1 Introduction
In the last decade, the insufficiency of fossil fuels and the coinciding intensification of the greenhouse effect have aroused intensive research into energy storage all over the world.
Among energy storage devices, supercapacitors are the most suited for high-power applications. Supercapacitors are intermediate systems between electrochemical batteries that can accumulate high levels of energy and convert them to low power through dielectric capacitors, which then can deliver high levels of power [1,2]. Supercapacitors have attracted much attention because they can be charged and discharged quickly, while batteries can only supply bulk energy. Compared to batteries, supercapacitors can store and deliver larger amounts of energy over a longer period of time [3]. These characteristics have spurred an increasing interest in energy storage applications such as: electric vehicles, backup power systems, electronic components and military fields.
Depending on the storage mechanisms, two types of electrochemical capacitors exist: electric double layer capacitors, storing charges by ion accumulation at the electrode/electrolyte interface (Fig. 1) [4] and pseudocapacitors, through electrochemical surface reactions [3].
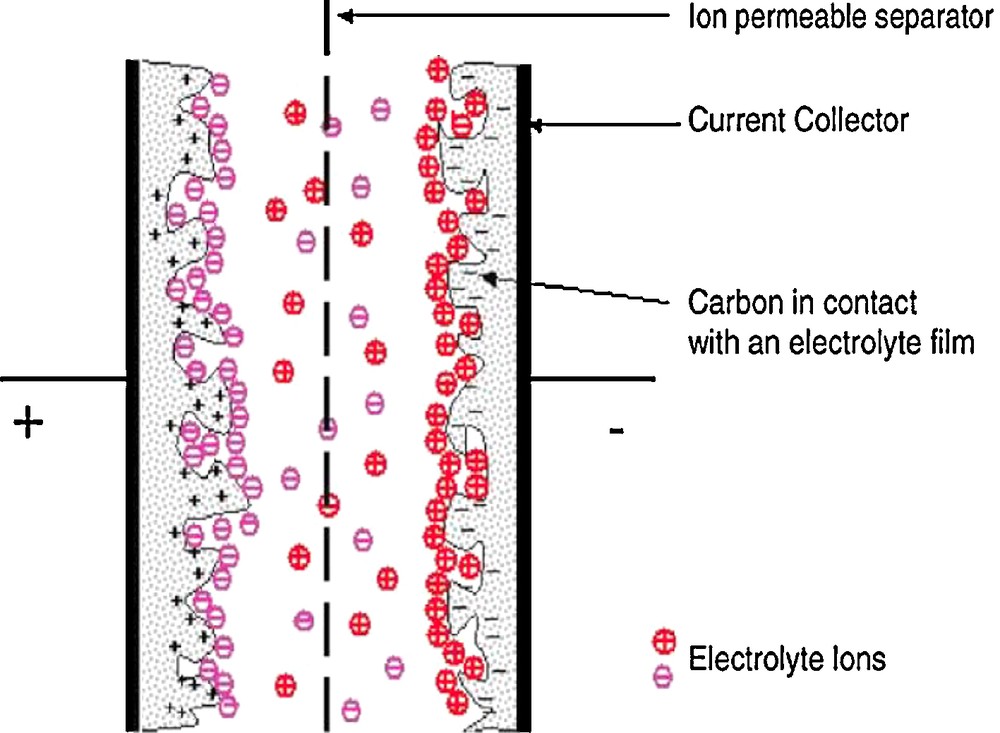
Representation of an electrochemical double layer capacitor (in its charged state).
Depending on the active materials, three different types of supercapacitors can be encountered:
This work is focused on carbon/carbon supercapacitors.
Carbon as an active material for supercapacitor electrodes has been extensively investigated because of its unique combination of chemical and physical properties, which include its high conductivity, high surface area, good corrosion resistance, and relatively low cost.
Seaweeds constitute a natural source of carbon that is very accessible and cheap. The chemical composition of seaweeds is very versatile, varying with the species and sometimes with the season. In general, they contain carbonate, proteins, lipids, fibre and ash [7]. Because of this adaptability, carbon materials can be prepared from seaweed with different physicochemical characteristics by simple pyrolysis.
Alginic acid, a fibrous substance located in the cellular wall of seaweeds, also offers interesting properties, such as a high oxygen content after pyrolysis. Thus, the oxygen trapped in these carbon electrodes can allow them to adsorb ionic electrolytes more easily [8].
The present work focuses on the feasibility of using carbon prepared from Caribbean seaweeds for electrode materials and studies the relationship between the obtained carbon properties and the performance of the assembled supercapacitor.
2 Experimental
2.1 Preparation of activated carbons
Turbinaria turbinata (Fig. 2) is a tropical brown algae of the Sargassaceae family. It generally grows on rocky substrates. The genus has shown promise as a method for removing pollutants from aqueous solutions [9].

Pictures of the Turbinaria turbinata seaweed.
T. turbinata biomass was collected in the French West Indies (Saint-François, Guadeloupe) and was sun-dried and crushed. Then, it was filtered to an average particle size ranging from 0.4 to 1 mm. The dried seaweed was pyrolysed in a tubular furnace (Thermolyne F 21100) under a nitrogen flow at temperatures between 600 and 900 °C. The heating rate was fixed, and the maximum temperature was held for 3 h. The resulting black solid was then thoroughly washed with 5 M HCl solution for a few hours at 80 °C and washed with water in Soxhlet extractor to ensure that pH stabilisation was reached. We used a similar method to that used in the work of Raymundo-Piñero et al. [10].
The carbons obtained were named CT600, CT700, CT800 and CT900.
2.2 Characterisations of activated carbons
2.2.1 Chemical and textural characterisations
The chemical composition of seaweeds is very versatile, fluctuating with the species and the harvest season. Seaweeds are lignocellulosic materials, which are complicated matrices composed of different ratios of cellulose, hemicellulose, lignin and intracellular substances. The chemical composition of the T. turbinata seaweeds was determined by performing a standard analysis for lignin (Klason lignin), pentosan (as hemicellulose) and cellulose (Kursshner and Hoffer method), for which the technical processes have already been described by Ouensanga [11].
The Boehm titration method is used to determine the number of acidic and basic surface groups [12]. The determination of the pHpzc of the activated carbons was carried out as previously described [13].
The yield of activated carbon is the percentage (%) amount of activated carbon produced at the end of the activation step. This value indicates the activation process efficiency. Characterisation of activated carbon was performed by nitrogen adsorption/desorption isotherms recorded at 77 K using a Micrometrics ASAP 2020 analyser. Prior to analysis, the samples were degassed in the analyser degassing port for 12 h at 300 °C. The specific surface area, SBET, was calculated using the standard Brunauer–Emmet–Teller (BET) equation [14].
2.2.2 Electrochemical measurements
The electrode was a pellet obtained by mixing 95% of the active material with 5% of the polytetrafluoroethylene (PTFE) binder, which was dispersed in ethanol [15]. After drying, a symmetric cell was built using a Teflon Swagelok construction, with a fibreglass separator between the electrode pellets. The mass loading and electrodes area were 15 mg/cm2 and 1.13 cm2, respectively.
Tetraethyl ammonium tetrafluoroborate (TEABF4, 1 mol/L) in acetonitrile served as the electrolytic solution.
The electrochemical cell was assembled in a glove box under an argon atmosphere to prevent any oxygen and water contamination (O2 and H2O contents were less than 1 ppm).
Cyclic voltammetry (CV), galvanostatic charge/discharge and electrochemical impedance spectroscopy (EIS) were performed using a VMP multichannel potentiostat-galvanostat (Biologic, France).
CV tests were performed between 0 and 2.5 V at rates of 5, 10 and 20 mV/s. Galvanostatic charge/discharge curves were measured in the potential range of 0 to 2.5 V at a constant current density of 5 mA cm−2. Impedance measurements were performed in the frequency range from 200 kHz to 10 mHz with a 5 mV voltage amplitude.
3 Results and discussions
3.1 Chemical composition
The results of the chemical analysis of the T. turbinata seaweeds are given in Table 1. Hollocellulose, which is cellulose plus hemicellulose, represents the major component at 59.5%, and the content of extractives are 5.1%. The T. turbinata has a lignin content of 15.8%.
Botanic composition of Turbinata turbinata seaweeds.
| Components | Weight (%) |
| Humidity | 13.0 |
| Cellulose | 47.3 |
| Hemicellulose | 12.2 |
| Lignin | 15.8 |
| Extractives | 5.1 |
| Ash | 6.1 |
During the carbonisation of the lignocellulosic precursors, hemicellulose, cellulose and lignin decompose at different rates within a distinct temperature range [16,17]. These values also allow the prediction that T. turbinata seaweeds could be a good candidate for activated carbon synthesis. This composition can play an important role in the properties found for the obtained activated carbons.
Table 2 shows the acidic or basic character of the activated carbons and the pH point of zero charge (pHpzc). The acidic character of a carbon is associated with oxygen surface complexes or oxygen functionalities such as carboxyls, lactones and phenols. Basic properties of the carbon surface are likely due to:
- • functional groups like pyrones, chromenes, ether and carbonyls [13] that contain less oxygen;
- • protonated amino groups for nitrogen-rich carbons;
- • graphene layers acting as Lewis bases that form electron donor–acceptor complexes with H2O molecules [18].
Results from chemical surface analyses.
| Activated carbons | pHpzc | Total acid groups (mequiv/g) | Total basic groups (mequiv/g) |
| CT600 | 2.85 | 0.836 | 0.051 |
| CT700 | 4.08 | 0.714 | 0.038 |
| CT800 | 3.15 | 0.959 | 0.050 |
| CT900 | 3.30 | 0.853 | 0.045 |
The Boehm titration was employed for the determination of active sites on the surface of the following carbon materials: CT600, CT700, CT800 and CT900.
The Boehm titration gives both qualitative and quantitative information about acidic groups (in the form of carboxyl, lactone and phenol) and basic groups. From the results obtained in Table 2, it can be observed that the amount of acidic groups was significantly higher than the total amount of basic groups. The results suggest that all carbon materials have an acidic character.
The pHpzc (the point of zero charge) is an important parameter that determines the range of pH sensibility and allows the surface active sites and adsorption capacities to be predicted [13]. The pHpzc values (Table 2) of the prepared activated carbons are between 2.85 and 4.08.
3.2 Surface and textural characterisations
The porous structure of the prepared carbons was determined by nitrogen adsorption/desorption measurements. Fig. 3 shows the nitrogen adsorption/desorption isotherms of T. turbinata seaweeds pyrolysed at 600 °C, 700 °C, 800 °C and 900 °C for 3 hours. Table 3 presents their textural and physical characteristics, including porosity, surface area and pore size.

Adsorption isotherm of nitrogen at 77 K by T. turbinata seaweeds pyrolysed at 600 °C, 700 °C, 800 °C, and 900 °C for 3 h.
Textural characteristics of T. turbinata activated carbons.
| Samples | Burn-off (%) | Bulk density (g/cm−3) | Mean pore diameter (nm) | BET surface area (m2/g) | Micropore surface area (m2/g) | Total volume pore (cm3/g) | Micropore volume (cm3/g) | Mesopore volume (cm3/g) |
| CT600 | 53 | 1.95608 | 4.1265 | 530 | 509 | 0.62 | 0.20 | 0.32 |
| CT700 | 58 | 2.13434 | 4.2406 | 724 | 638 | 0.76 | 0.26 | 0.50 |
| CT800 | 65 | 2.34718 | 4.3969 | 812 | 698 | 0.71 | 0.27 | 0.44 |
| CT900 | 70 | 2.03520 | 5.3518 | 258 | 241 | 0.24 | 0.09 | 0.15 |
Compared to the standard BDDT isotherm classification [19], the form of the isotherm may be of type IV for all samples, with an obvious increase in the N2 amount retained at low values of P/P0 (< 0.2) and higher relative pressure (0.1 < P/P0 < 1). It is characteristic of a mesoporous structure with a pore size between 2 and 50 nm. In a low-pressure region, the curve is of type II. This can be explained by the formation of a monolayer followed by a multilayer. The intermediate horizontal region in the isotherm corresponds to monolayer formation. The saturation level is reached at a pressure below the saturation vapour pressure. This can be explained by a possible condensation of adsorbent in the tiny capillary pores at pressures below the saturation pressure of the gas [20].
Table 3 shows that as burn-off increases from 53 to 70%, for CT600, CT700 and CT800, the BET surface increases, with the corresponding values of 530, 724 and 812 m2/g. The micropore volume also increases, with values of 0.20, 0.26 and 0.27 cm3/g for CT600, CT700 and CT800, respectively. For CT900, with a burn-off of 70%, a very low BET surface of 258 m2/g and a micropore volume of 0.09 cm3/g are obtained. The even lower BET surface area and micropore volume values compared to the values obtained for CT700 and CT800 carbons may be due to the achievement of the graphitisation limit and to porous structure collapse.
3.3 Electrochemical characterisations
3.3.1 Analysis of impedance measurements
Impedance measurements were performed in the frequency range from 200 kHz to 10 mHz. Fig. 4 presents the impedance spectra of the cells containing the CT600 (a,b), CT700 (c,d), CT800 (e,f), and CT900 (g,h) electrodes. The high frequency resistance values (when the imaginary part is close to zero) are 0.8, 0.65, 0.5 and 0.5 Ω cm2 for CT600, CT700, CT800 and CT900, respectively. Clearly, the shape of the plots is similar for CT700 and CT800; and it is likewise similar for CT600 and CT900. At low frequency, a vertical line close to 90°, indicating a pure capacitive behaviour, should be observed as well as a low ionic resistance in the structure of the electrodes; this ionic migration is observed between the high frequency and the knee frequency [2]. The closer the curve is to the vertical, the more the supercapacitor behaves as an ideal capacitor. Moreover, knee frequencies of 0.46 Hz, 0.73 Hz, 1.0 Hz and 3.5 Hz were measured for CT600, CT700, CT800 and CT900, respectively. A slightly different behaviour can be seen for CT900 and CT600: the vertical line is not close to 90°, indicating that a leakage resistance due to parasitic irreversible redox reactions is occurring at the electrode. Fig. 5 also shows the presence of a loop for the CT600 sample. The high amplitude of the loop explains the high resistance value. This loop could be ascribed to a pseudo-transfer resistance [21] related to a contact resistance between each activated carbon grain. But this could be also linked to electroactive functional groups at the carbon surface. The difference between the real part of the impedance at the knee frequency and the value of the ESR corresponds to the ionic resistivity of the electrolyte in the pores of the active material. The ionic resistivity is approximately 4.73, 1.13, 0.76, and 2.0 Ω cm2 for CT600, CT700, CT800 and CT900, respectively. Thus, CT800 is the best material in terms of impedance for several reasons: one of the lowest resistances, the lowest activated carbon ionic resistance, no high frequency loop (contact resistance or charge transfer resistance) and without any leakage resistance.

Impedance spectra between 200 kHz and 10 mHz of symmetric 2-electrode cells containing T. turbinata carbon electrodes. At 25 °C, in NEt4BF4 1M in acetonitrile. (a), (c), (e) and (g) for respectively CT600, CT700, CT800 and CT900. (b), (d), (f) and (h) are respectively a high frequency zoom-in for CT600, CT700, CT800 and CT900.
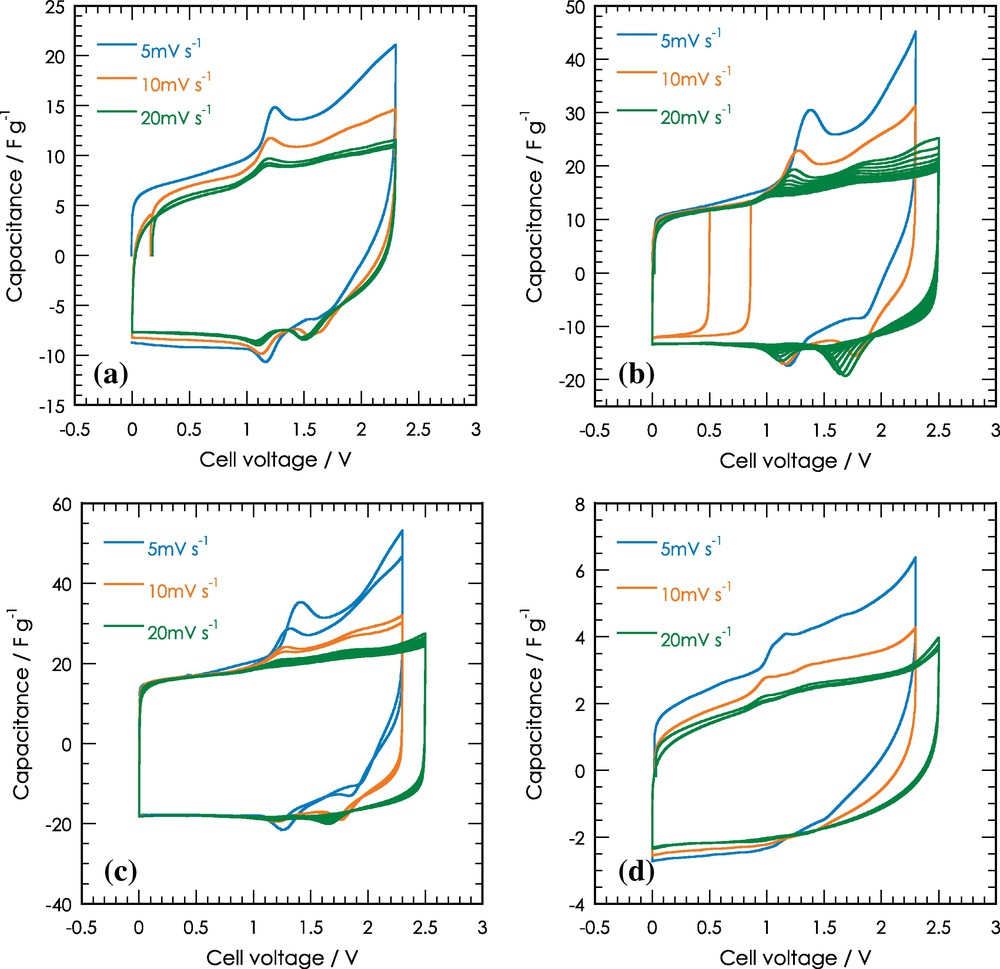
Cyclic voltammograms of symmetric two-electrode capacitors using CT600 (a), CT700 (b), CT800 (c) and CT900 (d) as electrodes in TEABF4 1 M in acetonitrile, scan rate 5 mV/s, 10 mV/s and 20 mV/s. Capacitance of cell is normalized respect to the total active material weight.
3.3.2 Cyclic voltammetry (CV)
CV measurements were reported between 0 and 2.5 V at different rates: 5, 10 and 20 mV/s. Symmetric supercapacitors were characterised by cyclic voltammetry to observe their profile differences and to determine the voltage window for charge/discharge cycling. The CV curves (Fig. 5) are quasi rectangular from 0 to 2.5 V. As seen on the CV, small reversible peaks are observed, which can be linked to the functional group at the carbon surface; likewise, it appears that those peaks decrease while the post-thermal treatment was higher. In Table 2, no total acidic group variation was observed, but the pHpzc may explain these results. This is likely because the acidic groups turned it into a less strong acid, and thus less reactive. Nevertheless, the redox potential decreases according to the increase in the number of cycles, leading to a rectangular shape regardless of the thermal treatment.
3.3.3 Galvanostatic cycling
Galvanostatic cycling of supercapacitor cells was performed using a VMP Biologic potentiostat at a constant current density of 5 mA cm−2 between 0 and 2.5 V. The electrolyte was used to perform this study in a solution of TEABF4 (1 M) in acetonitrile. Fig. 6 shows the galvanostatic charge/discharge curves of the prepared samples. The CT700 and CT800 curves are linear and symmetrical at a constant current density, meaning that no faradaic processes occur in the potential range studied (0–2.5 V). These are other typical characteristics of an ideal capacitor, and the small deviation to linearity is illustrative of a pseudocapacitance contribution.
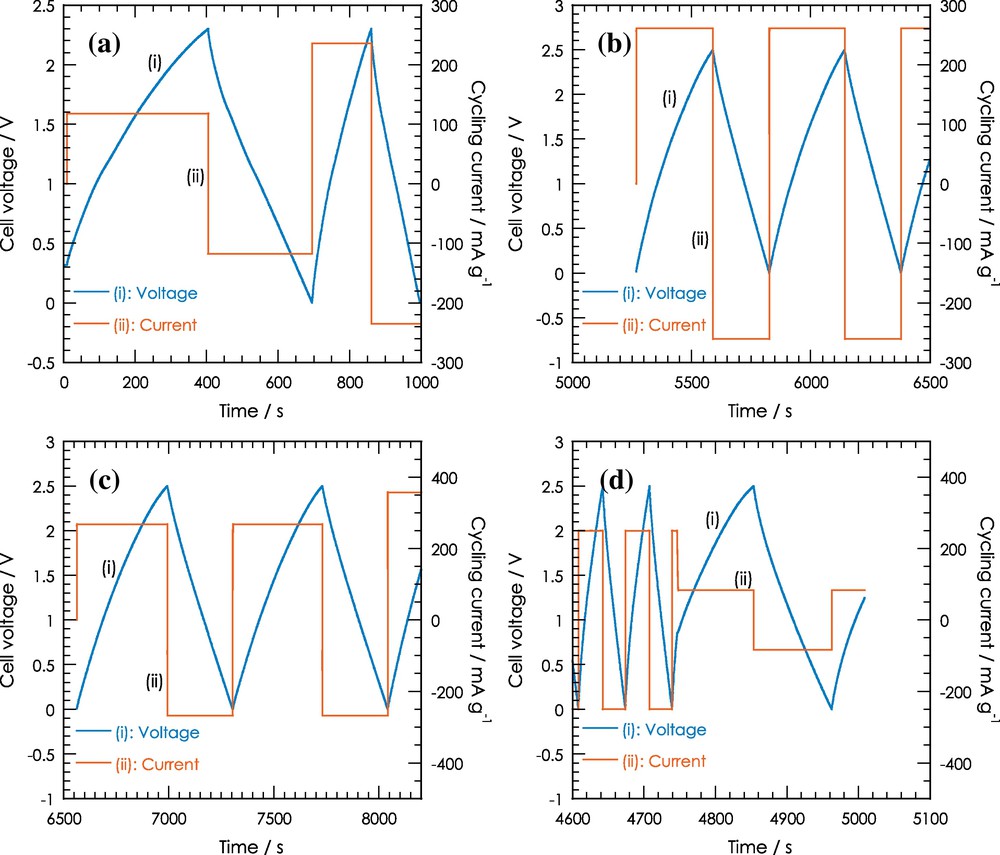
Charge/discharge galvanostatic cycling at 2.5 V of the seaweed carbon supercapacitor. (a) for CT600, (b) for CT700, (c) for CT800 and (d) for CT900.
However, the CT600 and CT900 curves are neither linear nor symmetrical.
The capacitances were calculated, and specific capacitances of 34.5, 55.0, 74.5 and 8.17 F/g are obtained for CT600, CT700, CT800 and CT900, respectively (Fig. 7).
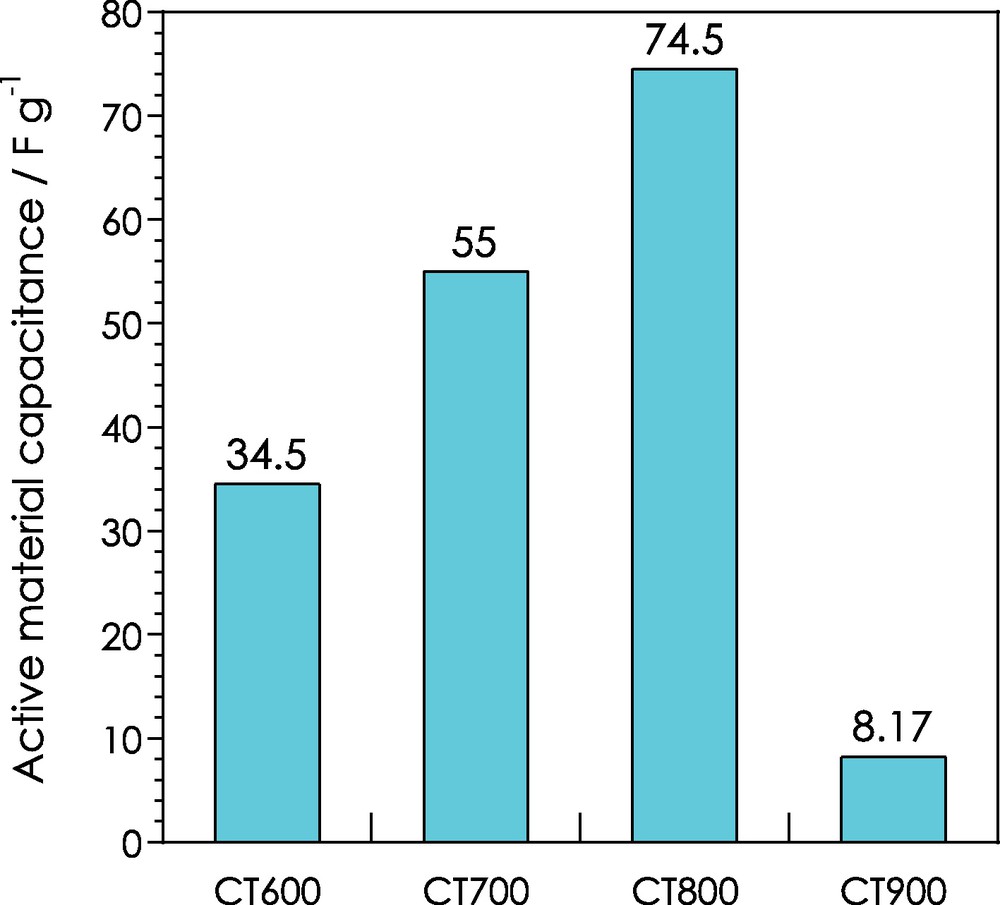
Evolution of the capacitance of assembled supercapacitors as a function of pyrolysis temperature.
4 Summary
Carbons with a moderate specific surface area and high density were produced by the pyrolysis of seaweeds. The CT600, CT700, CT800 and CT900 carbons were tested and electrochemically characterised by electrochemical spectroscopy impedance, cyclic voltammetry and galvanostatic cycling. The CV curves are quasi rectangular for all of the samples. The capacitance values are low except for CT800. A low ionic resistivity value was calculated for CT700 and CT800; both seem to have good capacitor behaviour. Textural characteristics making electrolyte ions accessible and enable their adsorption onto the carbon surface could explain this attractive behaviour. The CT800 carbon was found to be particularly interesting, with a specific capacitance of 74.5 F/g, an ESR of 0.5 Ω cm2 and an ionic resistivity of 0.76 Ω cm2 in 1.5 M TEABF4 in acetonitrile. CT800 seems to be an appropriate active carbon material in supercapacitors for energy storage applications. Further investigations are in progress to estimate the cyclability of such activated carbons.
Acknowledgments
Financial support from French Overseas Department Ministry and MESR for PhD funding is acknowledged.


