1 Introduction
Multicomponent reactions (MCRs) are excellent strategies, being employed in the synthesis of many natural products. These MCRs are generally defined as reactions where more than two starting materials react to form a product, incorporating more or less all the atoms of the starting materials [1]. The MCRs offer a wide range of advantages such as a single-step procedure, avoiding complicated purification processes and saving both solvents and reagents.
The MCRs have attracted considerable interest owing to their exceptional synthetic efficiency. Recently, there has been tremendous development in three- and four-component reactions [2].
Five-membered, N-containing heterocycles are important building blocks of an extensive number of biologically active compounds [3]. Among them, pyrroles are heterocycles of great importance because of their presence in numerous natural products like heme, chlorophyll, vitamin B12, and various cytochrome enzymes [4]. Some of the recently isolated pyrrole containing marine natural products has been found to exhibit considerable cytotoxicity and to function as multidrug-resistant reversal agents [5]. Many of these biologically active compounds have emerged as chemotherapeutic agents. In addition, polysubstituted pyrroles are molecular frameworks with immense importance in material science [6].
Consequently, a wide range of procedures have been devised for the synthesis of pyrroles [7]. However, many of the methods are associated with various drawbacks such as harsh reaction conditions, tedious experimental procedures, unsatisfactory yields, and long reaction times. Moreover, the number of methods for the synthesis of polysubstituted pyrroles is relatively limited. Herein we report an efficient, new approach for the synthesis of polysubstituted pyrroles.
As part of our program aimed at developing new reactions for the preparation of heterocyclic compounds [8], very recently, we have reported the synthesis of novel spiro[indoline-3,4′-pyridine]-3′-carboxylates 1 and spiro[indole-3,6′-[1,3]thiazin]-2-ones 2 (Fig. 1) by using of 1-phenyl-2-(1,1,1-triphenyl-λ5-phosphanylidene)-1-ethanone as a new important reagent in heterocyclic synthesis based on a one-pot reaction [9].
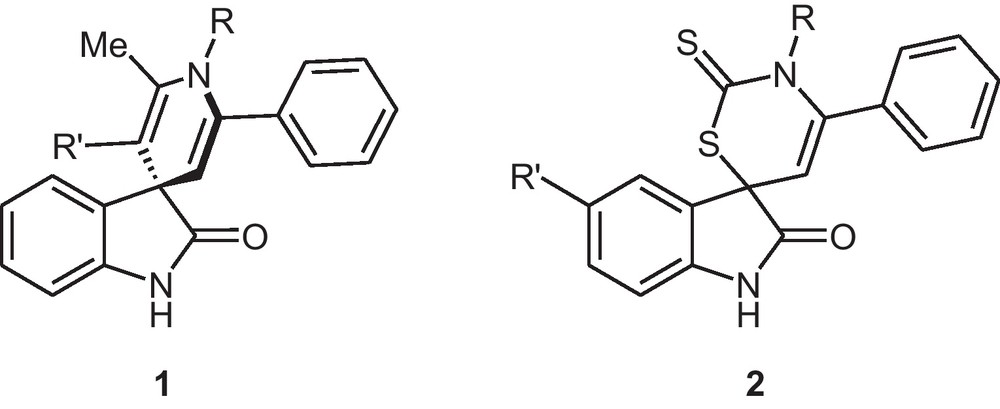
A new class of important spiroheterocyclic compounds.
Also, in continuation of our works on this reagent, we have observed that the reaction between ninhydrin, 1-phenyl-2-(1,1,1-triphenyl-λ5-phosphanylidene)-1-ethanone, and alkyl acetoacetae in presence of different amines in MeOH produced the corresponding substituted pyrroles at reflux condition (Scheme 1).
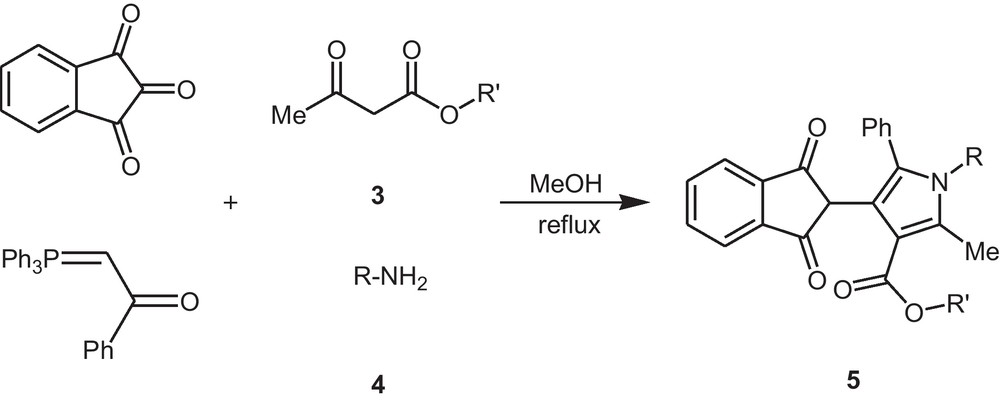
Synthesis of polysubstituted pyrroles.
2 Results and discussion
The choice of an appropriate reaction medium is of crucial importance for successful synthesis. Initially, the four-component reaction of ninhydrin and 1-phenyl-2-(1,1,1-triphenyl-λ5-phosphanylidene)-1-ethanone in the presence of benzylamine and methyl acetoacetate as a simple model substrate was investigated to establish the feasibility of the strategy and to optimize the reaction conditions.
Different solvents such as methanol, ethanol, acetonitrile, tetrahydrofuran (THF) and dichloromethane were explored. The results are summarized in Table 1.
Synthetic results of 5 under different reactions conditions.
| Entry | Solvent | Temp | Time/h | Yield (%)a |
| 1 | Methanol | Reflux | 12 | 87 |
| 2 | Ethanol | Reflux | 8 | 73 |
| 3 | Acetonitrile | Reflux | 8 | 61 |
| 4 | THF | Reflux | 10 | 65 |
| 5 | Dichloromethane | Reflux | 10 | 25 |
a Isolated yield
As can be seen in Table 1, the best result was obtained by refluxing the reaction mixture in methanol to yield product 5a in good yield (Table 1, Entry 1). Encouraged by this success, we extended this reaction of ninhydrin with 1-phenyl-2-(1,1,1-triphenyl-λ5-phosphanylidene)-1-ethanone, alkyl acetoacetate 3 and a range of aromatic and aliphatic amines 4 with both electron withdrawing and electron releasing substituents under similar conditions (MeOH), and corresponding pyrroles 5 were synthesized in high yields (81–87%) and the results are summarized in Table 2. We have shown that the use of a wide diversity of substituents in amines 4 and alkyl acetoacetate 3 in this four-component reaction makes possible the synthesis of libraries under similar circumstances (Table 2).
Synthesis of polysubstituted pyrroles.
| Product | R | R’ | Time | Yield (%) |
| 5a | C6H5CH2- | Me | 6 | 87 |
| 5b | C6H5CH2- | Et | 8 | 83 |
| 5c | 4-ClC6H4CH2- | Me | 6 | 84 |
| 5d | 4-ClC6H4CH2- | Et | 7 | 86 |
| 5e | 4-MeC6H4CH2- | Me | 8 | 81 |
| 5f | C6H5- | Me | 8 | 86 |
| 5g | n-Butyl | Me | 10 | 82 |
The structures of compounds 5a–g were deduced from their elemental analysis, IR and high-field 1H and 13C NMR spectra. The mass spectrum of 5a displayed the molecular ion peak at m/z = 449, which is in agreement with the proposed structure. The IR spectrum of this compound showed absorption bands due to the CH at 2947 and the C = O group at 1694 cm−1. The 1H NMR spectrum of 5a showed four singlet signals for the CH3, OCH3, CH, and CH2 groups at δ = 2.44, 3.05, 4.16 and 5.05 ppm respectively, and the aromatic moieties gave rise to multiplets in the aromatic region of the spectrum (δ = 6.93–8.01 ppm). The 1H-decoupled 13C NMR spectrum of 5a showed 21 distinct resonances in agreement with the suggested structure.
Although we have not established the mechanism of reaction experimentally, a possible explanation is proposed in Scheme 2. Compound 5 could result from the initial addition of the amine to alkyl acetoacetate and subsequent attack of the resulting reactive enaminone 7 on the compound 6 to yield intermediate 8. Cyclization of the intermediate 8 and subsequent loss of H2O lead to compound 5.

Probing the mechanism for the formation of title compounds.
3 Conclusions
In summary, a simple and easy approach has been developed for the quick construction of alkyl benzyl-4-(2,3-dihydro-1,3-dioxo-1H-2-indenyl)-1H-pyrrole-3-carboxylates 5 by the reaction of amines, alkyl acetoacetate, ninhydrin and 1-phenyl-2-(1,1,1-triphenyl-λ5-phosphanylidene)-1-ethanone. These types of heterocycles contain a number of functional groups and are therefore valuable precursors for diversity-oriented synthesis of pyrroles libraries, which are of potential uses in the facile preparation of biologically active molecules.
4 Experimental
4.1 Materials and techniques
All reactions were carried out in oven-dried glassware. Progress of reactions was monitored by thin layer chromatography while purification was effected by column chromatography, using silica gel (Merck 230-240 mesh). Melting points were measured on an Electrothermal 9100 apparatus. Elemental analyses for C, H and N were performed using a Heraeus CHN–O–Rapid analyzer. Mass spectra were recorded on a FINNIGAN-MATT 8430 mass spectrometer operating at an ionization potential of 70 eV. 1H and 13C NMR spectra were measured (CDCl3) with a Bruker DRX-500 AVANCE spectrometer at 500.1 and 125.8 MHz, respectively. IR spectra were recorded on a Shimadzu IR-460 spectrometer; absorbencies are reported in cm−1.
4.2 General procedure for the preparation of compounds 5a–g, exemplified on 5a
A solution of 1-phenyl-2-(1,1,1-triphenyl-λ5-phosphanylidene)-1-ethanone (0.38 g, 1 mmol), ninhydrin (0.16 g, 1 mmol) was magnetically stirred in 5 mL of MeOH for 20 min. Then, benzylamine (0.11 g, 1 mmol) and methyl acetoacetate (1.2 mmol) were added simultaneously. The reaction mixture was stirred for 6 h under reflux conditions and the progress of the reaction was followed by thin layer chromatography. When the reaction mixture was cooled to room temperature, a white solid precipitated. The precipitates were filtered and washed with diethyl ether to give product 5a in 87% yields. All products gave satisfactory spectral data in accordance with the assigned structures.
4.3 Spectral data
Methyl 1-benzyl-4-(1,3-dioxo-2,3-dihydro-1H-2-indenyl)-2-methyl-5-phenyl-1H-3-pyrrolecarboxylate (5a): White crystals (yield 87%); mp: 214-216 °C; IR (KBr) (νmax, cm−1): 2947 (CH), 1694 (C = O), 1447 (Ar). 1H NMR (500.13 MHz, CDCl3): δH (ppm) 2.44 (3H, s, CH3), 3.05 (3H, s, OCH3), 4.16 (1H, s, CH), 5.05 (2H, s, CH2), 6.93 (2H, d, 3JHH = 7.2 Hz, 2 CH of Ar), 7.25 (1H, t, 3JHH = 6.8 Hz, CH of Ar), 7.31 (5H, m, 5 CH of Ar), 7.40 (2H, m, 2 CH of Ar), 7.81-7.83 (2H, m, 2 CH of Ar), 8.00- 8.01 (2H, m, 2 CH of Ar). 13C NMR (125.8 MHz, CDCl3): δC (ppm) 11.85, 48.02, 49.50, 54.95, 109.30, 112.78, 122.90, 125.68, 127.31, 128.56, 128.61, 128.82, 130.30, 131.19, 134.77, 137.08, 137.19, 137.48, 141.73, 164.63, 199.00. MS (EI, 70 eV): m/z (%) = 449 (M+, 12), 417 (38), 326 (20), 73 (100). Anal. calcd for C29H23NO4 (449.50): C, 77.49; H, 5.16; N, 3.12%. Found: C, 77.42; H, 5.10; N, 3.06%.
Ethyl 1-benzyl-4-(1,3-dioxo-2,3-dihydro-1H-2-indenyl)-2-methyl-5-phenyl-1H-3-pyrrolecarboxylate (5b): White crystals (yield 83%); mp: 205-207 °C; IR (KBr) (νmax, cm−1): 2926 (CH), 1683 (C = O), 1448 (Ar). 1H NMR (500.13 MHz, CDCl3): δH (ppm) 0.84 (3H, t, 3JHH = 5.8 Hz, CH3), 2.44 (3H, s, CH3), 3.65 (2H, q, 3JHH = 5.8 Hz, CH2), 4.17 (1H, s, CH), 5.05 (2H, s, CH2), 6.94 (2H, d, 3JHH = 6.4 Hz, 2 CH of Ar), 7.25 (1H, m, CH of Ar), 7.32 (5H, m, 5 CH of Ar), 7.39 (2H, m, 2 CH of Ar), 7.81 (2H, m, 2 CH of Ar), 7.99 (2H, m, 2 CH of Ar). 13C NMR (125.8 MHz, CDCl3): δC (ppm) 11.52, 13.54, 47.53, 54.75, 58.46, 109.23, 112.17, 122.39, 125.22, 126.80, 128.06, 128.08, 128.32, 129.87, 130.69, 134.27, 136.53, 136.70, 136.75, 141.29, 163.94, 198.44. MS (EI, 70 eV): m/z (%) = 463 (M+, 46), 417 (68), 326 (42), 298 (38), 91(100). Anal. calcd for C30H25NO4 (463.53): C, 77.74; H, 5.44; N, 3.02%. Found: C, 77.69; H, 5.36; N, 2.98%.
Methyl 1-(4-chlorobenzyl)-4-(1,3-dioxo-2,3-dihydro-1H-2-indenyl)-2-methyl-5-phenyl-1H-3-pyrrolecarboxylate (5c): White crystals (yield 84%); mp: 210-212 °C; IR (KBr) (νmax, cm−1): 2924 (CH), 1710 (2 C = O), 1684 (CO2Me), 1453 (Ar). 1H NMR (500.13 MHz, CDCl3): δH (ppm) 2.43 (3H, s, CH3), 3.05 (3H, s, OCH3), 4.14 (1H, s, CH), 5.01 (2H, s, CH2), 6.85 (2H, d, 3JHH = 8.1 Hz, 2 CH of Ar), 7.28 (2H, d, 3JHH = 8.2 Hz, 2 CH of Ar), 7.29-7.37 (5H, m, 5 CH of Ar), 7.82-7.83 (2H, m, 2 CH of Ar), 8.00-8.01 (2H, m, 2 CH of Ar). 13C NMR (125.8 MHz, CDCl3): δC (ppm) 11.32, 46.90, 49.08, 54.40, 109.02, 112.48, 122.44, 126.55, 128.16, 128.25, 128.53, 129.60, 130.62, 132.72, 134.34, 135.20, 136.48, 136.75, 141.20, 164.05, 198.44. MS (EI, 70 eV): m/z (%) = 483 (M+, 26), 451 (74), 326 (64), 298 (58), 125 (100). Anal. Calcd. for C29H22ClNO4 (483.95): C, 71.97; H, 4.58; N, 2.89%. Found: C, 71.90; H, 4.54; N, 2.85%.
Ethyl 1-(4-chlorobenzyl)-4-(1,3-dioxo-2,3-dihydro-1H-2-indenyl)-2-methyl-5-phenyl-1H-3-pyrrolecarboxylate (5d): White crystals (yield 86%); mp: 256-258 °C; IR (KBr) (νmax, cm−1): 2960, 2922 (CH), 1713 (2 C = O), 1674 (CO2Et), 1446 (Ar). 1H NMR (500.13 MHz, CDCl3): δH (ppm) 0.84 (3H, t, 3JHH = 5.9 Hz, CH3), 2.43 (3H, s, CH3), 3.65 (2H, q, 3JHH = 5.9 Hz, CH2), 4.15 (1H, s, CH), 5.01 (2H, s, CH2), 6.86 (2H, d, 3JHH = 7.0 Hz, 2 CH of Ar), 7.28-7.36 (7H, m, 7 CH of Ar), 7.81 (2H, m, 2 CH of Ar), 7.99 (2H, m, 2 CH of Ar). 13C NMR (125.8 MHz, CDCl3): δC (ppm) 11.48, 13.53, 46.90, 54.69, 58.54, 109.45, 112.38, 122.42, 126.59, 128.14, 128.22, 128.52, 129.68, 130.62, 132.70, 134.32, 135.25, 136.42, 136.48, 141.26, 163.86, 198.35. MS (EI, 70 eV): m/z (%) = 497 (M+, 42), 451 (82), 326 (84), 298 (72), 125 (100). Anal. Calcd. for C30H24 ClNO4 (497.97): C, 72.36; H, 4.86; N, 2.81%. Found: C, 72.30; H, 4.80; N, 2.77%.
Methyl 4-(1,3-dioxo-2,3-dihydro-1H-2-indenyl)-2-methyl-1-(4-Methylbenzyl)-5-phenyl-1H-3-pyrrolecarboxylate (5e): White crystals (yield 81%); mp: 227-230 °C; IR (KBr) (νmax, cm−1): 2923 (CH), 1703 (C = O), 1445 (Ar). 1H NMR (500.13 MHz, CDCl3): δH (ppm) 2.33 (3H, s, CH3), 2.43 (3H, s, CH3), 3.05 (3H, s, OCH3), 4.17 (1H, s, CH), 5.01 (2H, s, CH2), 6.83 (2H, d, 3JHH = 7.2 Hz, 2 CH of Ar), 7.12 (2H, d, 3JHH = 7.2 Hz, 2 CH of Ar), 7.32 (3H, m, 3 CH of Ar), 7.41 (2H, m, 2 CH of Ar), 7.82 (2H, m, 2 CH of Ar), 8.00 (2H, m, 2 CH of Ar). 13C NMR (125.8 MHz, CDCl3): δC (ppm) 11.37, 20.51, 47.34, 48.99, 54.47, 108.71, 112.17, 122.41, 125.11, 128.05, 128.99, 129.04, 129.85, 130.68, 133.66, 134.28, 136.46, 136.58, 137.04, 141.23, 164.15, 198.56. MS (EI, 70 eV): m/z (%) = 463 (M+, 58), 431 (61), 326 (52), 298 (46), 105 (100). Anal. calcd for C30H25NO4 (463.53): C, 77.74; H, 5.44; N, 3.02%. Found: C, 77.69; H, 5.40; N, 2.98%.
Methyl 1-butyl-4-(1,3-dioxo-2,3-dihydro-1H-2-indenyl)-2-methyl-5-phenyl-1H-3-pyrrolecarboxylate (5f): White crystals (yield 86%); mp: 185-187 °C; IR (KBr) (νmax, cm−1): 2927 (CH), 1707 (C = O), 1533, 1439 (Ar). 1H NMR (500.13 MHz, CDCl3): δH (ppm) 0.78 (3H, t, 3JHH = 7.3 Hz, CH3), 1.15-1.19 (2H, m, CH2), 1.49-1.54 (2H, m, CH2), 2.57 (3H, s, CH3), 3.02 (3H, s, OCH3), 3.77 (2H, t, 3JHH = 8.2 Hz, CH2-N), 4.06 (1H, s, CH), 7.38-7.43 (3H, m, 3 CH of Ar), 7.48 (2H, d, 3JHH = 7.2 Hz, 2 CH of Ar), 7.80-7.81 (2H, m, 2 CH of Ar), 7.98-7.99 (2H, m, 2 CH of Ar). 13C NMR (125.8 MHz, CDCl3): δC (ppm) 11.23, 12.91, 19.27, 32.07, 43.76, 48.86, 54.42, 108.03, 111.89, 122.39, 128.01, 128.05, 130.33, 130.89, 134.23, 135.77, 136.41, 141.18, 164.13, 198.68. MS (EI, 70 eV): m/z (%) = 415 (M+, 82), 383 (100), 341 (42), 56 (20). Anal. Calcd. for C26H25NO4 (415.49): C, 75.16; H, 6.06; N, 3.37%. Found: C, 75.11; H, 6.01; N, 3.30%.
Methyl 4-(1,3-dioxo-2,3-dihydro-1H-2-indenyl)-2-methyl-1,5-diphenyl-1H-3-pyrrolecarboxylate (5g): White crystals (yield 82%); mp: 217-220 °C; IR (KBr) (νmax, cm−1): 2924 (CH), 1707 (CO2Me), 1526, 1441 (Ar). 1H NMR (500.13 MHz, CDCl3): δH (ppm) 2.37 (3H, s, CH3), 3.10 (3H, s, OCH3), 4.32 (1H, s, CH), 7.13-7.16 (5H, m, 5 CH of Ar), 7.22 (2H, m, 2 CH of Ar), 7.31 (3H, m, 3 CH of Ar), 7.84 (2H, m, 2 CH of Ar), 8.03 (2H, m, 2 CH of Ar). 13C NMR (125.8 MHz, CDCl3): δC (ppm) 12.41, 49.18, 54.49, 109.01, 112.25, 122.50, 127.23, 127.58, 127.61, 128.00, 128.42, 129.91, 130.37, 134.37, 136.56, 137.01, 137.78, 141.73, 164.23, 198.58. MS (EI, 70 eV): m/z (%) = 435 (M+, 34), 403 (100), 104 (24), 77 (76). Anal. Calcd. for C28H21NO4 (435.48): C, 77.23; H, 4.86; N, 3.22%. Found: C, 77.18; H, 4.80; N, 3.17%.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of Tarbiat Modares University.


