1 Introduction
N-(4-hydroxycyclohexyl)-acetamide, which was obtained by the catalytic hydrogenation of 4-acetamide-phenol, is commonly constituted by two diastereomers (cis- and trans-isomers) [1–6]. Because only the trans-isomer is an important medical intermediate, which could be used to synthesize many kinds of medicine, such as ambroxl hydrochloride [1–6], it is vital to separate the trans-isomer from the two diastereomers. The conventional separation methods are column chromatography [1] and crystallization [7], but those methods are inevitably associated with one or more disadvantages, such as a small amount of diastereomers separated, excessive solvent consumption and poor yield, which tend to confine the space that conventional methods are applied to in industrial processes.
Recently, Yang et al. found that N-(4-hydroxycyclohexyl)-acetamide could realized cis-trans epimerization only using Raney-Ni as catalyst under an atmosphere of hydrogen, so that the disadvantages mentioned above can be avoided and the trans-isomer is acquired [8]. So, it is of significance to explore the mechanism of N-(4-hydroxycyclohexyl)-acetamide cis-trans epimerization, which is helpful to obtain more trans-isomer by controlling the reaction conditions. As the intermediates are unstable and difficult to be detected in the experiments, theoretical studies seem to be more important to prove the rationality of the mechanism in the aid of discreet calculations.
The aim of this paper is to conduct theoretical studies on the mechanism of N-(4-hydroxycyclohexyl)-acetamide cis-trans epimerization with the density functional theory (DFT) method. To determine the conformational stability of the intermediates and products, we have carried out molecular geometry optimization calculations for all conformations using the 6-31G* basis set with the B3LYP method. In addition, temperature, pressure, and the solvent effect on all conformations were studied. Furthermore the computation outcomes were compared with the results obtained in the experiments.
2 Calculations
All calculations were performed with the Gaussian 98 program package [9]. The geometries of all the stationary points at different temperatures and pressures were fully optimized at the B3LYP/6-31G* level of the theory. The B3LYP functional is composed of Becke's three-parameter hybrid exchange functional (B3), as implemented in GAUSSIAN 98, and the correlation functional of Lee, Yang, and Parr (LYP). The solvation energies for the products and intermediates at different temperatures and pressures were computed using solvation model PCM with the permittivities of 78.39, 36.64, 24.55, 20.7, 8.93 and 2.02, for H2O, CH3CN, CH3CH2OH, CH3COCH3, CH2Cl2, and C6H12, respectively.
3 Experimental
To 40 g N-(4-hydroxycyclohexyl)-acetamide (cis:trans = 80:20), 100 mL of water and a freshly prepared Raney-Ni were added. The reaction mixture was refluxed under an atmosphere of hydrogen for several hours. The products (cis:trans = 30:70) were obtained after the reaction was completed. The products were recrystallized by methanol/acetone mixture to give cis:trans = 15:85, and the recovered mother liquid was reused in seven cycles to give cis:trans = 4:96.
4 Results and discussion
The experiment was initially tested in 100% ethanol; the yield of the trans-isomer was only 37.4%. When the solution is 95% ethanol (contains 5% water) and 100% water, the yield was 38.5% and 70.4%, respectively (Table 1). It indicates that the yield of the trans-isomer increases with increasing the polarity of the solvent. As it can be seen from Table 2, when the H2 pressure is 0.1 MPa, the yield is 70.47%, which is same when the H2 pressure is 0.4 MPa. It indicates that the H2 pressure has no effect on the yield of the trans-isomer in the experiments.
The selection of solvent.
| Solvent | Composition in equilibrium product (%) | trans/(trans+cis) × l00 % | ||
| cis | trans | Ketone | ||
| Ethanol | 60.0 | 37.4 | 2.6 | 38.40 |
| 95% ethanol | 61.3 | 38.5 | Trace | 38.58 |
| Water | 29.5 | 70.4 | Trace | 70.47 |
The effect of H2 pressure.
| H2 pressure (MPa) | Composition in equilibrium product (%) | trans/(trans+cis) × l00% | ||
| cis | trans | Ketone | ||
| 0.1 | 29.5 | 70.4 | Trace | 70.47 |
| 0.4 | 29.5 | 70.4 | Trace | 70.47 |
According to the experimental results, the mechanism of N-(4-hydroxycyclohexyl)-acetamide cis-trans epimerization was proposed (Scheme 1). For N-(4-hydroxycyclohexyl)-acetamide cis-trans epimerization, we can reasonably speculate that the cis-, trans-free radical intermediates and 4-acetamide-cyclohexanone are produced in the process. The cis- (1) and trans-isomers (2) can change, respectively, into free radical intermediates (3) and (4), (3) and (4) both can change into free radical intermediate (5), (5) changes into 4-acetamide-cyclohexanone (6), then the reaction has two routes to choose. Since the margin of energies of these two routes is only 1.88 kJ/mol, (6) can spontaneously change to either (7) or (8). Then, (7) or (8) can change, respectively, to (1) or (2). The whole pathway is cyclic and reversible. In Fig. 1, the ΔH298 of the I, II, III, IV, V, VI, VII, VIII, IX step in the reaction is given, respectively. In the whole process, the formation of (3) and (4) are the key steps.
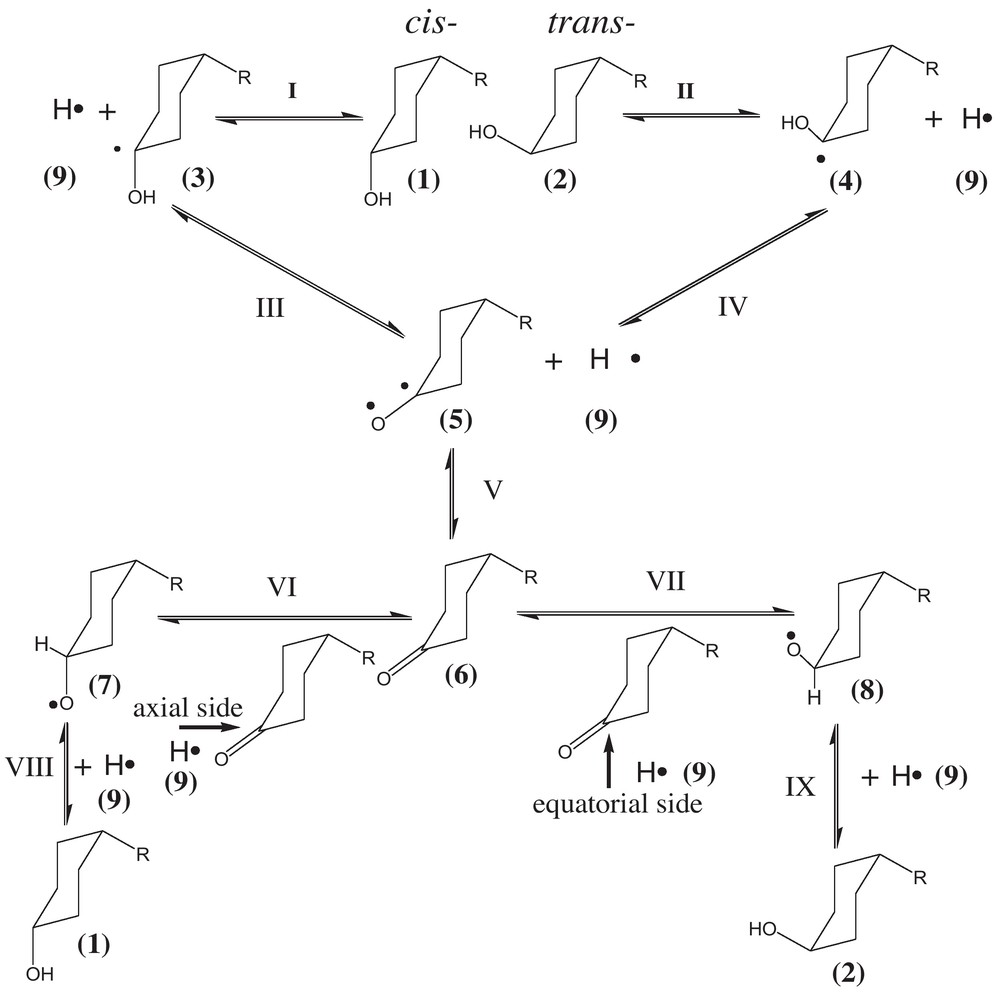
Mechanism of N-(4-hydroxycyclohexyl)-acetamide cis-trans epimerization R = –NHCOCH3.
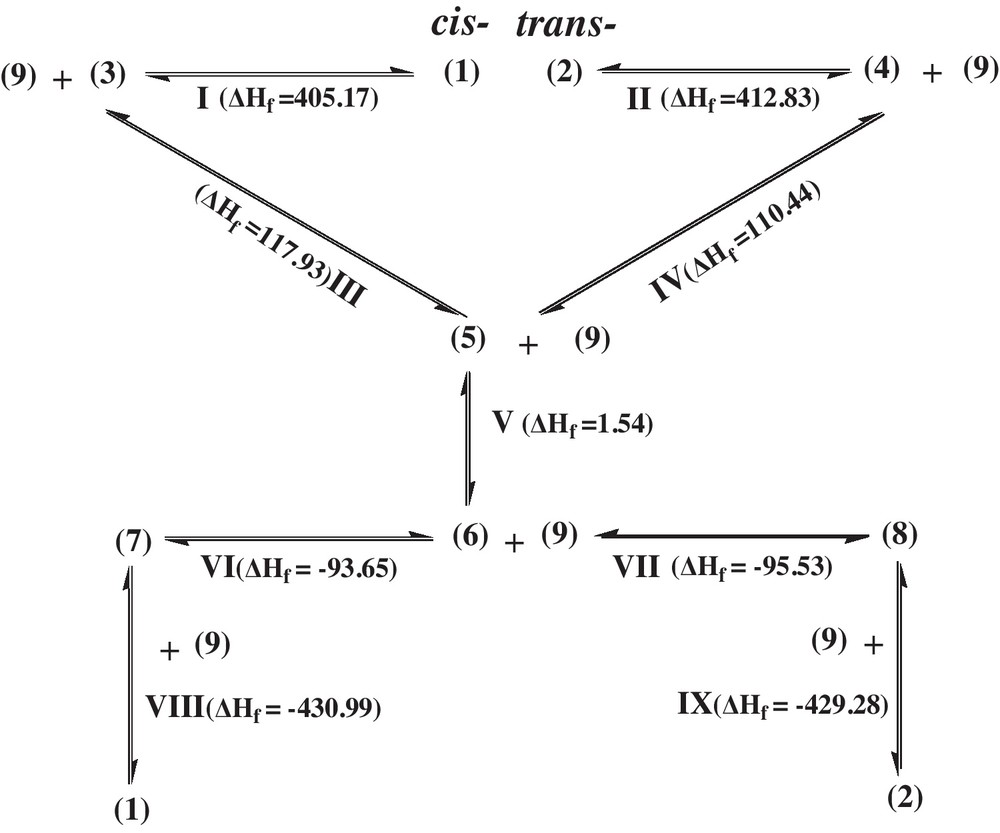
The energy change (KJ/mol) diagram of the reaction.
As it can be seen from Fig. 1, it is easier to change from (1) to (3) than (2) to (4), according to the free energy of reaction (ΔG298). So, (1), with the priority to trans-isomers (2), is more likely to change to (6). When (1) and (2) change to (6), (6) can choose to change either to (7) or (8). Taking into account the concept of catalyst hindrance, it is easier for (6) to be absorbed from the equatorial side than from the axial side of the molecule (Scheme 1) [10]. So, (8) is produced prior to (7). Certainly, the margin of energies of these two routes (1.88 kJ/mol) also makes it easier to change from (6) to (8) than (6) to (7), though it is not the major cause. Furthermore, the conformation of (2) is more stable than the conformation of (1), because the energy of (2) is lower than that of (1). So, it is easier to change from (6) to (2) than from (6) to (1). In the pathway, the cis-isomer (1) changes to the trans-isomer (2) easily. So the trans-isomer is the predominant product in the experiments. It must be noted that the value of free energy (ΔG298) is approximately represented by the value of enthalpy (ΔH298) for explaining the reaction equation, since the term of TS is commonly little, which determines that the value of enthalpy (ΔH298) is close to the value of free energy (ΔG298).
The interaction energies of the reactants, intermediates and solvents as well as products and solvents increase with increasing polarity of the solvent (Table 3). It explains that increasing the solvent polarity could facilitate the formation of the intermediates and products. In addition, temperature and pressure have no effect on the reaction pathway (Table 4). The computation results determine that water and 1.0 atm are the most optimized conditions for the experiments, which is in good accordance with the experimental results.
The absolute value of solvation energy (kJ/mol) of the reactants, intermediates and products in six distinct solvents.
| Entry | H2O | CH3CN | CH3CH2OH | CH3COCH3 | CH2CI2 | C6H12 | |
| l | (l) | 69.29 | 59.45 | 57.49 | 56.82 | 48.24 | 19.41 |
| 2 | (2) | 65.61 | 57.66 | 55.77 | 53.09 | 47.24 | 18.03 |
| 3 | (3) | 63.18 | 57.99 | 56.19 | 53.72 | 47.45 | 18.07 |
| 4 | (4) | 66.61 | 59.41 | 57.53 | 56.36 | 48.74 | 18.37 |
| 5 | (5) | 62.63 | 56.27 | 54.48 | 53.26 | 46.11 | 17.70 |
| 6 | (6) | 62.01 | 53.60 | 51.92 | 50.25 | 44.22 | 17.57 |
| 7 | (7) | 56.44 | 49.54 | 47.99 | 46.28 | 40.04 | 17.56 |
| 8 | (8) | 54.27 | 48.83 | 47.20 | 45.81 | 38.24 | 14.81 |
The calculated energies (kJ/mol) of the reactants, intermediates and products’ optimized conformations at different temperatures and pressures.
| (1) | (2) | (3) | (4) | (5) | |
| -273 K, 0 atm | -1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 348 K, 1.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 348K, 3.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 348 K, 5.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 348 K, 10.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 398K, 1.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 398 K, 3.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 398 K, 5.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 398 K, 10.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 448 K, 1.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 448 K, 3.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 448 K, 5.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| 448 K, 10.0 atm | −1362898.87 | −1362899.04 | −1361180.24 | −1361172.74 | −1359748.83 |
| (6) | (7) | (8) | (9) | ||
| -273 K, 0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 348 K, 1.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 348K, 3.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 348 K, 5.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 348 K, 10.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 398K, 1.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 398 K, 3.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 398 K, 5.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 398 K, 10.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 448 K, 1.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 448 K, 3.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 448 K, 5.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 | |
| 448 K, 10.0 atm | −1359747.29 | −1361154.41 | −1361156.29 | −1313.47 |
5 Conclusion
The mechanism of N-(4-hydroxycyclohexyl)-acetamide cis-trans epimerization was investigated with theoretical calculations at the B3LYP/6-31G* level. The calculation results indicate that the reaction proceeds through the cis-, trans-free radical and 4-acetamide-cyclohexanone intermediate pathway.
The effect of solvent on the reaction pathway that involves the formation of radicals was investigated using the PCM model. The calculation results indicate that the solvent plays an important role in the reaction by stabilizing the radicals and provide an alternate and lower energy pathway by which the reaction may proceed. From the analysis of the solvent effect on N-(4-hydroxycyclohexyl)-acetamide cis-trans epimerization, we can conclude that the ratio of the trans- to cis-isomer increases with increasing polarity of the solvent, which is in good agreement with the experimental results on solvent polarity. In addition, temperature and pressure has no effect on the reaction pathway.


