1 Introduction
Since a long time ago, environmental concerns have induced scientists to improve green systems in all the fields of science. Environmental pollution by hazardous organic solvents is a great challenge for organic chemists, which leads them to develop ecofriendly synthetic methodologies. By applying alternative reaction conditions, more efficient processes and new catalysts can be discovered, which redounds the preparation of innovative materials. Recently, through all the possible choices to reduce chemical pollution, ionic liquids (ILs) have attracted much attention due to their numerous advantages. IL, a unique alternative for organic solvents, is not only recyclable, stable and non-flammable, but also shows low vapor pressure. Unfortunately, some impediments, such as high cost and toxicity [1] have limited the chemical and industrial usage of ILs. On the other hand, deep eutectic solvents (DESs) are suitable alternatives. DESs are based on a combination of readily available and inexpensive components that are formed by mixing a quaternary ammonium salt with a simple hydrogen bond donor (HDB), such as urea, a carboxylic acid or a Lewis acid. Since their polar nature often makes DESs immiscible with organic solvents, work-up is easy. Furthermore, DESs show novel reactivity and selectivity for highly efficient synthesis of pharmaceutical products, agrochemicals, and natural products [2–11]
Tetraketones are important structural units in heterocyclic three-ring systems, such as xanthendione and acridindione. Meanwhile, tetraketones are also interesting due to their similar properties to those of 1,4-dihydropyridines, with similar structure to those of biologically important compounds, such as NADH and NADPH [12]. During the last few years, syntheses and biological application of tetraketone derivatives have received great interest. The tandem Knoevenagel condensations and Michael addition of aldehydes with active methylene compounds in the presence of acid or alkaline catalysts are widely used as important versatile precursors for the synthesis of tetraketones [2]. Because of their great practical importance, several methods have been proposed, employing different catalysts and promoters, such as NaOH [13], KOH [14], piperidine [15], proline [16], and cetyltrimethylammonium bromide (CTMAB) [17]. Furthermore, catalyst-free reactions in pure water [18], in the solid state, and in melts have been reported [19].
Natural products with xanthene heterocyclic motives have a great deal of importance because of their diverse ubiquitous pharmacological properties, such as antibacterial [20], antiviral and antinociceptive activities [21], In addition, due to their spectroscopic properties [22], they are used as fluorescent compounds in laser technology and in the monitoring of biomolecules. Considerable efforts have been made toward the one-pot, three-component synthesis of xanthenes annulated heterocyclic derivatives due to their wide applications. Among the numerous methods for the synthesis of xanthene, the condensation of aldehydes with active methylene in the presence of an acidic catalyst or a promoter is reported in the literature [23–32]. Some of these methods suffer from prolonged reaction times, high cost, or the catalysts’ sensitivity to moisture. Therefore, the development of simple and efficient procedures for the synthesis of xanthenes in the novel reaction media is a challenging task. Herein, we report an efficient three-component tandem synthesis of xanthenes and tetraketones from aldehydes and active methylene compounds in choline chloride-based deep eutectic solvents (DESs). To the best of our knowledge, this represents one of the first examples of the use of DES control in xanthenes and tetraketones synthesis by changing the DES component.
2 Results and discussions
As part of our continuous interest in developing green reaction media for the synthesis of useful compounds, we recently succeeded in developing the deep eutectic solvent as a green solvent and catalyst in organic synthesis [32–35]. Encouraged by these successful efforts and aiming to demonstrate the efficiency and generality of DES, we present in this paper a mild, reliable, efficient, and scalable synthesis of xanthene and tetraketone derivatives in a choline chloride-based deep eutectic solvent.
Choline chloride-based DESs were prepared according to the reported procedure and were used without further purification. Our initial effort was focused on the evaluation of choline chloride-based DESs systems for the model reaction of benzaldehyde 1 and dimedone 2 to optimize the reaction conditions and selectivity. The catalytic activities of various DESs were evaluated for this model reaction, and the results are summarized in Table 1. We found that all DESs catalyze this simple condensation reaction and provide the desired tetraketones 3 and xanthenes 4 in different proportions.
Investigation of the chemoselectivity of deep eutectic solvents (DES).
| Entry | DES | Yields (%) | |
| 3 | 4 | ||
| 1 | Choline chloride:urea (2:1) 1 mL | 92 | 00 |
| 2 | Choline chloride:malonic acid (1:1) 1 mL | 00 | 60 |
| 3 | Choline chloride:SnCl2 (1:2) 1drop | 85 | 00 |
| 4 | Choline chloride:ZnCl2 (1:2) 1 drop | 00 | 88 |
| 5 | Choline chloride:ZnCl2:SnCl2 (1:1:1) 1 drop | 00 | 85 |
| 6 | Choline chloride:LaCl3 (1:2) 1 drop | 60 | 30 |
| 7 | Choline chloride:PTSA (1:1) 1 mL | 20 | 60 |
| 8 | Choline chloride:glycerol(1:2) 1 mL | 75 | 10 |
The DES-based choline chloride–urea and choline chloride–SnCl2 provided tetraketones 3 in higher yields and shorter reaction times than methods using other DESs (Table 1, entry 8 vs entries 1–5). On the other hand, in choline chloride–ZnCl2 and choline chloride–malonic acid mixtures, the reaction proceeded selectively to generate xanthene derivatives 4.
With these optimized reaction conditions in hand, we continued to examine the substrate scope of the reaction using a range of functionalized aromatic aldehydes and dimedone condensation reaction in choline chloride–urea for tetraketones and choline chloride–ZnCl2 for xanthenes derivatives. The reaction was carried out in a simple manner using a mixture of aromatic aldehydes 1 (1.0 equiv) and dimedone 2 (2.0 equiv) in choline chloride–urea (1.0 mL) at 80 °C and was completed within 240 min to give the tetraketone derivatives 3 in good to excellent yields (Table 3). The reaction scope is quite broad with respect to a wide range of structurally varied aldehydes, and the electronic variation on the aryl aldehydes caused no appreciable changes in the efficiency of the condensations. Electron-rich, electron-poor, aromatic, heterocyclic, and sterically encumbered aldehydes were all well tolerated in these reaction conditions (Table 2).
Synthesis of xanthenes derivatives in ZnCl2:ChCl.
| Entry | ArCHO | R | Product | Yield (%)a | Mp | |
| Found | Reported | |||||
| 1 | C6H5 | Me | 4a | 88 | 203–205 | 203–204 |
| 2 | 4-OMe–C6H4 | Me | 4b | 78 | 222–223 | 222–223 |
| 3 | 4-NO2–C6H4 | Me | 4c | 75 | 242–245 | 242–244 |
| 4 | 4-Me–C6H4 | Me | 4d | 90 | 214 | 215–217 |
| 5 | 4-Cl–C6H4 | Me | 4e | 86 | 228 | 230–232 |
| 6 | 3-Cl–C6H4 | Me | 4f | 88 | 182–184 | 184–186 |
| 7 | 2-Cl–C6H4 | Me | 4g | 80 | 225–228 | 225–227 |
| 8 | 3-NO2–C6H4 | Me | 4h | 75 | 159 | 170–172 |
| 9 | 4-Br–C6H4 | Me | 4i | 90 | 238–241 | 240–241 |
| 10 | C6H5 | H | 4j | 82 | 261–263 | 269–270 |
a Isolated yields
Synthesis of tetraketone derivatives in urea–ChCl.
| Entry | ArCHO | Product | Yield (%)a | Mp | |
| Found | Reported | ||||
| 1 | C6H5 | 3a | 92 | 185 | 183–185 |
| 2 | 4-OMe–C6H4 | 3b | 88 | 145 | 142–145 |
| 3 | 4-NO2–C6H4 | 3c | 82 | 187–191 | 188–190 |
| 4 | 4-Me–C6H4 | 3d | 90 | 130–134 | 126–128 |
| 5 | 4-Cl–C6H4 | 3e | 82 | 141–143 | 136–142 |
| 6 | 3-Cl–C6H4 | 3f | 86 | 184–186 | 185–187 |
a Isolated yields.
In a further extension of these studies taking in consideration the impact of the DES on the products’ distribution, dimedone 2 (2.0 equiv) was treated with various aromatic aldehydes 1 (1.0 equiv) under identical conditions in the presence of ZnCl2–choline chloride (one drop); several representative examples are summarized in Table 3. The reaction was clean and proceeded smoothly to give the corresponding substituted xanthenes derivatives 4 in good to excellent yields. There were no remarkable difference in the yields and reaction time between aromatic aldehydes with electron-donating groups and those with electron-withdrawing groups.
In summary, we have developed a mild, fast and chemoselective procedure for the synthesis of various xanthenes and tetraketones with excellent yields in DES. This simple procedure is faster with high yields, and less laborious in isolation and purification procedures compared to the reported methods. Furthermore, the reaction was carried out in very mild conditions by just mixing the reactant in DES, with a simple purification method, as it involves filtration, washing with water, and recrystallization from ethanol in some cases.
The chemoselectivity of the reactions in DESs were established by analyzing the 1H NMR spectra of 3k and 4k. In urea–choline chloride-based DES, only 3k was isolated, in which 1H NMR analysis showed the presence of the OH group (11.88 ppm), which has vanished in 4k (Scheme 1)
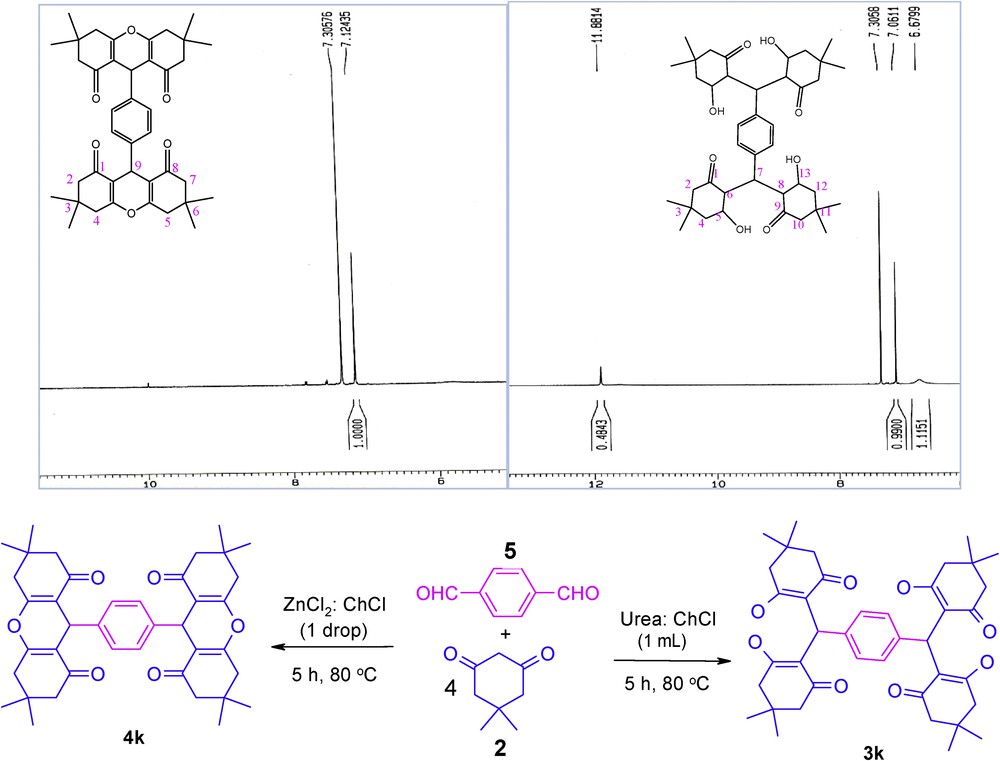
Synthesis of 3k and 4k and their 1H NMR spectrum.
3 Experimental
3.1 General
The melting points were determined on a Buchi 535 melting point apparatus and were uncorrected. 1H NMR spectra were recorded on a 500 MHz NMR spectrometer using CDCl3 as a solvent. Chemical shifts had been expressed in (ppm) downfield from TMS. All the starting materials and DES components, such as aldehydes, dimedone, choline chloride, malonic acid, SnCl2, ZnCl2, LaCl3, p-toluene sulfonic acid (PTSA), and glycerol were commercially available. All the reactions were monitored by thin layer chromatography (TLC) with UV light as a detecting agent.
3.2 Deep eutectic solvent preparations
Deep eutectic solvents were prepared according to the literature [10]. In all cases, choline chloride and the other components (Table 1) were mixed and heated; a clear and homogenized liquid was then obtained.
3.3 Typical experimental procedure
A mixture of an aldehyde (0.5 mmol) and dimedone (1 mmol) in DES (1 mL or 1 drop, Table 1) was heated at 80 °C under stirring for 2–6 h. After completion of the reaction (monitored with TLC and in some cases reaction solidified), water (10 mL) was added. The DES, being soluble in water, comes in the water layer. The solid was separated by filtration and recrystallized from ethanol and ethanol/water. DESs were reused for a second run without much loss of product yields after vaporization of water at a reduced pressure at 80 °C. All the compounds were characterized on the basis of their spectroscopic data (NMR) and melting points by comparison with those reported in the literature. In some cases, the pure products were pipetted off the top of the DES by adding ethyl acetate (10 mL), without adding water.
3.4 Selected spectral data
3.4.1 3b: 9-(4-methoxyphenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydro-2H-xanthene-1,8(5H,9H)-dione
Mp = 242–245 °C, 1H NMR (CDCl3, 500 MHz) δ = 1.03 (s, 6H, CH3), 1.14 (s, 6H, CH3), 2.19–2.28 (q, J = 9–15 Hz, 4H, CH2), 2.49 (s, 4H, CH2), 3.77 (s, 3H, CH3), 4.74 (s, 1H, CH), 6.78–6.80 (d, 2H, J = 10 Hz, ArH), 7.23–7.25 (d, J = 5 Hz, 2H, ArH).
3.4.2 4k: 3,4,6,7-tetrahydro-9-(4-(2,3,4,5,6,7,8,9-octahydro-3,3,6,6-tetramethyl-1,8-dioxo-1H-xanthen-9-yl)phenyl)-3,3,6,6-tetramethyl-2H-xanthene-1,8(5H,9H)-dione
Mp > 300, 1H NMR (500 MHz, CDCl3): δ H (ppm) 1.02 (s, 12H, CH3), 1.15 (s, 12H, CH3), 2.22 (s, 8H, CH2), 2.41–2.53 (m, 8H, CH2), 4.71 (s, 2H, CH), 7.12 (s, 4H, ArH).
3.4.3 3k: 2,2′-(1,4-phenylenebis((2-hydroxy-4,4-dimethyl-6-oxocyclohexyl)methylene))bis(3-hydroxy-5,5-dimethylcyclohex-2-enone)
Mp = 225 °C, 1H NMR (500 MHz, CDCl3): δ H (ppm) 1.12–1.16 (s, 12H, CH3), 1.26 (s, 12H, CH3), 2.26–2.53 (m, 16H, CH2), 3.30 (brs, 4H, CH2), 5.52 (s, 2H, OH), 6.67 (brs, 4H), 7.06 (s, 4H, ArH), 11.88 (s, 2H, OH).
4 Conclusion
In summary, we have developed an environmentally benign and green method for the synthesis of xanthenes and tetraketones in a biodegradable deep eutectic solvent based on choline chloride as a novel and efficient catalyst and reaction medium. Further studies in our laboratory are under way to develop multicomponent reactions in this green reaction medium.
Acknowledgment
Financial support of the Chemistry and Chemical Engineering Center of Iran is gratefully appreciated.


