1 Introduction
Thiazole and their derivatives are important heterocyclic compounds not only for intriguing biological activities and their versatile role in cell biochemistry, but also for particular importance as building blocks for medicinal chemistry and natural products [1,2]. Furthermore, they are estrogen receptors ligands and adenosine receptor antagonist and have been intensively studied as novel drug candidates for bacterial and HIV infections [3–5]. Therefore, many synthetic methods for the construction of thiazole rings in the presence of catalyst or activator have been developed [6–13]. Although these methodologies are useful tools and serve the synthetic requirements for simple thiazoles, most of them suffer from limitation, such as expensive catalyst, harmful organic solvent, and harsh reaction conditions and extended reaction times. In addition, one-pot condensation reactions of β-ketoesters, NBS and thioureas under catalyst-free conditions have not been reported. Due to their toxicity and flammability common organic solvents used in chemical, and separation processes, ionic liquids, also called molten salts, have been extensively replaced as environmentally friendly reaction media in the chemical industry and the laboratory. Ionic liquids have many properties that have led to their use in pharmaceutical, biomedical, and separation processes. They have many advantages, like low vapor pressure; they are also thermally stable in a wide temperature range. However, the main disadvantages of ionic liquids, such as toxicity, high cost, difficulty of preparation and biodegradability enforced the chemists to discover greener friendly reaction media. Related to ILs with similar properties with additional advantages are substances known as deep eutectic solvents (DESs) [14–22].
2 Experimental
2.1 General
All starting materials and DES components were commercially available or purchased from suppliers, such as Merck and Fluka. Melting points were determined on Buchi 535 and uncorrected. NMR spectra were recorded on Bruker 500 and 80 MHz spectrometers using DMSO and CDCl3 as solvents and TMS as an internal standard. FT–IR spectra were determined on a BrukerVector-22 infrared spectrometer using KBr disks. 1H NMR spectra were recorded at room temperature on a FT-NMR Bruker Ultra ShieldTM (500 MHz) or a Bruker AC 80 MHz instrument. Water and other solvents were distilled before use.
2.2 Choline chloride–urea-based DES preparation
The choline chloride–urea deep eutectic solvent was prepared according to the literature [15]. In a 250-mL Erlenmeyer flask with constant magnetic stirring, urea (200 mmol) and choline chloride (100 mmol) were mixed, and heated at 60 °C until a clear liquid appeared. The obtained deep eutectic solvent was used without any further purification (Fig. 1).
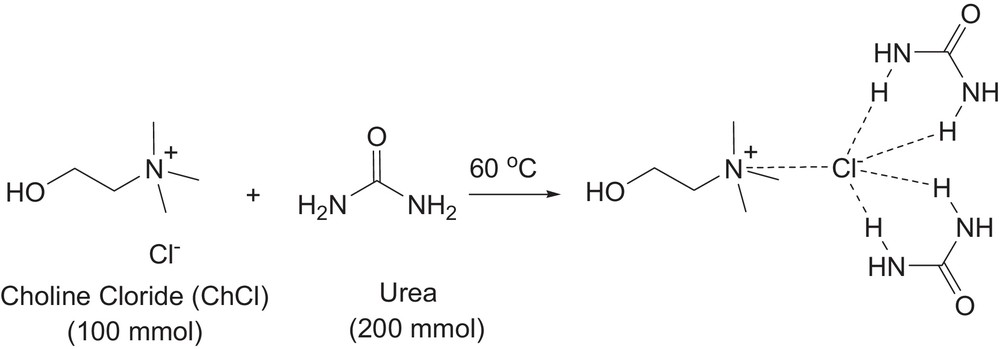
Deep eutectic solvent preparation from choline chloride and urea at 60 °C.
2.3 General procedure
A test tube equipped with a magnetic stir bar was charged with thiourea or urea (1.0 mmol), ethyl acetoacetate (1.0 mmol), NBS (1.0 mmol) and the DES (0.5 mL), and the resulting mixture was stirred at 60 °C until the reaction was complete. The reaction mixture was washed in water, and the solid residue was crystallized from ethanol or diethyl ether. The semisolid or liquid products were further purified by column chromatography (hexane/ethyl acetate). All compounds were known and were characterized by melting points found to be identical with the ones described in the literature.
3 Results and discussion
In continuation to our efforts in organic reactions in DES as green solvent and catalyst [23–27], herein we report the fast and catalyst-free tandem synthesis of 2-aminothiazole-5-carboxylates from β-ketoesters and thioureas with NBS in choline chloride-based DES. Our initial efforts were focused on the optimization of the reaction conditions for the synthesis of thiazole 3 by using ethyl acetoacetate 1, NBS and thiourea 2 in choline chloride–urea-based DES (0.5 mL) as model substrates. Control experiments on the model reaction showed that the reaction did not take place at room temperature and ethyl 2-bromoacetoacetate and thiourea were recovered after 2 h. Next, in the model reaction, the effect of temperature was investigated and the results suggest that temperature has a dramatic effect on the reaction yields. Increasing the reaction temperature to 60 °C afforded a quantitative yield of product within 20 min.
Under optimized reaction conditions, to extend the scope and generality of this procedure to the synthesis of thiazole derivatives, a range of β-ketoesters (1) were reacted with thiourea derivatives; the results are summarized in Table 1. Thiourea derivatives, such as thiourea, allylthiourea, thioacetamide, and thiobenzamide underwent cyclization reaction with a wide range of active methylene compounds such as ethyl acetoacetate, methyl acetoacetate, acetylacetone and 1,1,1-trifluoroacetylacetone under optimized reaction conditions.
Synthesis of substituted 2-aminothiazoles in a one-pot three-component reaction.
| Entry | Active methylene compounds | Thiourea | Product | Time (min) | Yields (%)a |
| 1 | R′′ = NH2 | 20 | 97 | ||
| 2 | R′′ = Ph | 100 | 65 | ||
| 3 | R′′ = CH3 | 80 | 82 | ||
| 4 | R′′ = N-allyl | 40 | 85 | ||
| 5 | R′′ = NH2 | 20 | 97 | ||
| 6 | R′′ = Ph | 100 | 72 | ||
| 7 | R′′ = CH3 | 70 | 78 | ||
| 8 | R′′ = N-allyl | 50 | 80 | ||
| 9 | R′′ = NH2 | 80 | 80 | ||
| 10 | R′′ = N-allyl | 100 | 78 | ||
| 11 | R′′ = NH2 | 100 | 75 | ||
| 12 | R′′ = N-allyl | 100 | 70 |
a Isolated yields.
In general, the reaction was carried out in a simpler manner, just by mixing three components in DES and heating the mixture at 60 °C, affording the corresponding thiazole 3 in quantitative yields after simple work-up. After the reaction was completed, water was added to the reaction mixture and the products were removed by filtration. The treatment of ethyl acetoacetate with NBS produces the ethyl 2-bromoacetoacetate intermediate, which then undergoes cyclization with thiourea to give the target products.
Functionalized oxazole derivatives are widespread structural units in natural products of various sources, synthetic intermediates, and pharmaceuticals [28–31]. Classical methods for oxazole synthesis include reaction of α-haloketones with urea under forcing conditions. Reaction of α-isocyanates with imines, or cycloadditions of acyl azides to alkynes and transition metal-catalyzed [3+2] cycloaddition reaction results were also reported in the literature [32–35].
Encouraged by this successful synthesis of thiazoles, the stage was set for a mild three-component synthesis of oxazoles from ketoesters and urea in the presence of NBS in deep eutectic solvent; the results are shown in Fig. 2. The reaction of various active methylene compounds with a wide variety of electronically various urea derivatives proceeds efficiently under mild conditions to give ethyl 2-amino-4-methyl-1,3-oxazole-5-carboxylate in good to excellent yields and short reaction times. This protocol for the synthesis of oxazoles requires two steps consisting of brominating of active methylene compounds, and cycloaddition of the urea to these intermediates in a one-pot reaction (Fig. 2).
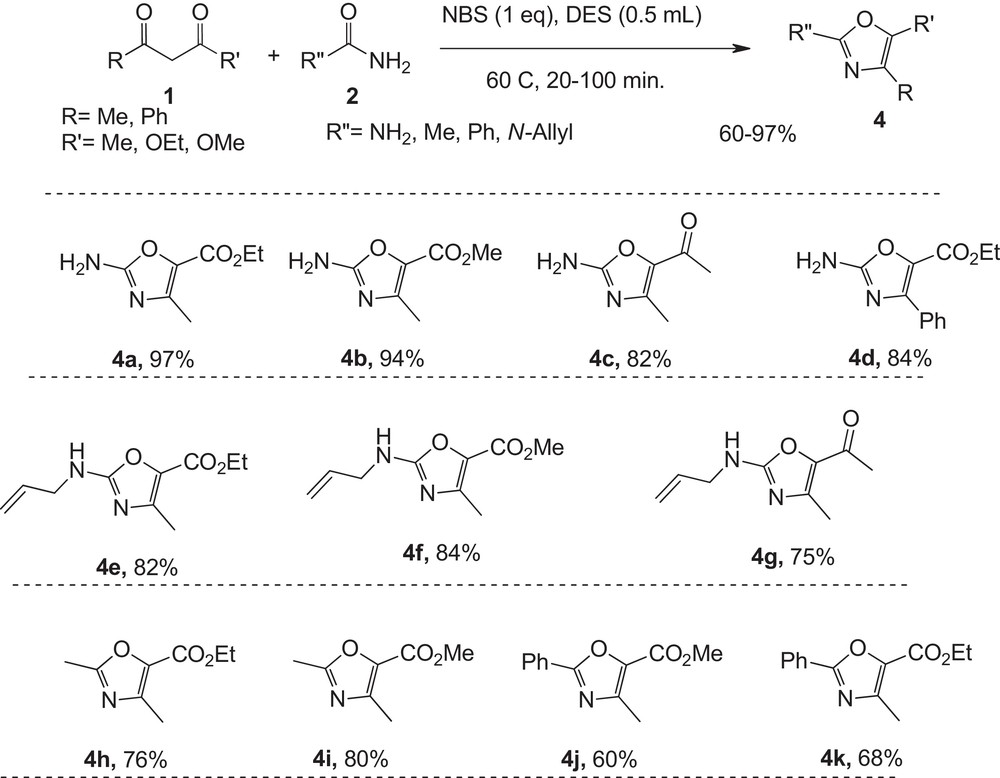
Green synthesis of 2-aminoxazole derivatives in DES.
4 Conclusion
In summary, we have developed a new and direct route to biologically relevant heterocyclic scaffolds of oxazoles and thiazole by an efficient consecutive three-component reaction involving active methylene compounds, urea or thiourea and NBS in a deep eutectic solvent. This method led to a catalyst-free route for the preparation of diversely functionalized 2-aminoxazoles and 2-aminothiazoles in good to excellent yields from readily available starting materials. In addition to short reaction times, wide scope of substrates, the use of biodegradable and inexpensive DES as solvent and catalyst are the distinct features of this procedure.
Acknowledgment
Financial support of Chemistry and Chemical Engineering Center of Iran is gratefully appreciated.


