1 Introduction
Ionic liquids (ILs) are versatile new media for many chemical synthesis, enzymatic catalysis and green engineering processes. In the last few years, room temperature ILs have been also used as clean solvents and catalysts for green chemistry, as electrolytes for batteries, in photochemistry and electrosynthesis [1–5]. Typically, these compounds have excellent chemical and thermal stability, negligible vapor pressure and many of them are described as environmentally friendly. In the last few years, ILs have been the subject of considerable interest in the context of green synthesis because of their wide acceptability as alternative reaction media [1,6,7] and have been found to act as selective catalysts for different reactions [8–13].
Apart from their potential advantages in recent years, there have been reasonable doubts about the overall safety and biodegradability of ionic liquids. The so-called protic ionic liquids (PILs) have shown a minor impact and a negligible toxicity, retaining many of the features previously observed in other analogous substances [14]. Due to this fact, the scope of this paper, and in general, our research line is focused on PILs and their potential applications in different industrial areas [15–21]. The main drawback in using ILs in homogeneous catalysis is the separation from the reaction media and the re-usability of the catalyst. The solution to this problem is the heterogenization of ILs on solid supports. Immobilization and supporting of ILs can be achieved by simple impregnation, covalent linking of the cation or the anion, polymerization, etc. [22–24]. Compared to pure ILs, immobilized ILs facilitate the recovery and reuse of the catalyst.
Previous reports describe the immobilization of ILs by adsorption or grafting onto silica surface and their use as catalysts for reactions, like Friedel–Crafts acylation [22], hydrogenation [25] and hydroformilation [26]. Organic polymers [12], natural polymers [27] and zeolites [28] have also been used as supports for ILs.
With this fact in mind, and as a continuation of a wider project to develop and study the applications of new PILs, in this work, we describe the catalytic activity and recycling of alanine-supported PILs for a series of aldol condensation processes. The PILs of this study consist of substituted amine cations of the form RXN+H (X is the number of alkyl substitutions) combined with organic anions of the form R′COO– (being of different nature R and R′). These short aliphatic chain PILs have low cost of preparation and only need simple synthesis/purification processes. Moreover, the very low toxicity and the degradability of this kind of PILs were verified [29]. Thus, sustainable processes can be originated from their use. By immobilization of the studied PILs on alanine, heterogeneous catalysts are obtained, which can be easily recycled and reused for several consecutive runs. The immobilization was done by impregnation of the support material with the PIL diluted with ethanol, followed by the removal of the solvent by evaporation.
On the other hand, it is a continuous challenge to find new catalysts able to perform with good activities and selectivity condensation reactions for the synthesis of pharmaceutical and fine chemicals and thus, replacing the homogenous catalysts used in the industrial production [30,31]. In order to determine the catalytic activity of the studied PILs, two condensation reactions of carbonyl compounds were carried out. The products obtained from these reactions are applied in pharmacological as well as flavor and fragrance industry.
In our previous two articles [15,18], we performed a complete study regarding the synthesis, the physicochemical properties, the catalytic activity and the recycling of this new family of PILs. An efficient recovery process of the PILs was developed, but it took about 17 h and for us, it represented an aspect of the process, which should be improved. Therefore, the main objective of the present study is to improve the recycling process by supporting the PILs onto a solid support, alanine.
2 Experimental
The studied PILs were 2-hydroxy ethylammonium formate (2-HEAF), 2-hydroxy ethylammonium acetate (2-HEAA), 2-hydroxy ethylammonium propionate (2-HEAPr), 2-hydroxy ethylammonium butanoate (2-HEAB), 2-hydroxy ethylammonium iso-butanoate (2-HEAiB) and 2-hydroxy ethylammonium pentanoate (2-HEAPe). The synthesis of these PILs, which is described elsewhere [15,18], is a simple acid–base neutralization reaction, creating the corresponding salt of ethanolamine, which in a general form, should be expressed as described in eq. (1). In this equation, X is the number of ethanol substitutions into the amine compound, Y is the number of protons (X + Y = 3) and R is the aliphatic end of the corresponding organic acid. For example, when X = 1, Y = 2, and R = CH3, this equation shows the chemical reaction for the reactants monoethanolamine + acetic acid and 2-hydroxy ethylammonium acetate (2-HEAA) as the neutralization product.
(1) (HOCH2CH2)XNHY + HOOC–R → (HOCH2CH2)X (–OOC–R)
The reagents, monoethanolamine (Merck Synthesis, > 99%) and the organic acids (Merck Synthesis, > 99%) were used without further purification.
In order to obtain the supported PILs, 1 g of PIL was dissolved in 7 mL of ethanol and, after stirring at room temperature for 30 min, 1 g of alanine was added. The mixture was stirred for 2 h and then, heated at 348 K under vacuum to remove ethanol. The supported PILs, thus, obtained were labeled hereafter as a-PILs.
The FT–IR spectra were taken by a Jasco FT/IR 680 plus model IR spectrometer, using a NaCl disk.
The studied reactions were the condensation between citral and acetone and the synthesis of benzylideneacetone. The reactions were performed in liquid phase using a 100-mL batch reactor equipped with a condenser system. To a stirred solution of substrate and ketone (molar ratio ketone/substrate = 4.4) was added 1 g of a-PIL, and the flask was maintained at 333 K using an oil bath. For the citral–acetone condensation, the reaction mixture contained 5.26 g of citral (34.55 mmol), 8.83 g of acetone (152.03 mmol), and 1.527 g of tetradecane (7.7 mmol). In the case of benzaldehyde–acetone condensation, the quantities were: 3.18 g of benzaldehyde (29.96 mmol), 7.65 g of acetone (131.71 mmol), and 1.527 g of tetradecane (7.7 mmol). Samples were taken at regular time periods and analyzed by gas chromatography using a flame ionization detector and an AG Ultra 2 column (15 m × 0.32 mm × 0.25 μm). Tetradecane was used as the internal standard. The reagents (citral 95%, acetone > 99.5%, benzaldehyde > 99%, tetradecane > 99.5%) were purchase from Sigma–Aldrich and used without further purification.
For the repeated runs at the end of the reaction, the catalyst was filtered from the reaction media and washed several times with acetone in order to eliminate the residues adsorbed on the catalyst surface.
3 Results and discussion
Alanine was chosen as support because it is a cheap, commercially available amino acid. Fig. 1 presents the IR spectrum of the a-2-HEAB and, for comparison purpose, the spectra of the free 2-HEAB and alanine.
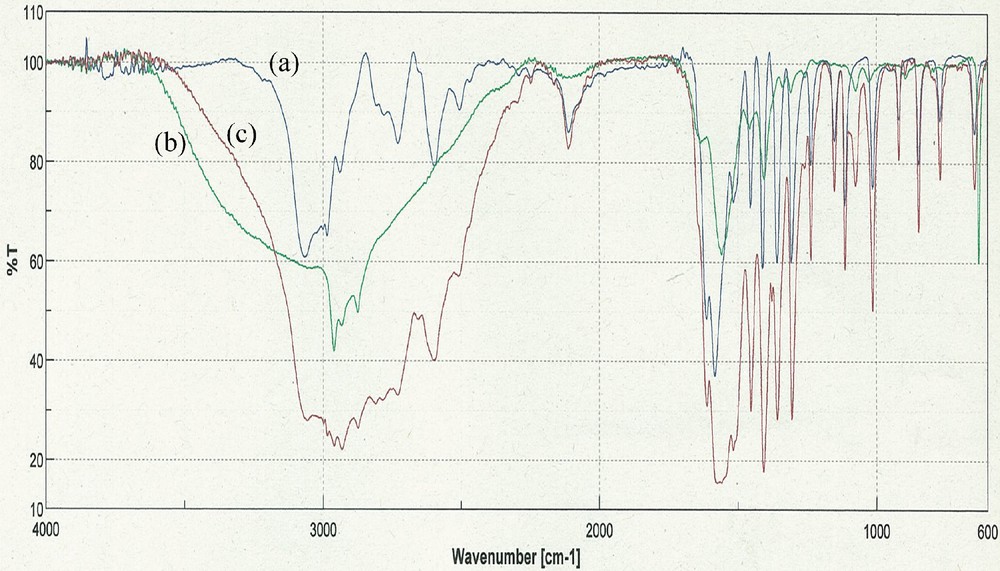
FT–IR spectra for: (a) alanine, (b) 2-HEAB and (c) a-2-HEAB. Colour available online.
The broad band in the 3500–2400 cm−1 range, characteristic of the ammonium structure of the PIL, is present in the supported PIL as well. Also, the broad band centered at 1600 cm−1, which is a combined band of the carbonyl stretching and N–H plane bending vibrations in the structure of the free PIL, is also present in the supported PIL. Based on this information, we can conclude that after the immobilization on alanine support, the structure of the PILs is conserved intact.
The same bands are present in the free PIL and in the supported PIL spectra; moreover, in the supported PIL spectra, the intensity of the bands is increased, indicating that the immobilization of the PIL on the alanine took place by adsorption on the support.
The catalytic activity of the alanine-supported PILs (a-PILs) was tested in the same reactions as the free PILs, see results of previous works [15,18].
The condensation reaction between citral and acetone (Scheme 1) leads to the formation of pseudo-ionone, an important precursor of the synthesis of vitamin A. Commercial citral consists of two isomers, neral and geranial, caused by the cis–trans isomerism at the CC bond near the aldehyde group [32].

Condensation reaction between citral and acetone.
The catalytic activity results of the a-PILs for the citral–acetone condensation are presented in Table 1. After 6 h of reaction, the two isomers of citral can be converted into the corresponding pseudo-ionone with conversions between 30 and 56%, except for a-HEAiB, for which a conversion of 9% was obtained. The most active PIL for this reaction is a-2-HEAA, which gives a conversion of 56%. The selectivity obtained in this reaction ranges between 48 and 80%. No trace of diacetone alcohol derived from the self-condensation of acetone was found, but other secondary products coming from the self-condensation of citral and oligomers derived from citral are detected in the reaction mixture. The support, alanine (entry 1), is not catalytically active.
Conversion after 6 h for citral–acetone condensation catalyzed by a-ILs.
| Entry | Catalyst | Conversion (%) | Selectivity (%) |
| 1 | Alanine | 0 | 0 |
| 2 | a-2-HEAF | 30 | 61 |
| 3 | a-2-HEAA | 56 | 74 |
| 4 | a-2-HEAPr | 49 | 80 |
| 5 | a-2-HEAB | 35 | 63 |
| 6 | a-2-HEAiB | 9 | 52 |
| 7 | a-2-HEAPe | 33 | 48 |
The catalytic activity of a-PILs was also tested in the production of benzylidenacetone (Scheme 2) from the condensation between benzaldehyde and acetone. In this reaction, the first step is the deprotonation of an acetone molecule to give the enolate anion whose nucleophilic attack on the CO group of benzaldehyde leads to the β-aldol. This latter is easily dehydrated on weak acid sites and benzylidenacetone is obtained.
Condensation reaction between benzaldehyde and acetone.
After 2 h of reaction, a conversion of 98–99% is achieved for the majority of a-PILs, while a lower conversion (78%) is obtained for a-2-HEAiB (Table 2). The selectivity toward benzylidenacetone is around 80–85%, due to the formation of dibenzylidenacetone as secondary product. The support, alanine (entry 1) is not active for citral–acetone condensation.
Conversion after 2 h for benzaldehyde–acetone condensation catalyzed by a-ILs.
| Entry | Catalyst | Conversion (%) | Selectivity (%) |
| 1 | Alanine | 0 | 0 |
| 2 | a-2-HEAF | 99 | 83 |
| 3 | a-2-HEAA | 99 | 82 |
| 4 | a-2-HEAPr | 99 | 85 |
| 5 | a-2-HEAB | 99 | 84 |
| 6 | a-2-HEAiB | 78 | 82 |
| 7 | a-2-HEAPe | 98 | 80 |
It is noteworthy that for both studied reactions, the conversions obtained with the a-PILs are in the same range as the ones obtained with free PILs [15,18] (Figs. 2 and 3).
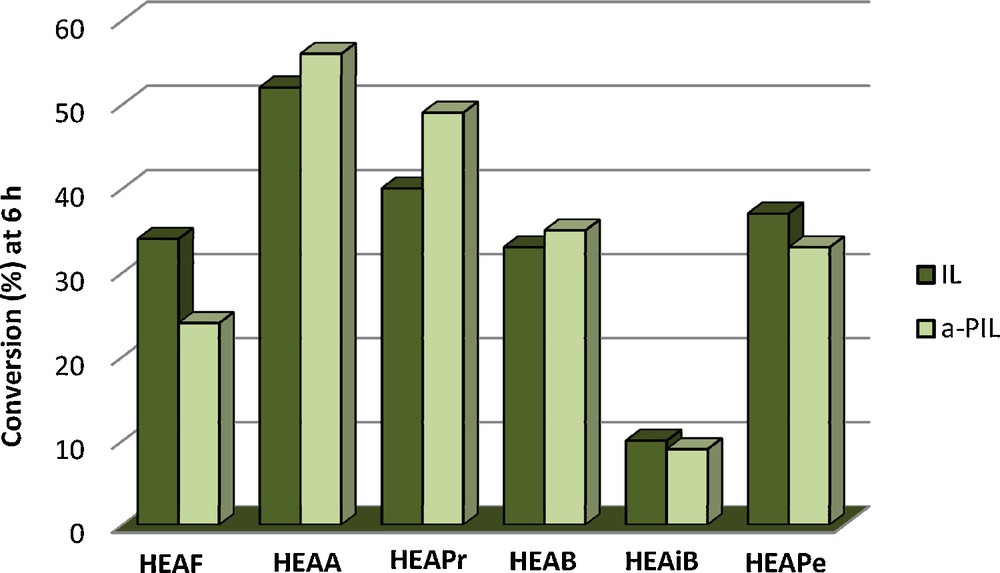
Conversion after 6 h for citral–acetone condensation for free PILs and a-PILs.
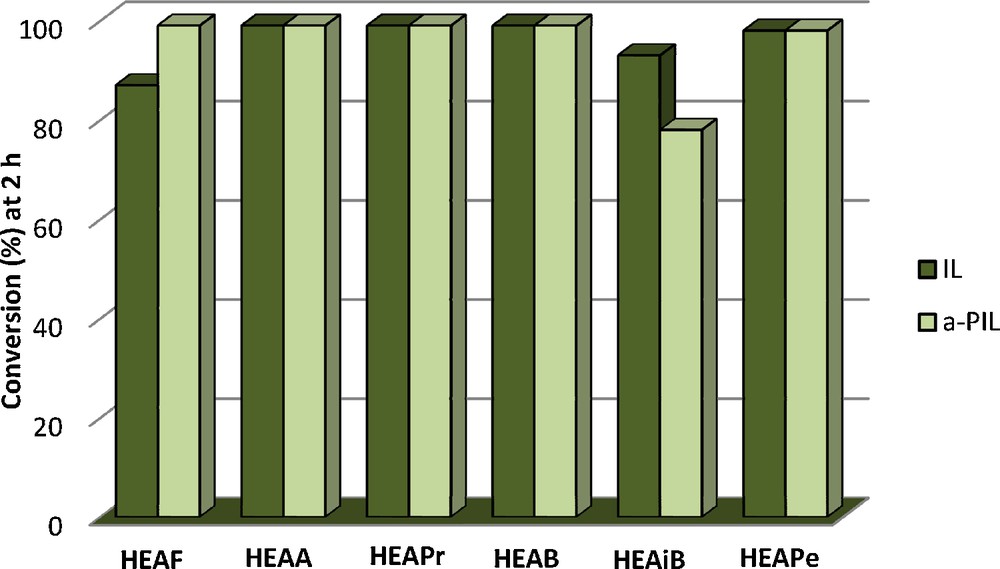
Conversion after 2 h for benzaldehyde–acetone condensation for free PILs and a-ILs.
The a-PILs are easily separated from the reaction mixture and reused. For the consecutive runs, we chose the condensation between benzaldehyde and acetone as a model reaction. The catalysts were recycled for three consecutive runs and a very good conversion was obtained in all the runs. The results are presented in Fig. 4.
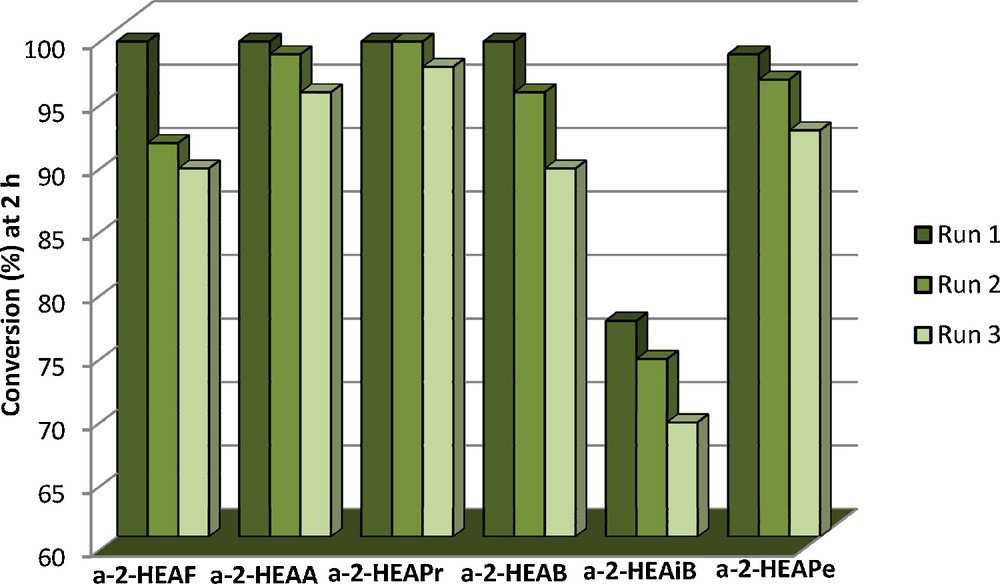
Benzaldehyde–acetone condensation: consecutive runs.
In the case of each PIL, only a negligible loss of activity is detected in the second and third run ranging between 6–10%, except in the case of a-2-HEAPr and a-2-HEAA, for which the activity decreased with only 2% and 4%. The activity loss can be attributed to the possible adsorption of the reactants or reaction products to the active sites of the catalyst which were not eliminated in the washing step and also, to some extent, to the leaching process.
4 Conclusions
A series of six PILs were immobilized on alanine, a cheap, readily available amino acid. These short aliphatic chain PILs have a low cost of preparation, need simple synthesis/purification processes, and moreover, have a very low toxicity. By their immobilization on the support, active heterogeneous catalysts were obtained.
Their catalytic potential was tested for citral–acetone and benzaldehyde–acetone condensations, two reactions with interest for fine chemistry industry. Good results were obtained in terms of conversion and selectivity; moreover, the catalysts can be recycled and reused for three consecutive cycles without significant loss of activity.
The recovery process of the PILs developed in the previous study [18] has been improved in the present study by supporting the PILs onto alanine and thus, obtaining heterogeneous catalysts that can be easily separated from the reaction media and reused. Taking into account that the recovery process was reduced from 17 h to a few minutes, we consider that the main objective of this work has been achieved.
Acknowledgments
Ministerio de Educación y Ciencia Spain CTQ2006-08196/PPQ and CTQ2009- 12520-C03-02 are acknowledged for financial support.
M. Iglesias would like to acknowledge the Fundacion Ibercaja (Programa de Excelencia en Investigacion 2009) for its support in developing this research.
R. Gonzalez-Olmos would like to acknowledge the Ministerio de Ciencia e Innovación (Spain) and the Juan de la Cierva program for their financial support (JCI-2010-07104)


