1 Introduction
Over the years, xanthenes and 14-aryl-14H-dibenzo[a,j]xanthene derivatives have emerged as a significant class of heterocyclic compounds that have attracted much synthetic interest due to their useful biological and pharmacological properties such as antiviral [1], antibacterial [2], and anti-inflammatory activities [3]. These compounds have also been used as luminescent sensors [4], dyes [5] and in laser technology [6]. Therefore, the synthesis of this class of heterocyclic compounds is practically important. Various catalysts such as Brönsted acids [7–23], Lewis acids [24–33], molecular iodine [34], nano-AgI [35], heteropolyacid [36,37], ionic liquids [38–41], nano-ZnO [42] and resin [43] have been developed for the synthesis of dibenzoxanthenes. However, several of these methodologies involve long reaction times, harsh conditions, use of toxic organic solvents, excess amount of catalyst, non-reusable catalysts, and tedious work-up confine the usefulness of some reported methodologies. Hence, introduction of new methods to overcome these problems is still in demand.
Carbon nanotubes (CNTs) have attracted much attention in the synthesis, characterization and other applications because of their unique structural, mechanical, thermal, optical and electronic properties [44–46]. Due to the high specific surface area of CNTs and other unique surface chemistry of them (e.g. activated carbons, silica, alumina, TiO2…); they are effective supports for the synthesis of solid acid catalysts. The covalent functionalization of CNTs with sulfonic groups provides stability, considerable solubility [47] and strong surface acidity [48]. The sulfonated single-walled carbon nanotube (SWCNT-SO3H) is useful as a solid acid catalyst in organic transformations.
In continuation of our interest towards green methodologies [49–53], we wish to report an efficient and green method for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes in the presence of reusable and robust SWCNTs-SO3H under solvent-free conditions (Scheme 1).

Synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes in the presence of SWCNTs-SO3H.
2 Result and discussion
2.1 Preparation of the catalyst
Scheme 2 shows the preparation procedure of SWCNTs-SO3H. As can be seen, first the SWCNTs, which are commercially available, were converted to SWCNTs-COOH with HCl (37%) and HNO3 (63%). This step was carried out in order to increase the reactivity and purity of SWCNTs and to remove the catalyst particles such as Fe, Co and Ni. Then sulforic acid (98%) was reacted with SWCNTs-COOH to afford the SWCNTs-SO3H.
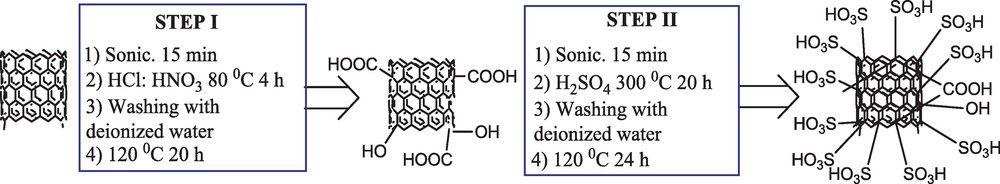
Preparation of the catalyst.
2.2 Characterization of SWCNTs-SO3H
The scanning electron microscopy (SEM) images of SWCNTs before and after H2SO4 treatment are shown in Fig. 1. The morphology, as observed by SEM, shows significant difference between SWCNTs and SWCNTs-SO3H. It is possible that the presence of –OH,–COOH and –SO3H groups on the side-wall of SWCNTs generate hydrogen-bond-type interactions.
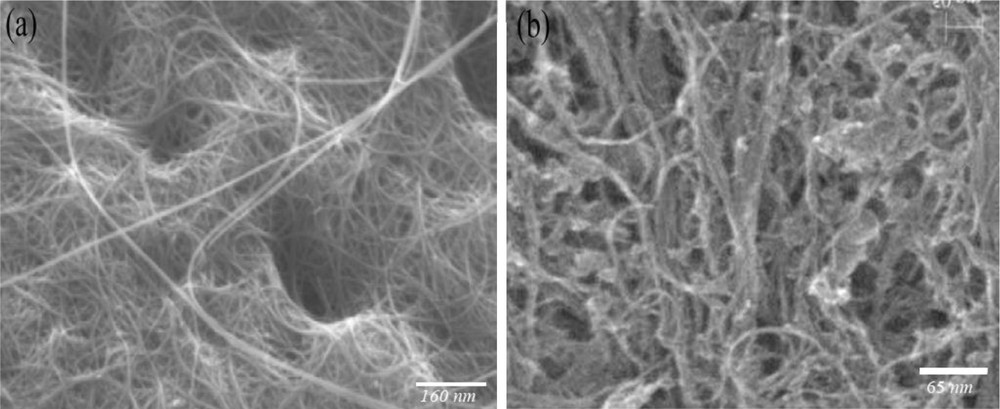
SEM images of (a): SWCNTs; (b): SWCNTs-SO3H.
The characterization of the SWCNTs-SO3H was performed by Fourier transform spectroscopy (FT-IR). As shown in Fig. 2, the high symmetry presented on pristine SWCNTs generates very weak infrared signals, due to the weak difference of charge state and very small induced electric dipole. After functionalization of SWCNTs, new bands are clearly seen. One of them appears at approximately 1550 cm−1 and is ascribed to the CC double bonds of CNTs. Another bands that become more prominent is the one at about 1720 cm−1, which can be assigned to the carbonyl group of the carboxylic acid group present in the SWCNTs-COOH. Although this band was present in the SWCNTs-SO3H, resulting from carboxylic acid groups, now it is much more intense. The presence of the sulfonic acid group is also demonstrated by the bands at 1120, 1300 and 590 cm−1, which correspond to the symmetric, asymmetric SO2 and CS stretching modes, respectively.

FT-IR spectra of (a): SWCNTs; (b): SWCNTs-COOH; (c): SWCNTs-SO3H.
Raman spectroscopy was also used to characterize the SWCNTs samples. Fig. 3 depicts the Raman spectra for SWCNTs, SWCNTs-COOH and SWCNTs-SO3H. In the region spectrum (1300–1600 cm−1); two bands are observed showing the characteristic of CNTs. These bands point the graphite band (G-band) and disorder or defects of the structure, named (D-band) [54]. The ratio between the intensity of the D-band and the G-band noted ID/G value corresponds to a higher proportion of sp3 carbon [55]. The large extent of CNTs functionalization is indicated by the significant increase in the D-band at about 1350 cm−1 (Fig. 3, spectra b and c) over that of pristine SWCNTs. We can calculate that ID/G values of SWCNTs, SWCNTs-COOH and SWCNTs-SO3H are 0.22, 0.34 and 0.80, respectively. This increasing value implies that a strong damage to the side-wall of SWCNTs is due to the functionalization.
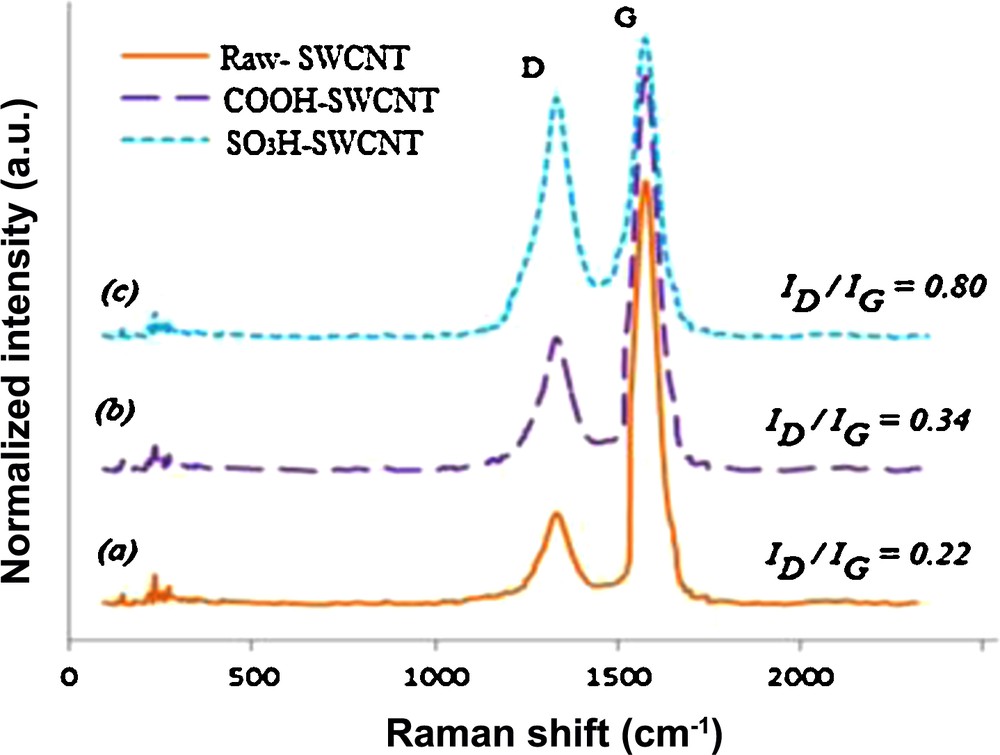
Raman spectra of (a): SWCNTs; (b): SWCNTs-COOH; (c): SWCNTs-SO3H.
The thermal gravimetric analysis (TGA) thermograms were used to determine the amount of sulfonic acid groups onto the SWCNTs. As shown in Fig. 4, the attached SO3H groups decomposed at ∼200 °C, while the SWCNTs remain stable up to 550 °C, at which temperature oxidation starts. Accordingly, these results indicate that about 30.3 wt% can be ascribed to the covalently of sulfonic acid groups and functionalization. Therefore, taking into account that the covalently SO3H measurements indicated one functionality every ∼30 C atoms, results obtained by TGA showed good agreement with those of FT-IR and Raman spectroscopy.

Thermograms of (a): SWCNTs-COOH; (b): SWCNTs-SO3H.
The dispersibility of SWCNTs is remarkably changed after functionalization. SWCNTs-SO3H is hydrophilic and well dispersed in polar solvent, but in non-polar solvent, SWCNTs-SO3H agglomerate and sediment at the bottom (Fig. 5). Thus, the sulfonation process can be used as an effective method to obtain dispersive CNTs for a variety of applications, e.g. composites and catalysts. In addition, the SWCNTs-SO3H act as a proton carrier; therefore, they can be used as solid acid catalysts in organic synthesis. The functional groups attached to SWCNTs are quantitatively determined by acid-base titration (–SO3H) in SWCNTs-SO3H 0.94 mmol g−1 (values calculated by the weight of SWCNTs-SO3H) [56].

Dispersibility of SWCNTs in (solvent) after 120 days. A: SWCNTs (CH3NO2). B: SWCNTs-COOH (CH3NO2). C: SWCNTs-SO3H (n-hexane). D: SWCNTs-SO3H (H2O). E: SWCNTs-SO3H (CH3CN). F: SWCNTs-SO3H (CH3NO2).
2.3 Synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes catalyzed by SWCNTs-SO3H under solvent-free conditions
The SWCNTs-SO3H was investigated in the synthesis of 14-aryl-14H-dibenzoxanthenes. First, the reaction parameters such as solvent, temperature and catalyst amount were optimized in the reaction of 4-nitrobenzaldehyde (2a) with 2-naphthol (1) in the presence of SWCNTs-SO3H as a model reaction (Table 1). Initially, the condensation of 2-naphthol (1) with 4-nitrobenzaldehyde (2a) in the presence of a catalytic amount of SWCNTs-SO3H under solvent-free conditions at 50 to 80 °C was examined (Table 1, entries 1–4). At 50 °C, the yield of the reaction was 60% and it increased to 91% at 60 °C (Table 1, entry 2). Entry 3 (70 °C) gave the best result. The effect of a higher temperature on the product yield was also examined and the reaction proceeded at 80 °C, but there was no significant change in the yield of the desired product and reaction time (Table 1, entry 4). In order to show the effect of solvent on this reaction, the reaction was carried out in different solvents such as n-hexane, CH3CN, CH3NO2 and also under solvent-free conditons (Table 1, entries 5–7). We observed that the best result was obtained under solvent-free conditions. In order to optimize the amount of the SWCNTs-SO3H, the model reaction was carried out in the presence of different amounts of the catalyst (Table 1, entries 3, 8–9). The maximum yield was obtained by the use of 50 mg of the SWCNTs-SO3H (Table 1, entry 3). Further increase in the amount of the catalyst did not affect the desired product yield (Table 1, entry 8), whereas the yield of the desired product was reduced by decreasing the amount of the SWCNTs-SO3H (Table 1, entry 9). When the same reaction was carried out in the absence of the SWCNTs-SO3H, the desired product was obtained in only 5% yield.
Optimization of the reaction conditions for the SWCNTs-SO3H catalyzed reaction of 2-naphthol with 4-nitrobenzaldehyde.
| Entry | Solvent | T (°C) | SWCNTs-SO3H (mg) | Time (min) | Yield (%)a |
| 1 | Solvent-free | 50 | 50 | 15 | 60 |
| 2 | Solvent-free | 60 | 50 | 15 | 91 |
| 3 | Solvent-free | 70 | 50 | 5 | 96 |
| 4 | Solvent-free | 80 | 50 | 5 | 96 |
| 5 | n-hexane | Reflux | 50 | 50 | 35 |
| 6 | CH3CN | Reflux | 50 | 50 | 50 |
| 7 | CH3NO2 | Reflux | 50 | 35 | 90 |
| 8 | Solvent-free | 100 | 55 | 5 | 96 |
| 9 | Solvent-free | 80 | 45 | 10 | 88 |
| 10 | Solvent-free | 80 | 0 | 60 | 5 |
a Isolated yields.
After optimization of the reaction conditions, synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes was carried out from the condensation of 2-naphthol (1) with different aromatic aldehydes utilizing SWCNTs-SO3H under solvent-free conditions at 70 °C. All reactions were completed within 3 to 15 min, as can be seen in Table 2. The electron-withdrawing substituents on the benzaldehyde afforded the desired product in high yields with short reaction times (Table 2, entries 1–5, 15), while electron-donating groups on benzaldehyde increased the reaction time (Table 2, entries 7–11). In addition, we observed that the steric effects between ortho-substituents of benzene ring and the xanthene ring decreased product yields (Table 2, entries 13–14). The proposed reaction mechanism for the SWCNT-SO3H-catalyzed synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes using 2-naphthol and aldehyde is shown in Scheme 3. The aldehyde is first activated by protonation with SWCNTs-SO3H to give I. Nucleophilic attack of 2-naphthol on I affords II, which in turn was activated by SWCNTs-SO3H to afford carbocation III. Nucleophilic attack of second molecule of 2-naphthol to carbocation III, gives the oxonium species IV and V, respectively, which is subsequently converted to the dibenzoxanthene VI and release SWCNTs-SO3H for the next catalytic cycle.
Synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes in the presence of SWCNTs-SO3H.
| Entry | RCHO | Product | Yield (%)a | Time (min) | Mp (°C) | |
| Found | Reported [Ref.] | |||||
| 1 | 4-Nitrobenzaldehyde (2a) | 3a | 96 | 5 | 312–313 | 309–310 [30] |
| 2 | 3-Nitrobenzaldehyde (2b) | 3b | 91 | 7 | 214–215 | 213–214 [30] |
| 3 | 4-Cyanobenzaldehyde (2c) | 3c | 94 | 6 | 338–339 | 336–338 [40] |
| 4 | 4-Chlorobenzaldehyde (2d) | 3d | 98 | 3 | 290–291 | 289–290 [25] |
| 5 | 2-Chlorobenzaldehyde (2e) | 3e | 85 | 6 | 213–214 | 214–216 [25] |
| 6 | Benzaldehyde (2f) | 3f | 93 | 8 | 182–183 | 184–185 [25] |
| 7 | 3-Methylbenzaldehyde (2 g) | 3 g | 90 | 8 | 197–198 | 197–198 [25] |
| 8 | 4-Methylbenzaldehyde (2 h) | 3 h | 89 | 10 | 226–228 | 227–229 [25] |
| 9 | 4-Isopropylbenzaldehyde (2i) | 3i | 80 | 15 | 240–241 | 240–241 [40] |
| 10 | 3-Hydroxybenzaldehyde (2j) | 3j | 92 | 10 | 263–264 | 262–263 [43] |
| 11 | 4-Methoxybenzaldehyde (2k) | 3k | 94 | 6 | 204–205 | 203–205 [25] |
| 12 | 2-Methoxybenzaldehyde (2l) | 3l | 87 | 15 | 258–259 | 258–259 [25] |
| 13 | 2-Hydroxy-4-methoxybenzaldehyde (2 m) | 3 m | 60 | 15 | 210-211 | – |
| 14 | 5-Bromo-2-hydroxybenzaldehyde (2n) | 3n | 55 | 15 | 219–221 | – |
| 15b | 4-Formylbenzaldehyde (2o) | 3o | 91 | 7 | 310–311 | 308–310 [26] |
a Isolated yields.
b Reaction conditions: 2-naphthol (8 mmol), 4-formylbenzaldehyde (2 mmol).
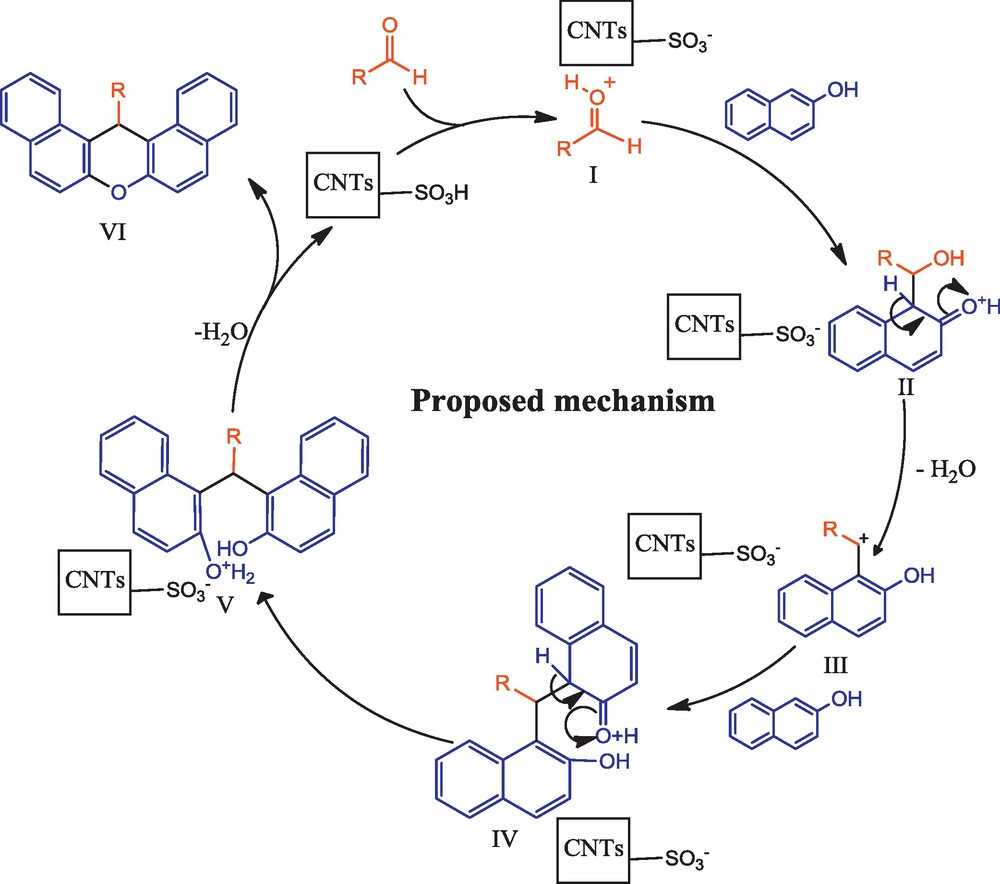
Proposed mechanism for the reaction.
As can be seen in Table 2 (entry 15), the practical synthetic efficiency of this reaction was investigated by the reaction of 4-formylbenzaldehyde (2o) (terephthaldehyde) with 2-naphthol (1). We expected that both of the formyl groups on the aromatic ring of terephthaldehyde (2o) would react with 2-naphthol (1). But we observed that one of the formyl groups reacted with 2-naphthol and another formyl group was unreacted, because of the steric effects between ortho-hydrogens of the benzene ring and the xanthene rings (Scheme 4).

Reaction of terephthaldehyde with 2-naphthol in the presence of SWCNTs-SO3H.
In order to show the efficiency and advantage of the new method in the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes, we have compared the obtained results in the synthesis of 14-(4-nitrophenyl)-14H-dibenzo[a,j]xanthene catalyzed by SWCNTs-SO3H with those of some works in the literature (Table 3). It seems that the presented new method is superior in terms of catalyst amount, time of reaction, reaction temperature, product yield and reusability of the catalyst.
Comparison of the results obtained for the synthesis of 14-(4-nitrophenyl)-14H-dibenzo[a,j]xanyhene catalyzed by SWCNTs-SO3H with those obtained by the recently reported catalysts.
| Entry | Catalyst | Catalyst (mol%) | T (°C) | Time | Yield (%)a | Ref. |
| 1 | SWCNTs-SO3H | 12.5 mg | 70 | 5 min | 96 | This work |
| 2 | Cellulose sulfonic acid | 80 mg | 115 | 2 h | 90 | [7] |
| 3 | SBA-15-SO3H | 40 mg | 85 | 24 h | 91 | [8] |
| 4 | Oxlic acid | 100 | 80 | 35 min | 95 | [9] |
| 5 | Silica sulfuric acid | 30 mg | 80 | 60 min | 94 | [10] |
| 6 | NH4H2PO4-SiO2 | 100 mg | 125 | 20 min | 75 | [12] |
| 7 | H3BO3 | 20 | 120 | 2 h | 98 | [13] |
| 8 | TCCA | 5 | 110 | 50 min | 74 | [15] |
| 9 | Sulfamic acid | 20 | 125 | 11 h | 94 | [16] |
| 10 | Saccharin sulfonic acid | 15 | 90 | 100 min | 96 | [17] |
| 11 | Cyanuric chloride | 10 | 110 | 45 min | 90 | [18] |
| 12 | SiO2-Pr-SO3H | 20 mg | 125 | 40 min | 98 | [19] |
| 13 | [C4DABCOC4SO3H][BF4][HSO4] | 3 | 100 | 10 min | 88 | [20] |
| 14 | HBF4-SiO2 | 0.2 g (10) | 120 | 1 h | 94 | [21] |
| 15 | ZrO (OTf)2 | 1 | 120 | 2 min | 95 | [24] |
| 16 | Fe (HSO4)3 | 10 | 60 | 1.2 h | 95 | [25] |
| 17 | I2 | 20 | 90 | 2.5 h | 85 | [34] |
| 18 | KAl (SO4)2.12 H2O | 50 | 100 | 4 h | 90 | [26] |
| 19 | Yb (OTf)3 | 1 | 110 | 3 h | 91 | [27] |
| 20 | Mg (HSO4)2 | 10 | 60 | – | 90 | [28] |
| 21 | Nano-TiO2 | 15 | 90 | 10 min | 90 | [29] |
| 22 | TaCl5 | 10 | 110 | 1 h | 90 | [30] |
| 23 | Sc [N(SO2C8F17)2]3 | 1 | 110 | 2 h | 95 | [32] |
| 24 | BF3-SiO2 | 80 mg | 60 | – | 93 | [33] |
| 25 | Nano-AgI | 20 | 140 | 42 min | 95 | [35] |
| 26 | HPA | 1 | 100 | 60 min | 89 | [36] |
| 27 | n-Bu4NBr | 10 | 125 | 60 min | 96 | [38] |
| 28 | MPA-DAZY | 0.6 | 100 | 110 min | 93 | [31] |
| 29 | [MIMPS]HSO4 | 5 | 100 | 6 min | 94 | [40] |
| 30 | SAFIS | 10 | 110 | 5 min | 98 | [41] |
| 31 | Dowex-50 W | 100 mg | 100 | 2 h | 84 | [43] |
a Isolated yields.
2.4 Reusability and stability of the catalyst
The reusability of the SWCNTs-SO3H is of great importance from synthetic and economical points of view. Therefore, the reusability of the catalyst was checked by separating the SWCNTs-SO3H from the reaction mixture by simple filtration, washing with ethanol and chloroform, and drying in a vacuum oven at 80 °C for 10 h prior to reuse in subsequent reactions. The recovered catalyst can be reused at least eight times in subsequent reactions without significant loss in the product yield (Fig. 6).
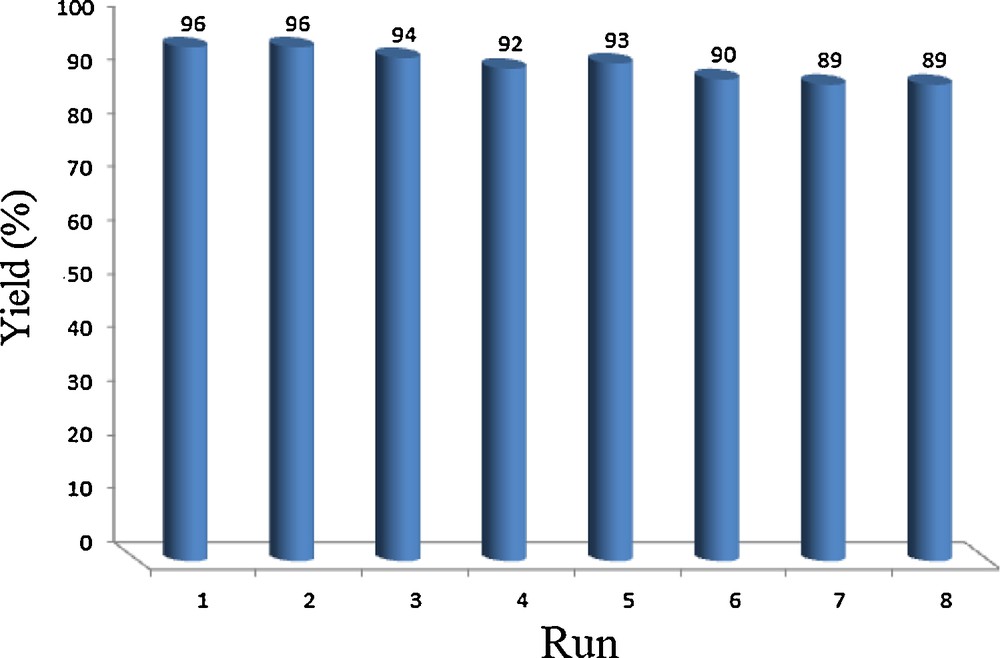
Reusability of the SWCNTs-SO3H in the synthesis of 14-(4-nitrophenyl)-14H-dibenzo[a,j]xanthene.
2.5 A comparison study with MWCNTs-SO3H as catalyst
The catalytic properties of multi-walled and single-walled carbon nanotubes were compared with the synthesis of 14-(4-nitrophenyl)-14H-dibenzo[a,j]xanthene. The results showed that there is no significant difference between these two types of catalysts. For comparison, multi-walled carbon nanotube was also used for the reaction of 2-naphthol (1) with 4-nitrobenzaldehyde (2a). Observations showed no improvement in the reaction efficiency. But the time of the reaction was somewhat more as compared to SWCNTs-SO3H (5 min vs 7 min).
3 Conclusions
In conclusion, in this research a heterogeneous and stable SWCNTs-SO3H was prepared and characterized by SEM, FT-IR spectroscopy, Raman spectroscopy, TGA and acid-base titration. For the first time, we report a new and efficient method for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes via condensation of 2-naphthol with different aromatic aldehydes using SWCNTs-SO3H. To the best of our knowledge, the use of SWCNTs-SO3H as a catalyst for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes has not been previously reported. We also introduced a robust, stable, eco-friendly and reusable catalyst as an efficient catalyst for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes. In addition, short reaction time, high yield of the desired product, solvent-free and non-toxicity of the catalyst are other worthwhile advantages of the present work.
4 Experimental method
4.1 Materials
The CNTs used in this study (L = 20–30 μm, D = 1–3 nm) have been purchased from Research Institute of Petroleum Industry (RIPI-Iran). The chemicals used in this work were purchased from Fluka and Merck companies.
4.2 Apparatus
Melting points were determined using melting point IA 8103 apparatus and are uncorrected. The thermal analysis (C, H, N) was carried out using a Carlo ERBA Model EA 1108 analyzer. FT-IR spectra were obtained with KBr pellets in the range of 400 to 4000 cm−1 with a Nicolet-860 spectrometer. FT-NMR spectra were recorded in CDCl3 solvent on a Brucker-Avance 500 MHz spectrometer using TMS as an internal standard. The Raman spectra were recorded with an Almega Thermo Nicolet Dispersive Raman spectrometer excited at 532 nm. TGA was performed on a mettle TA 4000 instrument at a heating rate of 10 K min−1. SEM images of CNTs were taken on a Philips XL30 SEM instrument.
4.3 Preparation of SWCNTs-SO3H
In a typical experiment; SWCNTs (1 g) and 100 ml of deionized water were added in a well becher and were sonicated for 15 min. After the sonication, the solvent was removed under reduced pressure and the obtained SWCNTs was transferred to another flask containing (HCl [37%], 25 ml; HNO3 [63%], 25 ml) and stirred at 80 °C for 4 h under a nitrogen atmosphere. Then the solution was filtered under reduced pressure and the obtained materials were first washed thoroughly with deionized water and then dried at 120 °C for 20 h. In this step (I), the SWCNTs-COOH was obtained. After the step (I), the SWCNTs-COOH (0.5 g) and 50 ml of deionized water were sonicated for 15 min. Then, the water was filtered and 40 ml H2SO4 (98%) was added to the set-up at 250 to 270 °C (the sulfuric acid boils at 335 °C) for 20 h under a nitrogen atmosphere. After cooling the solution to room temperature, the liquid was filtered and washed completely with deionized water for several times. The obtained solid materials were dried at 120 °C for 24 h. After this step (II), the SWCNTs-SO3H was obtained and used in the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes as a catalyst.
4.4 General procedure for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes in the presence of SWCNTs-SO3H under solvent-free conditions
In a 25-ml flask equipped with a condenser and magnetic stirring bar, a mixture of 2-naphthol (8 mmol), aldehyde (4 mmol) and SWCNTs-SO3H (50 mg) was heated at 70 °C under solvent-free conditions for the appropriate time according to Table 2. The progress of the reaction was monitored by TLC (EtOAc/n-hexane, 5:1). After completion of the reaction, the mixture was cooled to the room temperature and 30 ml CHCl3 was added. The catalyst was filtered by simple filtration. The obtained liquid was heated by evaporation on a rotary evaporator. The remained solid materials were recrystallized from ethanol to afford the pure products. All of the pure products were identified by FT-IR, 1H-NMR and melting points.
4.5 Acidity of SWCNTs-SO3H
The content of sulfonic acid groups in the SWCNTs-SO3H was determined by acid-base titration according to the literature [56]. The acid-base titration showed that the amount of SO3H attached to SWCNTs is 0.94 mmol g−1.
4.6 Reusability of the catalyst
Recovery and reusability of the SWCNTs-SO3H is a very important factor in practice and also from an economic point of view. The reusability of the SWCNTs-SO3H was investigated in the reaction of 2-naphthol (1) and 4-nitrobenzaldehyde (2a). At the end of the reaction, the catalyst was isolated by filtration, washed exhaustively with chloroform and ethanol, and dried at 80 °C for 10 h before being used with fresh 2-naphthol and aldehyde. The catalyst can be reused eight times without any loss in its catalytic activity.
4.7 Spectroscopic and physical data
14-(4-Nitrophenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 1, compound 3a): IR (KBr) (ν cm−1): 3070, 1621, 1589, 1513, 1340, 1239, 961, 806. 1H-NMR (500 MHz, CDCl3), δ (ppm): 6.63 (1H, s, CH), 7.46–7.49 (2H, t, 3J = 8 Hz, Ar-H), 7.53–7.55 (2H, d, 3J = 7.8 Hz, Ar-H), 7.62–7.65 (2H, t, 3J = 8 Hz, Ar-H), 7.70–7.71 (2H, d, 3J = 7.8 Hz, Ar-H), 7.86–7.89 (4H, m, Ar-H), 8.02–8.03 (2H, d, 3J = 8 Hz, Ar-H), 8.30–8.32 (2H, d, 3J = 7.8 Hz, Ar-H). Anal. Calcd for C27H17NO3: C, 80.38; H, 4.25; N, 3.47%. Found: C, 80.40; H, 4.33; N, 3.41%.
14-(3-Nitrophenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 2, compound 3b): IR (KBr) (ν cm−1): 3066, 1590, 1519, 1397, 1352, 1238, 1079, 958, 804, 745. 1H-NMR (500 MHz, CDCl3), δ (ppm): 6.64 (1H, s, CH), 7.30–7.34 (1H, t, 3J = 7.9 Hz, Ar-H), 7.46–7.49 (2H, t, 3J = 8 Hz, Ar-H), 7.54–7.56 (2H, d, 3J = 8 Hz, Ar-H), 7.63–7.66 (2H, t, 3J = 7.9 Hz, Ar-H), 7.83–7.89 (6H, m, Ar-H), 8.33–8.35 (2H, d, 3J = 8 Hz, Ar-H), 8.45 (1H, s, Ar-H). Anal. Calcd for C27H17NO3: C, 80.38; H, 4.25; N, 3.47%. Found: C, 80.45; H, 4.30; N, 3.37%.
14-(4-Cyanophenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 3, compound 3c): IR (KBr) (ν cm−1): 3026, 2221, 1635, 1591, 1558, 1500, 1449, 1374, 1245, 1169, 960, 810. 1H-NMR (500 MHz, CDCl3), δ (ppm): 6.54 (1H, s, CH), 7.12–7.15 (4H, m, Ar-H), 7.30–7.33 (4H, m, Ar-H), 7.55–7.58 (2H, m, Ar-H), 7.84–7.87 (4H, t, 3J = 8.2 Hz, Ar-H), 8.27–8.30 (2H, t, 3J = 8.2 Hz, Ar-H). Anal. Calcd for C28H17NO: C, 87.71; H, 4.47; N, 3.65%. Found: C, 87.55; H, 4.65; N, 3.64%.
14-(4-Chlorophenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 4, compound 3d): IR (KBr) (ν cm−1): 3068, 3026, 1620, 1591, 1484, 1242, 1082, 961, 807. 1H-NMR (500 MHz, CDCl3), δ (ppm): 6.50 (1H, s, CH), 7.13–7.15 (2H, d, 3J = 8 Hz, Ar-H), 7.44–7.52 (6H, m, Ar-H), 7.60–7.63 (2H, t, 3J = 7.9 Hz, Ar-H), 7.83–7.84 (2H, d, 3J = 7.9 Hz, Ar-H), 7.86–7.88 (2H, d, 3J = 8 Hz, Ar-H), 8.34–8.36 (2H, d, 3J = 7.8 Hz, Ar-H). Anal. Calcd for C27H17ClO: C, 82.54; H, 4.36%. Found: C, 82.59; H, 4.44%.
14-(2-Chlorophenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 5, compound 3e): IR (KBr) (ν cm−1): 3057, 1622, 1591, 1400, 1243, 1030, 960, 808. 1H-NMR (500 MHz, CDCl3), δ (ppm): 6.84 (1H, s, CH), 6.92–6.98 (2H, m, Ar-H), 7.28–7.30 (1H, m, Ar-H), 7.41–7.48 (3H, m, Ar-H), 7.51–7.53 (2H, d, 3J = 8 Hz, Ar-H) 7.64–7.67 (2H, t, 3J = 8 Hz, Ar-H), 7.82–7.86 (4H, m, Ar-H), 8.77–8.79 (2H, d, 3J = 7.9 Hz, Ar-H). Anal. Calcd for C27H17ClO: C, 82.54; H, 4.36%. Found: C, 82.62; H, 4.04%.
14-(Phenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 6, compound 3f): IR (KBr) (ν cm−1): 3069, 3016, 1591, 1513, 1398, 1247, 1076, 960, 808, 696. 1H-NMR (500 MHz, CDCl3), δ (ppm): 6.53 (1H, s, CH), 7.01–7.04 (1H, t, 3J = 7.8 Hz, Ar-H), 7.17–7.20 (2H, t, 3J = 7.9 Hz, Ar-H), 7.43–7.46 (2H, t, 3J = 7.8 Hz, Ar-H), 7.52–7.54 (2H, d, 3J = 8.1 Hz, Ar-H), 7.56–7.58 (2H, d, 3J = 8 Hz, Ar-H), 7.60–7.63 (2H, t, 3J = 8.1 Hz, Ar-H), 7.82–7.84 (2H, d, 3J = 7.9 Hz, Ar-H), 7.85–7.87 (2H, d, 3J = 8 Hz, Ar-H), 7.43–7.45 (2H, d, 3J = 8 Hz, Ar-H). Anal. Calcd for C27H18O: C, 90.47; H, 5.06%. Found: C, 90.55; H, 4.94%.
14-(3-Methylphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 7, compound 3 g): IR (KBr) (ν cm−1): 3047, 3016, 1619, 1587, 1515, 1249, 809. 1H-NMR (500 MHz, CDCl3), δ (ppm): 2.18 (3H, s, CH3), 6.46 (1H, s, CH), 6.80–6.82 (2H, d, 3J = 8 Hz, Ar-H), 7.05–7.08 (2H, d, 3J = 8.1 Hz, Ar-H), 7.40–7.61 (6H, m, Ar-H), 7.79–7.84 (4H, m, Ar-H), 8.40–8.42 (2H, d, 3J = 8 Hz, Ar-H). Anal. Calcd for C28H20O: C, 90.29; H, 5.41%. Found: C, 90.35; H, 5.35%.
14-(4-Methylphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 8, compound 3 h): IR (KBr) (ν cm−1): 3070, 3019, 1591, 1511, 1399, 1245, 962, 811, 741. 1H-NMR (500 MHz, CDCl3), δ (ppm): 2.16 (3H, s, CH3), 6.49 (1H, s, CH), 6.97–6.99 (2H, d, 3J = 8 Hz, Ar-H), 7.42–7.45 (4H, m, Ar-H), 7.50–7.52 (2H, d, 3J = 7.8 Hz, Ar-H), 7.59–7.62 (2H, t, 3J = 8 Hz, Ar-H), 7.80–7.82 (2H, d, 3J = 7.8 Hz, Ar-H), 7.84–7.86 (2H, d, 3J = 8 Hz, Ar-H), 8.42–8.44 (2H, d, 3J = 7.8 Hz, Ar-H). Anal. Calcd for C28H20O: C, 90.29; H, 5.41%. Found: C, 90.25; H, 5.15%.
14-(4-Isopropylphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 9, compound 3i): IR (KBr) (ν cm−1): 3048, 1621, 1578, 1510, 1244, 806. 1H-NMR (500 MHz, CDCl3), δ (ppm): 2.22 (6H, d, 2CH3), 3.1 (1H, m, CH), 6.46(1H, s, CH), 6.84–6.86 (2H, d, 3J = 8 Hz, Ar-H), 7.12–7.41(4H, m, Ar-H), 7.48–7.53 (4H, m, Ar-H), 7.68–7.71 (4H, m, Ar-H), 7.86–7.88 (2H, d, 3J = 8 Hz, Ar-H). Anal. Calcd for C30H24O: C, 89.97; H, 6.04%. Found: C, 90.25; H, 5.85%.
14-(3-Hydroxyphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 10, compound 3J): IR (KBr) (ν cm−1): 3451, 3056, 1593, 1512, 1237, 813, 697. 1H-NMR (500 MHz, CDCl3), δ (ppm): 4.55 (1H, s, OH), 6.47–6.50 (2H, m, CH, Ar-H), 6.91 (1H, s, Ar-H), 7.04–7.07 (1H, t, 3J = 8.1 Hz, Ar-H), 7.21–7.23 (1H, d, 3J = 8 Hz, Ar-H), 7.43–7.47 (2H, t, 3J = 7.8 Hz, Ar-H), 7.50–7.52 (2H, d, 3J = 8 Hz, Ar-H), 7.61–7.63 (2H, t, 3J = 8.1 Hz, Ar-H), 7.79–7.81 (2H, d, 3J = 7.8 Hz, Ar-H), 7.85–7.87 (2H, d, 3J = 8.1 Hz, Ar-H), 8.40–8.42 (2H, d, 3J = 8 Hz, Ar-H). Anal. Calcd for C27H18O2: C, 86.61; H, 4.85%. Found: C, 87.01; H, 4.84%.
14-(4-Methoxyphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 11, compound 3k): IR (KBr) (ν cm−1): 3042, 1625, 1594, 1512, 1251, 1236, 811. 1H-NMR (500 MHz, CDCl3), δ (ppm): 3.62 (3H, s, OCH3), 6.45 (1H, s, CH), 6.67–6.69 (2H, d, 3J = 7.9 Hz, Ar-H), 7.27–7.40 (4H, m, Ar-H), 7.42–7.44 (4H, m, Ar-H), 7.78–7.80 (2H, d, 3J = 8 Hz, Ar-H), 8.02–8.04 (2H, d, 3J = 8 Hz, Ar-H), 8.38–8.40 (2H, d, 3J = 7.9 Hz, Ar-H). Anal. Calcd for C28H20O2: C, 86.57; H, 5.19%. Found: C, 87.22; H, 4.79%.
14-(2-Methoxyphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 12, compound 3l): IR (KBr) (ν cm−1): 3055, 3023, 1592, 1404, 1242, 1156, 1100, 960, 807, 744. 1H-NMR (500 MHz, CDCl3), δ (ppm): 4.30 (3H, s, OCH3), 6.65–6.68 (1H, t, 3J = 7.8 Hz, Ar-H), 6.89–6.91 (1H, d, 3J = 8 Hz, Ar-H), 6.99 (1H, s, CH), 7.00–7.04 (1H, t, 3J = 7.8 Hz, Ar-H), 7.22–7.23 (1H, d, 3J = 8 Hz, Ar-H), 7.40–7.43 (2H, t, 3J = 8.1 Hz, Ar-H), 7.49–7.51 (2H, d, 3J = 8 Hz, Ar-H), 7.55–7.58 (2H, t, 3J = 8.1 Hz, Ar-H), 7.78–7.84 (4H, m, Ar-H), 8.60–8.62 (2H, d, 3J = 7.9 Hz, Ar-H). Anal. Calcd for C28H20O2: C, 86.57; H, 5.19%. Found: C, 86.95; H, 5.55%.
14-(2-Hydroxy-4-methoxyphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 13, compound 3 m): IR (KBr) (ν cm−1): 3398, 3156, 3057, 1594, 1508, 1462, 1399, 1228, 1102, 1010, 812, 746. 1H-NMR (500 MHz, CDCl3), δ (ppm): 4.30 (3H, s, OCH3), 5.90 (1H, s, OH), 6.65–6.68 (1H, m, Ar-H), 6.89–6.91 (1H, m, Ar-H), 7.14 (1H, s, CH), 7.00–7.04 (1H, m, Ar-H), 7.22–7.23 (1H, m, Ar-H), 7.40-–7.43 (2H, m, Ar-H), 7.49–7.51 (2H, m, Ar-H), 7.55–7.58 (2H, m, Ar-H), 7.78–7.84 (4H, m, Ar-H), 8.60–8.62 (1H, m, Ar-H). Anal. Calcd for C28H20O3: C, 83.15; H, 4.98%. Found: C, 83.65; H, 4.15%.
14-(5-Bromo-2-hydroxyphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 14, compound 3n): IR (KBr) (ν cm−1): 3535, 3431, 3057, 1603, 1460, 1405, 1253, 1189, 1120, 812. 1H-NMR (500 MHz, CDCl3), δ (ppm): 5.98 (1H, s, OH), 6.65–6.99 (2H, m, Ar-H), 6.89–6.93 (1H, m, Ar-H), 7.20 (1H, s, CH), 7.22–7.24 (1H, m, Ar-H), 7.26–7.28 (1H, m, Ar-H), 7.40–7.43 (2H, m, Ar-H), 7.49–7.51 (2H, m, Ar-H), 7.55–7.58 (2H, m, Ar-H), 7.78–7.82 (3H, m, Ar-H), 8.60–8.62 (1H, m, Ar-H). Anal. Calcd for C27H17BrO2: C, 71.54; H, 3.78%. Found: C, 70.98; H, 4.11%.
14-(4-Formylphenyl)-14H-dibenzo[a,j]xanthene (Table 2, entry 15, compound 3o): IR (KBr) (ν cm−1): 3060, 2923, 2852, 2765, 1691, 1595, 1513, 1243, 819. 1H-NMR (500 MHz, CDCl3), δ (ppm): 6.58 (1H, s, CH), 7.17–7.19 (2H, d, 3J = 8 Hz, Ar-H), 7.42–7.45 (2H, t, 3J = 7.8 Hz, Ar-H), 7.50–7.68 (4H, m, Ar-H), 7.70–7.78 (4H, m, Ar-H), 7.82–7.86 (2H, t, 3J = 7.6 Hz, Ar-H), 8.33–8.35 (2H, d, 3J = 8 Hz, Ar-H), 9.79 (1H, s, CHO). Anal. Calcd for C28H18O2: C, 87.02; H, 4.69%. Found: C, 87.15; H, 4.65%.
Acknowledgments
We gratefully acknowledge the financial support by the Malek-Ashtar University of Technology (MUT). We thank Dr. Rashidi from the Research Institute of Petroleum Industry (RIPI-Iran) for providing CNTs and SEM images of the catalyst.


