 6 | p-(OCH3)C6H4CH2OH | 1:1.1 | p-(OCH3)C6H4CH2OAc | 27 | 94 |
| p-(OCH3)C6H4CH2OH | 1:1.2 | p-(OCH3)C6H4CH2OCOPh | 50 | 90 | |
| 7 | n-CH3(CH2)6CH2OH | 1:1.1 | n-CH3(CH2)6CH2OAc | 40 | 95 |
| n-CH3(CH2)6CH2OH | 1:1.2 | n-CH3(CH2)6CH2OCOPh | 55 | 89 | |
| 8 | n-CH3(CH2)5CH2OH | 1:1.1 | n-CH3(CH2)5CH2OAc | 40 | 94 |
| n-CH3(CH2)5CH2OH | 1:1.2 | n-CH3(CH2)5CH2OCOPh | 55 | 88 | |
| 9 | CH3(CH2)3CH(OH)CH3 | 1:1.1 | CH3(CH2)3CH(OAc)CH3 | 40 | 93 |
| CH3(CH2)3CH(OH)CH3 | 1:1.2 | CH3(CH2)3CH(OCOPh)CH3 | 50 | 86 | |
| 10 | (CH3)2CHOH | 1:1.1 | (CH3)2CHOAc | 40 | 92 |
| (CH3)2CHOH | 1:1.2 | (CH3)2CHOCOPh | 50 | 87 | |
| 11 | C6H5CH(CH3)CH2OH | 1:1.1 | C6H5CH(CH3)CH2OAc | 55 | 92 |
| C6H5CH(CH3)CH2OH | 1:1.2 | C6H5CH(CH3)CH2OCOPh | 75 | 88 | |
| 12 | C6H5CH(CH3) OH | 1:1.2 | C6H5CH(CH3) OAc | 55 | 94 |
| C6H5CH(CH3) OH | 1:1.2 | C6H5CH(CH3) OCOPh | 80 | 85 | |
| 13 | C6H5C(CH3)2 OH | 1:1.3 | C6H5C(CH3)2 OAc | 70 | 94 |
| C6H5C(CH3)2 OH | 1:1.4 | C6H5C(CH3)2 OCOPh | 90 | 86 | |
| 14 | 1:1.2 | 65 | 92 | ||
| 1:1.3 | 85 | 87 | |||
| 15 | CH2 = CHCH2OH | 1:1.2 | CH2 = CHCH2OAc | 90 | 90 |
| CH2 = CHCH2OH | 1:1.3 | CH2 = CHCH2OCOPh | 90 | 86 | |
| 1:1.3 | 90 | 92 | |||
| 16 | 1:1.5 | 100 | 83 | ||
| 17 | C6H5OH | 1:1.3 | C6H5OAc | 50 | 94 |
| C6H5OH | 1:1.4 | C6H5OCOPh | 80 | 86 | |
| 18 | p-(OCH3)C6H4OH | 1:1.2 | p-(OCH3)C6H4OAc | 45 | 94 |
| p-(OCH3)C6H4OH | 1:1.3 | p-(OCH3)C6H4OCOPh | 50 | 89 | |
| 19 | p-(CH3)C6H4OH | 1:1.2 | p-(CH3)C6H4OAc | 45 | 93 |
| p-(CH3)C6H4OH | 1:1.3 | p-(CH3)C6H4OCOPh | 50 | 88 | |
| 20 | p- (CHO)C6H4OH | 1:1.2 | p-(CHO)C6H4OAc | 65 | 91 |
| p- (CHO)C6H4OH | 1:1.3 | p-(CHO)C6H4OCOPh | 120 | 82 | |
| 21 | 1:1.3 | 70 | 91 | ||
| 1:1.4 | 100 | 82 | |||
| 22 | 1:1.3 | 70 | 92 | ||
| 1:1.4 | 100 | 81 | |||
| 23 | HOCH2(CH2)3CH2OAc | 1:1.1 | AcOCH2(CH2)3CH2OAc | 45 | 92 |
| 24 | HOCH2(CH2)6CH2OBn | 1:1.1 | AcOCH2(CH2)6CH2OBn | 40 | 92 |
| 25 | HOCH2(CH2)6CH2OBz | 1:1.1 | AcOCH2(CH2)6CH2OBz | 40 | 92 |
| 26 | HOCH2(CH2)3CH2OTs | 1:1.1 | AcOCH2(CH2)3CH2OTs | 45 | 91 |
a All acetylation reactions were carried out in CH2Cl2 (5 mL) in the presence of PS/GaCl3 (0.1 mmol) at room temperature.
b All benzoylation reactions were carried out in refluxing CH2Cl2 (5 mL) in the presence of PS/GaCl3 (0.1 mmol).
c The mole ratio of (substrate/Ac2O and substrate/(PhCO)2O).
d All the acetate and benzoate products were identified by comparison of their physical and spectral data with those of authentic samples.
e Isolated yield.
2.4 Catalyst recovery and reuse
The reusability of the catalyst was checked in the multiple acetylation of benzyl alcohol. At the end of each reaction, the solvent was evaporated, ether (Et2O, 10 mL) was added and the catalyst was filtered. The recovered catalyst was used with fresh benzyl alcohol, Ac2O and CH2Cl2.
3 Results and discussion
3.1 Preparation of PS/GaCl3 complex
First, cross-linked polystyrene (5–7% divinylbenzene, mesh size: 20–75 mm) was prepared via suspension polymerization as reported in the literature [48]. Then, PS/GaCl3 was prepared by addition of anhydrous gallium chloride to polystyrene (8% divinylbenzene) in carbon disulfide under reflux conditions. The loading capacity of the polymeric catalyst obtained by gravimetric method and checked by atomic absorption technique was 0.391 mmol GaCl3/g of complex beads catalyst [50]. The data obtained by these two techniques showed, within experimental error, that the catalyzing species are in the form of GaCl3 supported on the polymeric support. The UV spectrum of the solution of PS-GaCl3 complex in CS2 showed a new strong band at 470 nm, which is due to the formation of a stable π→p type coordination complex between the benzene rings in the polystyrene carrier with gallium trichloride. The FT IR spectrum of PS/GaCl3 showed new absorption peaks due to the C-C stretching vibration and the C-H bending vibration of the benzene ring at 1500 to 1560 and 400 to 800 cm−1, by which complex formation was demonstrated. The structure of the PS-GaCl3 complex is similar to that of the PS-AlCl3 complex as suggested by Neckers et al. [51], because the Lewis acid GaCl3 is complexed with the benzene rings of the polystyrene and the GaCl3 is stabilized due to the decreased mobility of the benzene rings hindered by the long polystyrene chain. The PS-GaCl3 complex catalyst is a non-hygroscopic, water-tolerant, and especially stable species. In addition, this polymeric catalyst is easy to prepare, stable in air for a long time (over 2 years) without any change, easily recycled and reused without appreciable loss of its activity.
3.2 Acetylation and benzoylation of alcohols and phenols with Ac2O and (PhCO)2O catalyzed by PS/GaCl3
In our initial evaluations, we used as the model reaction, the reaction of benzyl alcohol (1 equiv.) with acetic anhydride (1.1 equiv.). The choice of the solvent appeared crucial in order to maximize yield. Among the various solvents surveyed, the highest yield was obtained in CH2Cl2 (Table 1, entry 7). The highest yield obtained by using CH2Cl2 as the solvent can be ascribed to the fruitful swelling of the polymer network of the catalyst in this media, allowing the metal particles located inside the polymer matrix to effect the catalysis. The optimum molar ratio of the polymeric catalyst to hydroxyl compound was found to be 0.1:1. The key role played by the Lewis acidity of the heterogeneous catalyst PS/GaCl3 was proved by employing the polystyrene beads (Table 1, entry 9) and the GaCl3-benzene complex as catalysts. In fact, while in the former case no reaction occurred, which indicated that polystyrene itself did not promote the reaction, in the latter, the desired product was isolated in low yield 46% (Table 1, entry 10). Also, PS/GaCl3 was found to be a more effective catalyst than PS/AlCl3 in terms of reaction time and the yield of the product for acetylation of benzyl alcohol under identical conditions (Table 1, entry 12). We then turned our attention on whether the same catalyst could be employed for acetylation of alcohols using acetic acid and ethyl acetate as acylating agents. For this purpose, the model reaction was carried out with acetic acid and ethyl acetate under identical conditions, and only 36% and 23% of the corresponding acetates were produced, respectively.
Reaction conditions optimization in the acetylation of benzyl alcohol with Ac2O catalyzed by PS/GaCl3 at room temperature.a
| Entry | Solvent | Catalyst (mol %) | Time (min) | Yieldb (%) |
| 1 | n-Hexane | 10 | 25 | 17 |
| 2 | THF | 10 | 25 | 21 |
| 3 | CH3Cl | 10 | 25 | 40 |
| 4 | CH3CN | 10 | 25 | 58 |
| 5c | CH2Cl2 | – | 60 | NR |
| 6 | CH2Cl2 | 5 | 25 | 60 |
| 7 | CH2Cl2 | 10 | 25 | 97 |
| 8 | CH2Cl2 | 20 | 25 | 97 |
| 9d | CH2Cl2 | – | 25 | NR |
| 10e | CH2Cl2 | – | 25 | 46 |
| 11f | CH2Cl2 | 10 | 15 | NR |
| 12g | CH2Cl2 | 10 | 60 | 76 |
a Reaction conditions: benzyl alcohol (1 mmol), Ac2O (1.1 mmol), solvent (5 mL).
b Isolated yield.
c No catalyst.
d PS was used as catalyst.
e The toluene-GaCl3 complex was used as catalyst.
f Catalyst was filtered after 15 min.
g PS/AlCl3 was used as catalyst.
When using a heterogeneous and metal-supported catalyst one of the most important issues is the possibility of leaching of the reactive center into the reaction mixture, particularly for practical applications of the reaction. Our preliminary investigations demonstrated that PS/GaCl3 catalyst (a stable π complex) is very stable to air and moisture. To rule out the contribution of concurrent homogeneous catalysis in the results shown in Table 2, the catalyst was allowed to react with dry methylene chloride for 15 min under the operated reaction conditions and then filtered off. We observed that the filtrate (the catalyst-free liquid) showed no catalytic activity in acetylation of benzyl alcohol under the operated reaction condition (Table 1, entry 11). On the other hand, after each run, the filtrates were used for determination of catalyst leaching (gallium content), which showed a negligible release of GaCl3 by atomic absorption spectroscopy or ICP measurement. The capacity of the catalyst after six uses was 0.38 mmol of GaCl3 per gram. Also, the catalytic behavior of the separated liquid was tested by addition of fresh benzyl alcohol and Ac2O to the filtrates after each run. Execution of the acetylation reaction under the same reaction conditions, as with catalyst, showed that the obtained results were as same as blank experiments. These findings confirmed that PS/GaCl3 is stable and no significant leaching of Lewis acid moieties is operating under our reaction conditions and that the observed catalysis is truly heterogeneous in nature.
Further, we carried out the reactions between Ac2O and various alcohols to explore the reaction scope of PS/GaCl3-catalyzed acetylation, the results are summarized in Table 2. As can be seen, all reactions proceeded very cleanly (checked by GC) in good to excellent yields. A broad selection of alcohols, including primary (benzylic and linear ones), secondary (including aliphatic and aromatic alcohols), tertiary and allylic alcohols, were converted to their corresponding acetates successfully at room temperature (Table 2). It is noteworthy that in the case of both tertiary and allylic alcohols, which are acid-sensitive alcohols, no elimination product or migration of C = C bonds (rearrangement) was observed when PS/GaCl3 was used, probably due to its mild catalytic activity and they were also converted to their corresponding acetates in high yields (Table 2, entries 13, 15, 16). Protection of phenolic hydroxyl group was also achieved under the same reaction conditions described for alcohols and the corresponding acetates were produced in high yields (Table 2). As can be seen, a phenol whose benzene ring substituted with a strong electron-donating group, reacted quickly with excellent yield (Table 2, entries 18, 19). We then turned our attention on whether the same catalyst could be useful for acetylation of substrates containing other protecting groups. We observed that various protected alcohols (Table 2, entries 23–26) containing protecting groups such as acetyl, benzyl, benzoyl and tosyl were smoothly converted into the corresponding acetates without affecting the other protecting groups.
In order to extend the scope of the catalyst further, the benzoylation of alcohols and phenols with benzoic anhydride, a less reactive anhydride, was also investigated. The results of this investigation are shown in Table 2. Treatment of a series of alcohols and phenols with benzoic anhydride in the presence catalytic amount of PS/GaCl3 provided the corresponding benzoates in good yields (Table 2, entries 1–22).
In order to show that whether the presented method is potentially applicable for chemoselective conversion of alcohols and phenols to the corresponding acetates or benzoates, a set of competitive reactions was allowed between a primary alcohols and a secondary or tertiary alcohols, or phenol with Ac2O and (PhCO)2O in the presence of PS/GaCl3 (Table 3). The results indicated that PS/GaCl3 was able to discriminate between different types of alcohols and or phenols from each other, a transformation that is difficult to accomplish via conventional methods (Table 3, entries 1–9).
| Entry | Subst. 1 | Subst. 2 | Subst. 1/Subst. 2/(Ac2O) or (PhCO)2O | Time (min) | Yield (%)c | |
| Ester 1 | Ester 2 | |||||
| 1 | 1:1.1 | 50 | 3 | 93 | ||
| 2 | 1:1:1.1 (1.2)d | 50 (75)e | 4 (7)f | 94 (91)f | ||
| 3 | 1:1:1.1 (1.2) | 50 (85) | 4 (8) | 93 (90) | ||
| 4 | 1:1:1.1 (1.2) | 65 | 5 | 92 | ||
| 5 | 1:1:1.1 (1.2) | 60 (85) | 4 (8) | 92 (89) | ||
| 6 | 1:1:1.1 | 55 | 3 | 93 | ||
| 7 | 1:1:1.1 | 90 | 4 | 92 | ||
| 8 | 1:1:1.2 (1.4) | 85 (100) | 4 (9) | 92 (87) | ||
| 9 | 1:1:1.2 (1.4) | 60 (85) | 3 (6) | 94 (90) |
a All acetylation reactions were carried out in CH2Cl2 (5 mL) in the presence of PS/GaCl3 (0.1 mmol) at room temperature.
b All benzoylation reactions were carried out in refluxing CH2Cl2 (10 mL) in the presence of PS/GaCl3 (0.1 mmol).
c Yields based on GC and NMR.
d ,e,fThe molar ratios, times and yields in the parentheses refer to benzoylation reactions.
The definite mechanism of the reaction is unclear. However, a plausible mechanism for the formation of acetates in the presence of PS/GaCl3 as a catalyst is shown in Scheme 2. The acetic anhydride is first activated by Ga(III) as a Lewis acid to afford 1. The hydroxyl compound attacks 1 which in turn converts to the final product and simultaneously releases the catalyst for the next catalytic cycle.

The proposed catalytic mechanism for the formation of acetates.
In order to show the efficiency and applicability of the present method, the catalytic activity of PS/GaCl3 was compared with that of some reported catalysts in the literature, the results are summarized in Table 4. The results have been compared with respect to the reaction times, mol-% of the catalyst used, and yields. As demonstrated in Table 4, PS/GaCl3 is an equally or more efficient catalyst for this acetylation reaction in terms of yield and reaction rate.
Comparison of the results obtained for the acetylation of benzyl alcohol catalyzed by PS/GaCl3 with those obtained by some reported catalysts.
| Entry | Catalyst (mol %) | Conditions | Time (min) | Yield (%) | Ref. |
| 1 | BiCl3 (10) | CH3CN, reflux | 35 | 98 | [19] |
| 2 | Bi(TFA)3 (5) | CH3CN, reflux | 60 | 96 | [19] |
| 3 | CoCl2 (0.5) | CH3CN, RT | 240 | 98 | [14] |
| 4 | Montmorillonite KSF (20 mg) | Solvent-free, RT | 60 | 90 | [16] |
| 5 | ZeoliteHSZ-360 (20 mg) | Solvent-free, 60 °C | 60 | 84 | [17] |
| 6 | Saccharinsulfonic acid (13 mg) | CH2Cl2/reflux | 120 | 90 | [39] |
| 7 | Gd(OTf)3 (0.1) | CH3CN, RT | 30 | 97 | [43] |
| 8 | Cobalt(II) salen complex (1) | Solvent-free, 50 °C | 45 | 99 | [42] |
| 9 | Cu(BF4)2.XH2O | Solvent-free, RT | 60 | 96 | [31] |
| 10 | Cp2ZrCl2 (1) | Solvent-free, RT | 10 h | 93 | [26] |
| 11 | TaCl5 (10) | CH2Cl2, RT | 120 | 96 | [20] |
| 12 | Mn(III) salen complex (7) | Toluene, 80 °C | 6 h | 93 | [42] |
| 13 | HBF4-SiO2 (1) | Solvent-free, RT | 60 | 94 | [30] |
| 14 | InCl3 (0.1) | Solvent-free, RT | 30 | 85 | [44] |
| 15 | Ph3P+CH2COCH3Br− (5) | Solvent-free, RT | 35 | 96 | [45] |
| 16 | [Mn(III)(haacac)Cl] complex | CH3NO2, RT | 5 h | 97 | [46] |
| 17 | LiOTf (20) | Solvent-free, RT | 12 h | 97 | [47] |
| 18 | PS/GaCl3 (10) | CH2Cl2, RT | 25 | 97 | Table 2 |
3.3 Catalyst reusability
Finally, the reusability of the catalyst was tested in the acetylation reaction of benzyl alcohol with acetic anhydride as a model substrate. For each of the repeated reactions, the catalyst was filtered, washed exhaustively with methylene chloride and diethyl ether, respectively, and dried before using with fresh benzyl alcohol and Ac2O. The catalyst was consecutively reused six times with negligible loss in its activity or a negligible catalyst leaching, and also there is no need for regeneration (Table 5).
| Run | Benzyl acetate (%)c | Time (min) |
| 1 | 97 | 25 |
| 2 | 97 | 25 |
| 3 | 96 | 25 |
| 4 | 96 | 25 |
| 5 | 95 | 25 |
| 6 | 94 | 25 |
a The capacity of the catalyst after six uses was 0.38 mmol GaCl3 per gram.
b Reaction conditions: benzyl alcohol (1 mmol), Ac2O (1.1 mmol), PS/GaCl3 (0.1 mmol), CH2Cl2 (5 mL)
c Isolated yield.
4 Conclusion
In conclusion, in this article a simple, mild, efficient and highly chemoselective method for both acetylation and benzoylation of alcohols and phenols using stable and heterogeneous polystyrene-supported gallium trichloride is reported. The short reaction times, high to excellent yields, low cost, easy preparation and handling of the polymeric Lewis acid catalyst are the advantages of the present method. In addition, the use of water-tolerant PS/GaCl3 has resulted in a reduction in the unwanted and hazardous waste and minimum amount of product contamination with metal that is produced during conventional homogeneous processes. Most importantly, the work-up is reduced to a mere filtration and evaporation of the solvent. Finally, this catalytic system showed a good catalytic activity in these reactions and this polymeric catalyst can be recovered unchanged and used again at least six times with negligible loss in its activity. The other applications of PS/GaCl3 is currently under investigation and will be reported in due course.


