1 Introduction
According to the World Cancer Report 2008 issued by the World Health Organization, there were an estimated 12.4 million new cases of cancer worldwide in 2008 and 7.6 million deaths from the disease [1]. In contemporary drug therapy, most drugs still lack selectivity for specific sites in the human body; they can cause great damage to the patients. In order to improve the therapeutic effect, the dose of the drug has to be increased, which raises the cost of the therapy as well as the risk of side-effects. Therefore, it would be desirable to explore targeted drug delivery [2].
In recent years, a wide range of different nanoscale drug delivery vectors have been evaluated. Nanoscale particles generated using organic molecules as building blocks have been widely investigated for drug and gene delivery. For example, liposomes, polymersomes, polymeric micelles, and long-circulating polymeric nanoparticles are in various stages of preclinical and clinical development [3]. Moreover, due to their unique physicochemical properties, many inorganic materials, such as calcium phosphate, gold, carbon materials, silicon oxide, iron oxide and layered double hydroxide [4], have gained significant attention recently. Specifically, optical, magnetic, physical and other features such as inertness, stability, easy of functionalization make them attractive alternatives to organic nanoparticles. Also, multifunctional nanoscaled particulates in a single entity have attracted a great deal of attention for their diagnostic and biomedical researches. Particularly, magnetic and luminescent nanocomposites provide a new platform for both bioimaging and treatment of disease due to their enhanced multifunctional properties in contrast with their single use [5]. So far, various approaches have been developed to synthesize magnetic and fluorescent nanocomposites [6]. For example, quantum dots (QDs) were deposited onto the surface of polymer-coated Fe2O3 beads through thiol chemistry [7], gold nanoparticles were immobilized on the surface of Fe3O4 nanoparticles via electrostatic interactions [8], water-based CdSe/SiO2/Fe3O4 superparamagnetic luminescent nanocomposite particles via the electrostatic interactions between carboxyl groups and amino groups were also described [9].
Carbon nanotubes (CNTs), as a kind of inorganic nanocarriers, exhibit potential in biological systems due to their distinct properties in cell membrane penetration, loading and release of molecular cargoes [10]. Furthermore, it has been discovered that CNTs could be metabolized by neutrophil myeloperoxidase [11] and the functionalized CNTs (f-CNTs) have less cytoxicity [12]. In order to develop the targeting ability of the f-CNTs, conjugation with targeted moieties of folate (FA), antibodies, receptors or nanoparticles (NPs) is necessary. Many researches have been carried out, such as using FA-modified single-walled carbon nanotubes (SWCNTs) as targeted carrier to delivery of cisplatin into Ntera-2 cells [13], phosphatidylserine-modified SWCNTs targeted cytochrome C into phagocytic cells [14], NPs-modified multiwall carbon nanotube (MWCNT) targeted gemcitabine into lymph nodes [15], antibody-modified SWCNTs transport doxorubicin (DOX) into WiDr cells [16] and other related researches [17].
Despite excellent progress in using CNTs as drug delivery vehicles has appeared, most of them can only afford people with only single function, more research is needed to further optimize their ability to be multifunctionally modified. Here, we aim to develop a drug delivery system using Fe3O4 and CdSe quantum dots bi-functionalized CNTs as a drug nanocarrier. The assembled bi-functional CNTs, which could arrive at a certain site following the external magnetic field and trace at the same time, would have great application prospects. Due to their multifunctional property, they might be developed as novel drug delivery carriers for the treatment of brain, liver, lung, spleen diseases and so on.
2 Materials and methods
2.1 Materials
Carbon nanotubes (CNTs, purity > 98%) were provided by Tsinghua University. Ferric chloride hexahydrate (FeCl3·6 H2O, 99%), ethylene glycol (EG), polyethylene glycol (PEG), selenium powder (Se), sodium sulfite (Na2SO3), cadmium chloride (CdCl2·2.5 H2O), mercaptoacetic acid were purchased from Huadong Huabo Co. Ltd., China. Poly (diallyldimethylammonium chloride) (PDDA), sodium polystyrene sulfonate (PSS), chitosan (CHI), N,N′-dicyclohexyl carbodiimide (DCC) and anhydrous dimethyl sulfoxide (DMSO) were obtained from Hefei Bomei Biotechnology Co. Ltd., China. All reagents were of analytical grade.
2.2 Pretreatment of CNTs
The CNTs were treated in the mixture of concentrated HNO3 and H2SO4 (1:3, v/v) solution at 80 °C with constant stirring for 6 h. Afterwards, the mixture was diluted with distilled water and rinsed for several times until the pH value reached neutral, and then filtered and dried at 80 °C under vacuum, the as-made product named treated-CNTs.
2.3 Preparation of Fe3O4/CNTs
Firstly, PDDA (1 mL) and PSS (0.24 g) were orderly mixed with treated-CNTs (0.12 g) in distilled water (100 mL) and stirred for 2 h; a mixture of CNTs decorated with PDDA and PSS was obtained. Next, the modificatory CNTs and FeCl3·6 H2O (1.0 g) were added to a stirred solution of EG (50 mL) for 2 h, the resulting mixture of CNTs with attaching Fe3+ was centrifuged and reserved, named Fe3+/CNTs. And then, the Fe3+/CNTs were dispersed to EG and ultrasonicated for 30 min to form a homogenous suspension. Following, PEG (1.0 g) and sodium acetate (3.6 g) were added to the above solution and ultrasonicated for 30 min, then stirred for another 12 h, so the hydrothermal precursor was obtained; the hydrothermal reaction was then carried out at 200 °C for 12 h. After that, the product was collected by magnet, repeatedly washed by ethyl alcohol (EtOH) and dried at 40 °C.
2.4 Preparation of CdSe@ Fe3O4/CNTs
Scheme 1 is the schematic illustration of the preparation of CdSe@Fe3O4/CNTs. Specified volumes of Fe3O4/CNTs (0.1 g) and CHI (0.2 g) were dispersed to distilled water, ultrasonicated for 30 min to form Fe3O4/CNTs-CHI and centrifuged. At the same time, Se (0.05 g) was dissolved in supersaturated sodium sulfite (100 mL) to get a selenosulfate solution, CdCl2·2.5 H2O (0.15 g) was dissolved in 100 mL distilled water, then mercaptoacetic acid was added dropwise to form lots of white precipitate, NaOH was added until the precipitate completely dissolved to form a mercaptoacetic acid cadmium solution. After, according to the proportion of mixed selenosulfate and mercaptoacetic acid cadmium solution, ultrasound dispersing for a period of time and heat under reflux of the mixture, then the CdSe was formed. Subsequently, a solution of CdSe and DCC in anhydrous DMSO was prepared and stirred at ambient temperature for 1 h. Following that, this solution was added to a solution of Fe3O4/CNTs–CHI in acetate buffer (pH 4.7) and vigorously stirred at room temperature for 8 h. It was then brought to pH of 9 by aqueous NaOH and dialyzed first against PBS (pH 7.4) for 12 h and then against water for 12 h. Finally, the CdSe@Fe3O4/CNTs was obtained.
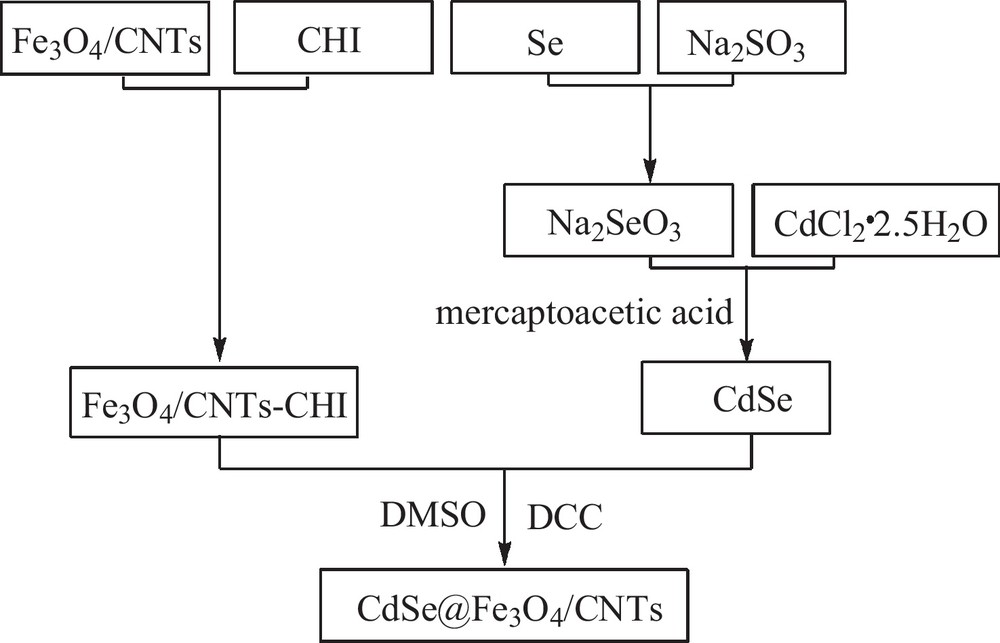
A schematic illustration of the formation of CdSe@Fe3O4/CNTs.
2.5 Measurements
The morphology and structure of the product was observed by transmission electronic microscopy (TEM, Hitachi, Japan). XRD spectrum was recorded using a Bruker D8 diffractometer with Cu Kα radiation (λ = 1.5418 Å) at a scanning rate of 0.02°/s and time step of 2 s, 2θ ranging from 5 to 70°. A vibrating sample magnetometer (VSM, Yangzhou University Instrument Plant, LH-3) was used to measure the magnetic moment. Photoluminescence spectra were obtained using an F-96 spectrofluorimeter.
3 Results and discussion
3.1 The formation mechanism of CdSe@ Fe3O4/CNTs
For the synthesis reaction, the possible formation mechanism of CdSe@ Fe3O4/CNTs is shown in Scheme 2.
- • First of all, the PDDA with positive charge and PSS with negative charge were adsorbed on CNTs through static electricity interaction, and the surfactant can prevent the agglomeration of suspension. The resulting effects include:
- ∘ with the negative CNTs surface, it was easy for Fe3+ ion to be adsorbed on the surface of CNTs by electrostatic attraction,
- ∘ due to the diameter increase of functional CNTs, there were more physical attachment points for the formation of Fe3O4 or loading drug;
- • after that, solvothermal method was adopted to prepare Fe3O4 magnetic particle. EG was used as deoxidizer to reduce partly Fe3+ to Fe2+ and PEG played a role of surfactant to prevent aggregation of Fe3O4;
- • then the Fe3O4/CNTs were coated with chitosan to ensure the surface with amino;
- • following selenosulfate solution was prepared used Se and supersaturated Na2SO3, which reacted with CdCl2 and mercaptoacetic acid to form CdSe with carboxylic acid groups. Here CdSe was difficult to conjugate with the amino of CHI because of the low reactivity of the carboxylic acid group. So DMSO and DCC were used as the solvent and condensing agent, respectively, to conjugate CdSe with CHI;
- • finally, CdSe and Fe3O4/CNTs-CHI were conjugated with the amino bond to form CdSe@Fe3O4/CNTs.
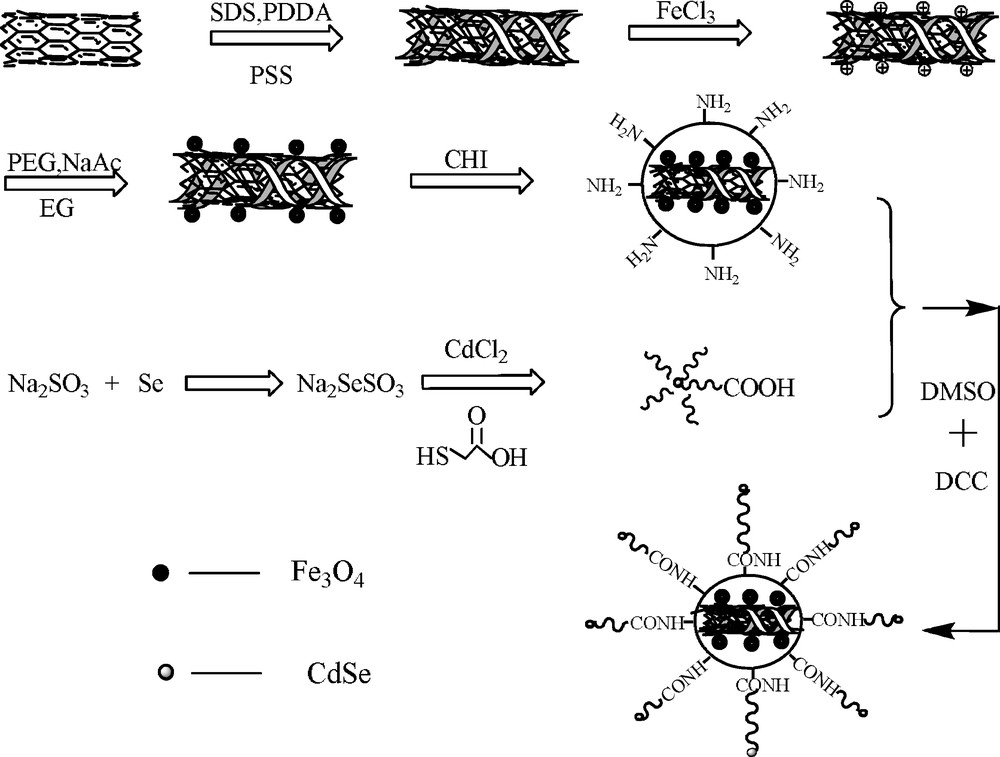
The formation mechanism scheme of CdSe@ Fe3O4/CNTs.
3.2 Characterization of sample
The morphology of the as-produced samples was investigated by TEM, as shown in Fig. 1. Fig. 1(a) reveals that the treated-CNTs, with a diameter of about 20 nm, present well-graphitized walls and basically have no extra materials. Obviously, unlike the treated-CNTs, CdSe@Fe3O4/CNTs nanocomposites show many additional tiny particles attached to the surface of the CNTs (Fig. 1(b)). The size of nanoparticles is about 10 nm, the particle size distribution is uniform, and the distribution of particles in the surface of CNTs is relatively uniform and compact.

TEM micrograph of (a) treated-CNTs and (b) CdSe@Fe3O4/CNTs.
The XRD pattern on Fig. 2 indicates that the crystal structure of magnetic nanocomposites comprises cubic Fe3O4 (pdf card: 65-3107) and CdSe (pdf card: 65-3436). The diffraction peak at 2θ = 26.4° is the typical Bragg peak of pristine CNTs and can be indexed to the (002) reflection of CNTs. Well-resolved diffraction peaks reveal the good crystallinity of the Fe3O4 specimens, which located at 2θ of 30.02°, 35.6°, 43.32°, 53.5°, 57.08°, 62.92° and correspond to Fe3O4 cubic crystal (220) (311) (400) (422) (511) (440) crystal faces. The peaks located at 2θ of 25.9°, 42.5° and 50.3° can be attributed to the (111), (220) and (311) planes of the zinc-blend phase of CdSe. This was in good agreement with the formation of CdSe@Fe3O4/CNTs.
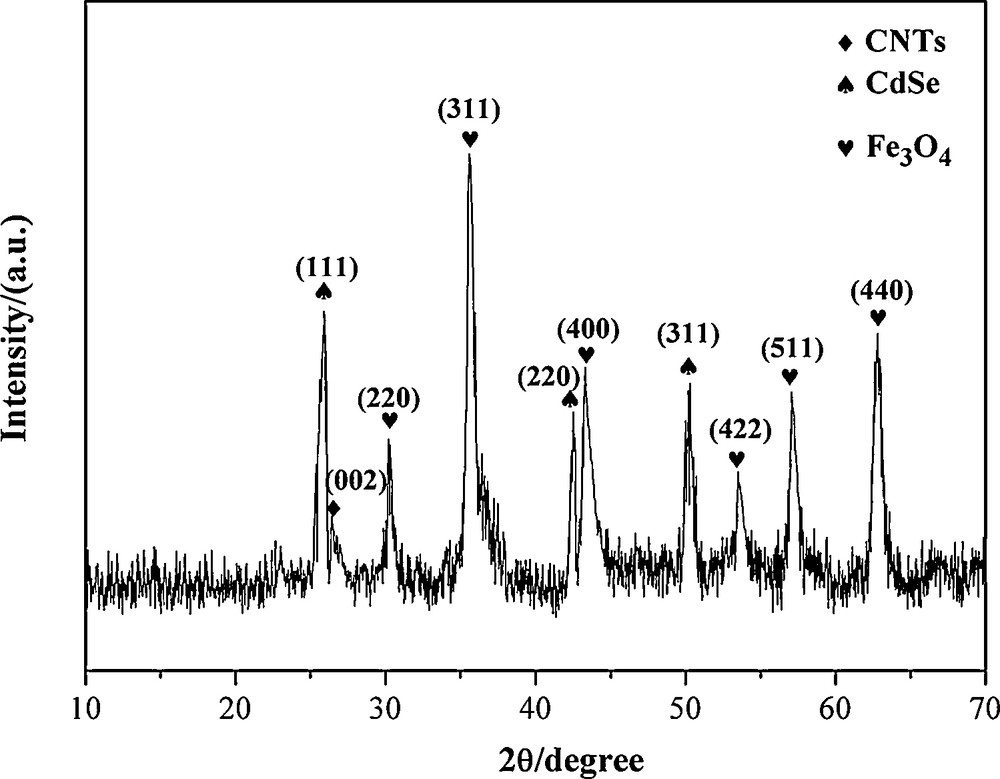
X-ray diffraction (XRD) of CdSe@Fe3O4/CNTs.
Fig. 3(a) exhibits the hysteresis loop of the CdSe@Fe3O4/CNTs at room temperature. It can be seen that the saturation magnetization of the composite is about 44 emu/g. With its unique superparamagnetic property, it can be used as a promising vehicle for magnetic field-directed drug delivery systems. Localization of CdSe@Fe3O4/CNTs without magnet (L) and with magnet (R) was shown in Fig. 3(b). The as-prepared sample could be easily well-dispersed in aqueous solution by transitory agitation and remained stable for a long time (Fig. 3(b) L), this is attributed to the existence of chitosan. Under an external magnetic field (Fig. 3(b) R), the composite hybrids were easily collected together, which indicates that they have a good magnetism. This is relevant for the use of CdSe@Fe3O4/CNTs to carry drugs to targeted positions under an external magnetic field.
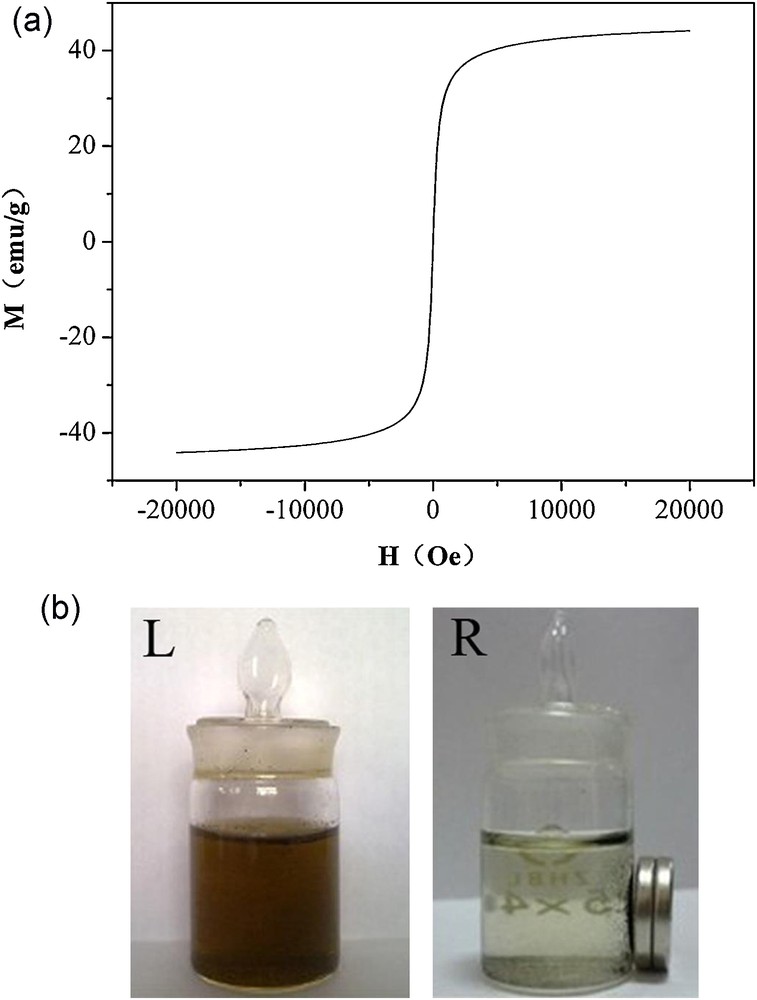
(a) Hysteresis loop of CdSe@Fe3O4/CNTs. (b) Localization of CdSe@Fe3O4/CNTs (L) without magnet and (R) with magnet.
The optical properties of CdSe, CdSe@Fe3O4/CNTs and Fe3O4/CNTs were characterized by fluorescence spectroscopy. As shown in Fig. 4, there is an observable luminescence peak at 518 nm of CdSe QDs, whereas the CdSe@Fe3O4/CNTs exhibit photoluminescence with a maximum emission at 518 nm similar to the luminescence peak of CdSe QDs, which suggests that the assembly of CdSe onto Fe3O4/CNTs does not change the fluorescence emission wavelength of CdSe. Moreover, the fluorescence intensity of CdSe@Fe3O4/CNTs is lower than the single CdSe, which is probably because of fluorescence quenching derived from CNTs. A possible pathway for this phenomenon is that the electrons of the excitons can be partly transferred to CNTs by an electron-injection mechanism while the reminder of electrons renders a reduced emission by an electron-hole recombination process.

Fluorescence spectra of (a) CdSe, (b) CdSe@Fe3O4/CNTs and (c) Fe3O4/CNTs.
4 Conclusions
We have fabricated a multifunctional nanocarrier by conjugating Fe3O4 nanoparticles and CdSe on the surfaces of CNTs. The CdSe@Fe3O4/CNTs shows good magnetism and fluorescence, which could anchor at the sites of cancer cells via Fe3O4 NPs by an external magnetic field and be used for in vivo imaging. The formation mechanism of CdSe@Fe3O4/CNTs suggested that the carrier can keep good stability and magnetism for the cladding by chitosan. In addition, quantum dots connect with the matrix by a covalent bond, which ensures the stability as well as the fluorescence characteristic. This novel nanocarrier provides excellent targeted properties as well as fluorescence for in vivo imaging, which indicates that it could be applied as a promising bi-functionalized drug nanocarrier in cancer chemotherapies.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (authorization numbers: 20975056, 21275082 and 81102411), Shandong (ZR2011BZ004 and ZR2011BQ005); JSPS and NSFC under the Japan–China Scientific Cooperation Program (21111140014); the Taishan Scholar Program of Shandong Province (TS20070711); and the National Key Basic Research Development Program of China (973 special preliminary study plan) (Grant No. 2012CB722705).
Appendix A Description
This manuscript reports the preparation of a novel cancer drug targeting carrier (CdSe@Fe3O4/CNTs) was prepared, in this system, chitosan was used as a bridge to link CdSe and magnetic CNTs, which improved the stability of the entirety; CdSe was linked up with chitosan using covalent bond steadily and kept a good fluorescence. Scheme 1 and Scheme 2 show schematic illustration and formation mechanism of the CdSe@Fe3O4/CNTs, respectively. Fig. 1 and Fig. 2 display the results of TEM and XRD studies, which indicate the formation of CdSe@Fe3O4/CNTs nanocomposites. Fig. 3 and Fig. 4 show that the nanocomposites display good superparamagnetic behavior and fluorescent properties, which indicate that the novel carrier has the potential to meet the specific needs in cancer in vivo imaging and targeted cancer therapy.


