1 Introduction
The resistance towards available drugs is rapidly becoming a major worldwide problem. The need to design new compounds to deal with this resistance has become one of the most important areas of research today. Thiadiazoles have been of great interest as antitumor compounds for several scores of years [1–3]. Recent literature shows that 1,3,4-thiadiazole derivatives have received considerable attention due to their synthesis and biological importance. They exhibit a wide spectrum of interesting pharmacological properties, such as anticonvulsant [4], antituberculosis [5], analgesic [6], and leishmanicidal [7] activities, in addition to cytotoxic effects (on human non-small cell lung cancer A549) [8] as well as potent and selective PDE7 inhibitors [9]. Moreover, compounds bearing the 1,2,3-thiadiazole ring system have been reported to show antifungal [10], antitumor [11], antihistaminic [12], insecticidal [13], and antithrombotic activity [14]. The fused chromene-2-thione derivatives show various biological properties, including anticoagulant [15], anti-inflammatory and HIV-1[16] activities.
1,3-Dipolar cycloaddition is one of the most useful reactions for the synthesis of five-membered ring heterocycles [17,18], due to a high degree of site-, regio- and stereoselectivity. Furthermore, these reactions have attracted the attention of chemists from the standpoint of reaction mechanisms. Nitrilimines are known to be among the active dipoles in 1,3-dipolar reactions, and have been extensively investigated from the viewpoint of synthetic utility and of elucidation of the reaction mechanism of 1,3-dipolar reactions [19,20].
The present work is prompted by the previously well-established results, describing the regioselective behavior of aromatic nitrilimines with thione-containing compounds. In this context, we investigated the alkylation of 4-mercapto-2H-chromene-2-thione 1, as this was expected to provide dipolarophiles 2 and 3 with better yields and was successfully used as suitable starting materials for implementing this goal. The cycloaddition of diarylnitrilimines to the CS double bond compound 2 was strictly regioselective and afforded a mixture of both regioisomer of new spiro cycloadduct thiadiazoles 5a–d and 6a–d. In similar reaction conditions, 4-(allylthio)-2H-chromene-2-thione 3 proceeded with exclusive site selectivity, via 1,3-dipolar cycloaddition, and afforded only the major regioisomers of the mixture spiro-chromene[1,3,4]thiadiazoles 7a–d and 9a–d.
2 Results and discussion
Our first key intermediate was the 4-(methylthio)-2H-chromene-2-thione 2, which was obtained in good yield (70%) by stirring a mixture of 4-mercapto-2H-chromene-2-thione 1 and (5 equiv) N,N,dimethylformamide dimethyl acetal (DMFDMA) [21] during 3 h in dry toluene (Scheme 1). The mass spectrum, recorded in ESI–HRMS, showed for product 2 the molecular ion peak [M+H]+ at m/z 209.0096, which is consistent with the molecular formula of C10H9OS2, as well as the 1H NMR spectrum showed a singlet at 2.56 ppm, corresponding to the methyl group.
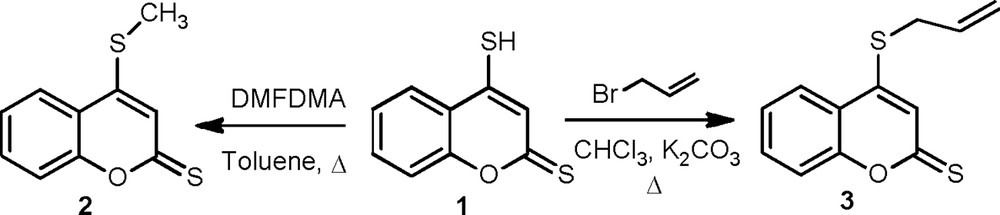
S-Alkylation of 4-mercapto-2H-chromene-2-thione 1.
Likewise, we have studied the behavior of derivative 1 with an excess of allyl bromide [22] in the presence of potassium carbonate under refluxing dry CHCl3; the S-alkylation process took place and gave the 4-(allylthio)-2H-chromen-2-thione 3 (Scheme 1). Compatible ESI–HRMS and spectral measurements for this compound were gained (See Experimental section).
A survey of the literature revealed a lack of information related to the synthesis of two regioisomer spirothiadiazoles via the 1,3-dipolar cycloaddition reaction. Most of the work that we have found described the condensation of dipolarophile (CS) with an equivalent amount of a diarylnitrilimine as a route to the only spiro cycloadduct [23–25].
Thus, prompted by these findings, we studied the condensation of the 4-(methylthio)-2H-chromene-2-thione 2, under refluxing dry chloroform, with a stoichiometric amount of diarylnitrilimines (generated in situ via triethylamine dehydrohalogenation of the corresponding hydrazonoyl chlorides 4a–d) [26], completed in 24 h as shown by TLC, and afforded, in each case, two regioisomer products (Scheme 2). The structure of the isolated products were established to be 4′-(4-R-phenyl)-4-(methylthio)-2′-phenyl-2′H-spiro[chromene-2,5′-[1,2,3]thiadiazole] 5a–d and 5′-(4-R-phenyl)-4-(methylthio)-3′-phenyl-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] 6a–d based on the spectroscopic measurements (1H, 13C NMR, 2D and ESI–HRMS).
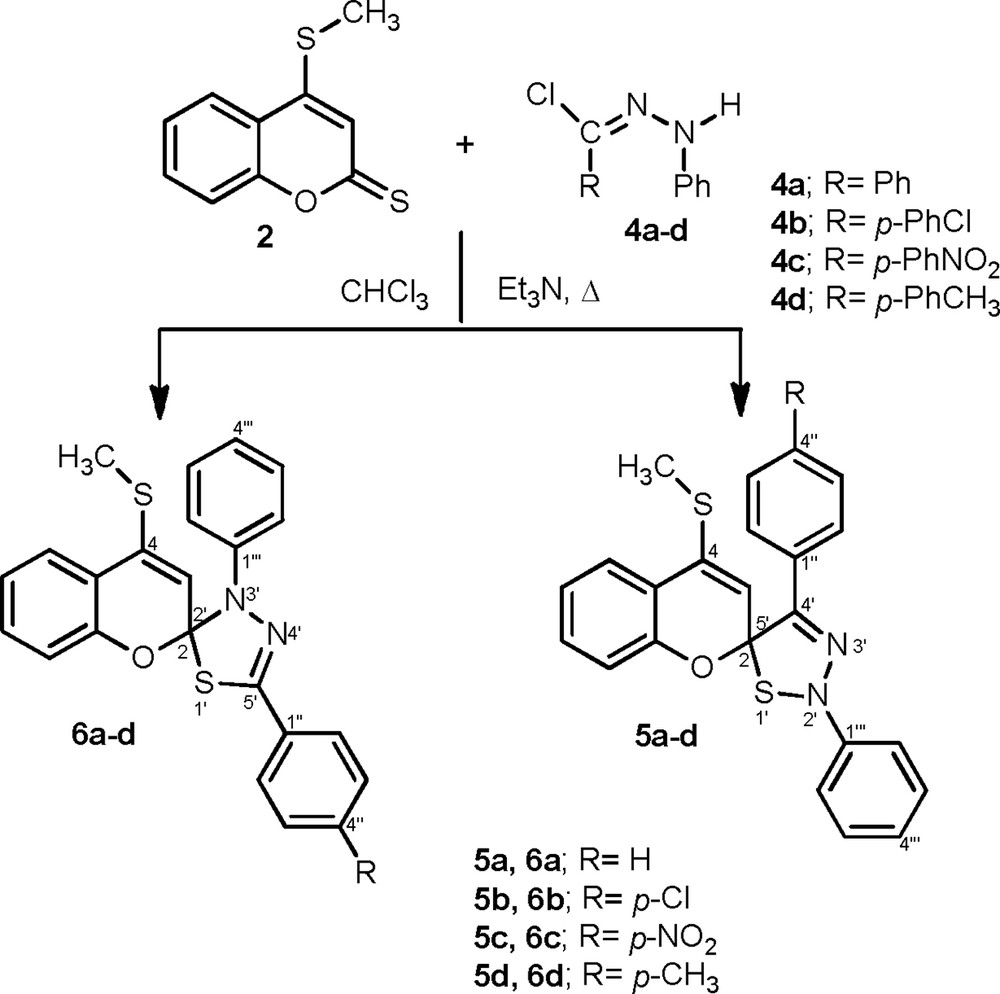
Synthesis of spiro-chromene[1,2,3]thiadiazole 5a–d and spiro-chromene[1,3,4]thiadiazole 6a–d.
The comparison of the spectral data of the cycloadducts 5c and 6c shows that the two compounds have the same spirothiadiazoles skeleton. Indeed, the mass spectra recorded in ES+ mode are identical and showed for both compounds the molecular ion peaks [M+H]+ at m/z 448.1. Moreover, the assignment of regiochemistry to 5c and 6c cannot be differentiated by analysis of standard 1H and 13C NMR spectra. For this reason, the only difference in cyclization between the two compounds occurred at the C-2 spiro center, as judged by the NOESY experiments, by the observation of a dipolar interaction between the ethylenic proton H-3 and aromatic protons fixed at the nitrogen, which confirms the structure of the cycloadduct 6c.
On the other hand, the 13C NMR, reinforced by HMBC for derivative 6c, indicates the presence of characteristic signals based on their chemical shifts, readily assigned the new spiro center C-2 and the iminic carbon C-5′ at δC 112.5 and at 138.6 ppm, respectively. Additional supports for our assignments were provided by a HMBC experiment that revealed significant correlations between the aromatic protons H-2′′,6′′ with carbons C-5′ (138.6 ppm) and C-4′′ (146,7 ppm). The same spectrum showed the correlation of the ethylenic proton H-3 with both quaternary carbons C-2 and C-4a at 138.6 ppm and at 118,6 ppm, respectively.
On the other hand, the comparison of the 13C NMR data of the spiro cycloadduct 5c to those of 6c suggest that this compound did not have the same regiochemistry, showing the new generated spirocyclic quaternary carbon C-2 at δC 100.9 ppm and the iminic carbon C-4′ at δC 149.0 ppm. However, the relative deshielding of the iminic carbon (C-4′ δC 149.0 ppm) in cycloadduct 5c, due to the absence of the sulfur atom, reinforces the proposed structure. The reaction is thus regioselective.
Analogously, 4-(allylthio)-2H-chromene-2-thione 3 reacted with a series of diarylnitrilimines 4a–d [26] under the same previously described reaction conditions, and gave two types of spiroheterocycles, 7a–d and 9a–d. For example, the two spiroheterocycles 7c and 9c were obtained in the amount of the product ratio 14:75. Once again, the spiroheterocycles 7c and 9c were obtained preferentially. Indeed, the 1,3-dipolar cycloaddition reaction did not afford the corresponding regioisomer cycloadducts 8c and 10c, which could be formed as minors; it was difficult to isolate them quantitatively (Scheme 3).
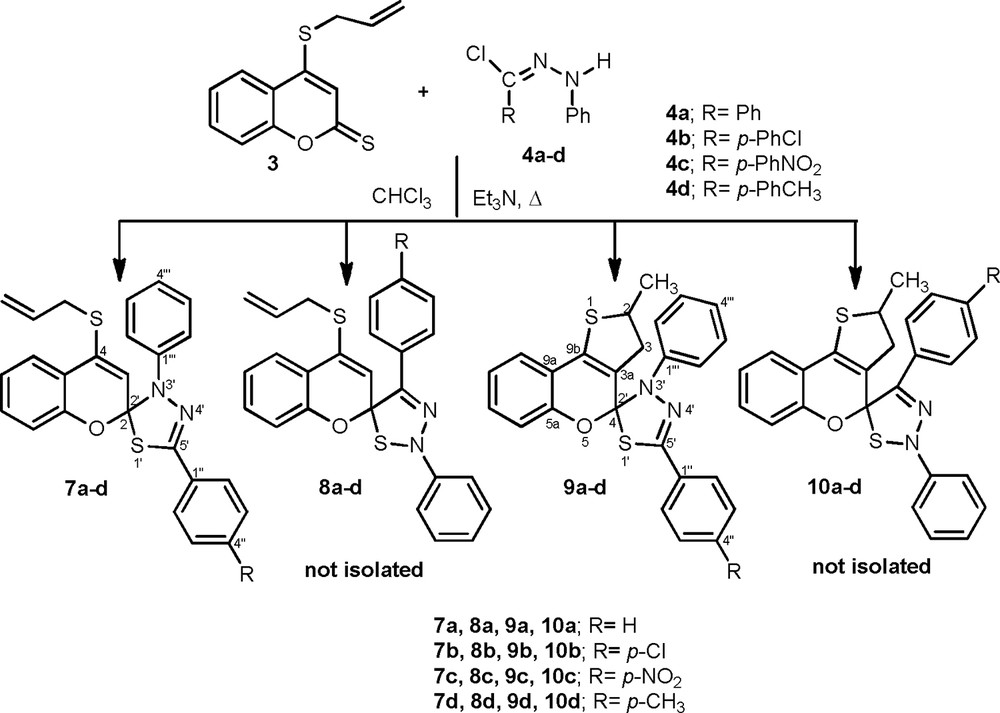
Synthesis of spiro-chromene[1,3,4]thiadiazoles 7a–d and 9a–d.
The structures of cycloadducts 7a–d and 9a–d were confirmed on the basis of their spectroscopic data (ES–MS, 1H, and 13C NMR). Indeed, the mass spectrum recorded in ES+ mode showed for both products 7c and 9c molecular ion peaks [M+H]+ at m/z = 474.1. Furthermore, the structural assignment of the products 7c and 9c was made on the basis of 1H and 13C NMR spectral analysis and by comparison of their spectroscopic data with those reported for related systems. In particular, 1H NMR analysis clearly indicated that the cycloadduct 7c maintains the allylic system. In addition, the 13C NMR spectrum showed two signals at δC 113.3 and 139.6 ppm, attributable to the spirocarbon C-2 and to the iminic carbon C-5′, respectively. This value located the spiro center near the electronegative nitrogen atom of the thiadiazole ring.
Indeed, the 1H NMR spectrum of 9c revealed the two methyl groups as two doublet signals at δH 1.06 and 1.37, corresponding to the major (CH3b, J = 6.6 Hz) and minor (CH3a, J = 6.6 Hz) diasterioisomers, respectively, together with a multiplet signal at δH 3.81 relative to the proton H-2a,b. In addition, the major diasterioisomer H-3b exhibits two closely spaced doublet of doublets (δH 2.76 J = 15.6 Hz, J = 6.9 Hz and δH 2.83 J = 16.2 Hz, J = 8.1 Hz), while the minor diasterioisomer H-3a exhibits two widely spaced doublet of doublets (δH 2.28 J = 16.5 Hz, J = 3.6 Hz and δH 3.21 J = 16.5 Hz, J = 8.7 Hz). The same spectrum showed two doublets at δH 7.98 (J = 8.1 Hz, H-2′′,6′′) and 8.17 (J = 8.1 Hz, H-3′′,5′′) in addition to the multiplet for the aromatic protons at δH 6.91–7.39 ppm.
The 13C NMR spectrum of 9c was also a good support for the proposed structure, since it exhibited characteristic signals at δC 22.5; 23.5, 44.0; 44.3 and 44.4 ppm, corresponding to the carbons of the methyl groups, to C-3a,b and C-2a,b of the 2-methyl-2,3-dihydrothiophene moiety, respectively. Furthermore, the comparison of the 13C NMR data of spirothiadiazole 9c with those of compound 7c confirmed that compound 9c shows the same regiochemistry, with the spiro carbon C-4 appearing at δC 113.8 ppm. Moreover, the regiochemistry was also confirmed by the NOE observed between the protons CH3a,b and H-2a,b and the aromatic protons fixed at the nitrogen.
With the aim of understanding if compounds 9a–d were obtained starting from compounds 7a–d or directly starting from the rearranged compound 13 formed as explained in Scheme 4 [27,28], we subjected compound 3 under reflux of chloroform in the absence of the diarylnitrilimines, and we also applied the same conditions to compound 7c. This allowed us to conclude that cycloadducts 9a–d could be obtained at the same time from the reaction of cycloaddition on the rearranged 2-methyl-2H-thieno[3,2-c]chromene-4(3H)-thione 13 and from the rearrangement of compounds 7a–d.
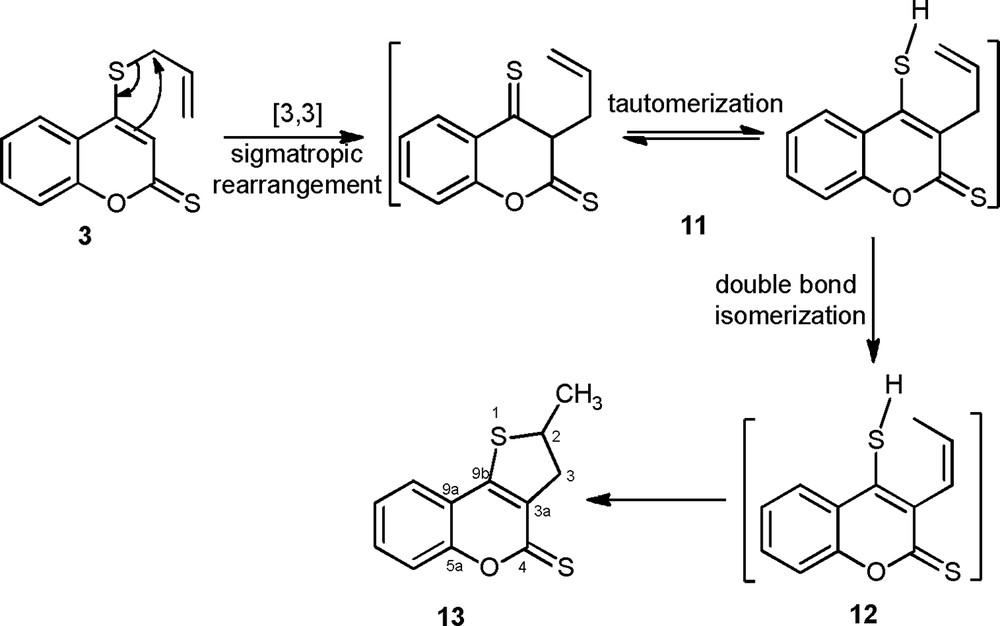
Proposed mechanism for the synthesis of 2-methyl-2H-thieno[3,2-c]chromene-4(3H)-thione 13.
3 Conclusion
In this paper, we described the two-step synthesis of an interesting rigid heterosystem with a strategy that allows the development of a new series of novel spiro-chromene thiadiazole derivatives in a fast way through the cycloadditions of diarylnitrilimines to the CS double bond. The fusion of two biologically relevant systems as they are, the thiadiazole ring along with the S-alkyl chromene-2-thione system spirojoined, may make them excellent compounds for bioactivity trials.
4 Experimental
Melting points were taken on a Büchi-510 capillary melting point apparatus. 1H (300 MHz) and 13C (75 MHz) NMR spectra were recorded with AC-300 Bruker spectrometers. HRMS spectra were acquired with an ESI–TOF (LCT Premier XE, Waters) using the reflectron mode in the positive ion mode. Leucine–enkephaline peptide was employed as the LockSpray lockmass. Commercial TLC plates (Silica gel 60, F254, SDS) were used to monitor the progress of the reaction. Column chromatography analysis was performed with silica gel 60 (particle size 40–63 μm, SDS).
4.1 General procedure for the reaction of the 4-mercapto-2H-chromene-2-thione 1 with DMFDMA
To a stirred suspension of compound 1 (1 g, 1 mol) in dry toluene (70 mL), an excess of N, N-dimethylformamide dimethylacetal (12 mL, 5 equiv.) was added. The mixture was heated at 100 °C for almost 3 h. After the removal of the volatile components in vacuo, the purification of the obtained brownish oily residue on silica gel (ether petroleum/ethyl acetate as 7:3 v/v) afforded 2 as an orange powder.
4.1.1 4-(Methylthio)-2H-chromene-2-thione (2)
Orange powder, yield 70%, mp 179 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.56 (s, 3H, CH3), 7.06 (s, 1H, H-3), 7.32 (t, 1H, J = 8.1 Hz, H-6), 7.47 (m, 1H, H-8), 7.59 (t, 1H, J = 8.4 Hz, H-7), 7.79 (d, 1H, J = 8.1 Hz, H-5). 13C NMR (75 MHz, CDCl3): δC 14.0 (CH3), 117.2 (C-3), 119.3 (C-8), 120.6 (C-7), 123.6 (C-5), 125.2 (C-4a), 132.6 (C-6), 151.5 (C-8a), 154.3 (C-4), 194.2 (C-2). ESI–HRMS: m/z [M+H]+ calcd for (C10H9OS2)+: 209.0095; found: 209.0096.
4.2 Reaction of compound 1 with allylic halide
An excess of allyl bromide was added to a solution of compound 1 (0.5 g, 0.25 mmol) and 0.11 mmol of anhydrous potassium carbonate in dry CHCl3 (30 mL). The mixture was refluxed for 24 h. The residue obtained after removing the solvent in vacuo was chromatographed on silica gel, employing ether petroleum/dichloromethane; 1:1, as an eluent. Crystallization from (CH2Cl2/EP, 2:8) gives orange cottony 3.
4.2.1 4-(Allylthio)-2H-chromene-2-thione (3)
Orange cottony, yield 64%, mp 131 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 3.75 (d, 2H, J = 7.5 Hz, CH2), 5.37 (dd, 1H, J = 12.0 Hz, J = 1.2 Hz, –CHCH2(a)), 5.48 (dd, 1H, J = 16.8 Hz, J = 1.2 Hz,–CHCH2(b)), 5.95 (m, 1H,–CH2–CH), 7.12 (s, 1H, H-3), 7.33 (m, 1H, H-6), 7.46 (dd, 1H, J = 8.4 Hz, J = 1.2 Hz, H-8), 7.60 (m, 1H, H-7), 7.76 (dd, 1H, J = 8.1 Hz, J = 1.5 Hz, H-5). 13C NMR (75 MHz, CDCl3): δC 34.1 (CH2), 117.5 (C-3), 119.8 (C-4a), 120.7 (C-8), 121.8 (–CHCH2), 124.0 (C-6), 125.5 (C-5), 130.3 (–CH2–CH), 132.9 (C-7), 150.1 (C-4), 154.9 (C-8a), 194.4 (C-2). ESI–HRMS: m/z [M+H]+ calcd for (C12H11OS2)+: 235.0251; found: 235.0242.
4.3 Synthesis of compounds 5a–d and 6a–d (general procedure)
A mixture of 2 (1 mmol) and the appropriate hydrazonoyl chloride 4a–d (1 mmol) in anhydrous CHCl3 (20 mL) containing triethylamine (1 mmol) was boiled under reflux for 24 h. The formed triethylamine hydrochloride was separated by filtration and the clear reaction mixture was evaporated till dryness under reduced pressure. After completion of the reaction, the resulting crude brownish oil was chromatographed on silica gel and eluted with (ether petroleum/dichloromethane; 1:1). This allowed the separation of compounds 5a–d and 6a–d.
4.3.1 4-(Methylthio)-2′,4′-diphenyl-2′H-spiro[chromene-2,5′-[1,2,3]thiadiazole] (5a)
Orange solid, yield 15%, mp 103 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.35 (s, 3H, CH3), 5.23 (s, 1H, H-3), 6.89–7.13 (m, 5H, H-2′′′,4′′′,6′′′,6,8), 7.32–7.63 (m, 7H, H-3′′,3′′′,4′′,5′′,5′′′,5,7), 7.94 (d, 2H, J = 8.1 Hz, H-2′′,6′′). 13C NMR (75 MHz, CDCl3): δC 14.4 (CH3), 101.0 (C-3), 101.0 (C-2), 116.0 (C-6), 117.6 (C-4′′′), 122.0 (C-4a), 123.5 (C-2′′′,6′′′), 124.8 (C-8), 126.1 (C-3′′′,5′′′), 128.2 (C-2′′,6′′), 128.2 (C-3′′,5′′), 128.6 (C-5), 129.5 (C-7), 137.2 (C-4), 142.0 (C-1′′), 146.7 (C-1′′′), 148.0 (C-4′′), 149.0 (C-4′), 150.0 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C23H19N2OS2)+: 403.0939; found: 403.0937.
4.3.2 4′-(4-Chlorophenyl)-4-(methylthio)-2′-phenyl-2′H-spiro[chromene-2,5′-[1,2,3]thiadiazole] (5b)
Pale yellow solid, yield 16%, mp 115 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.35 (s, 3H, CH3), 5.23 (s, 1H, H-3), 6.89–7.13 (m, 5H, H-2′′′,4′′′,6′′,6,8), 7.32–7.63 (m, 6H, H-2′′′,3′′′,5′′′,5,6′′,7), 7.94 (d, 2H, J = 8.1 Hz, H-3′′,5′′). 13C NMR (75 MHz, CDCl3):δC 14.4 (CH3), 101.8 (C-3), 101.8 (C-2), 116.0 (C-6), 117.6 (C-4′′′), 120.0 (C-4a), 123.5 (C-2′′′,3′′,5′′,6′′′), 124.8 (C-8), 126.1 (C-3′′′,5′′′), 128.2 (C-2′′,6′′), 128.6 (C-5), 130.0 (C-7), 137.2 (C-4), 142.0 (C-1′′), 147.0 (C-1′′′), 148.0 (C-4′′), 149.0 (C-4′), 150.0 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C23H18N2OS2Cl)+: 437.0549; found: 437.0553.
4.3.3 4-(Methylthio)-4′-(4-nitrophenyl)-2′-phenyl-2′H-spiro[chromene-2,5′-[1,2,3]thiadiazole] (5c)
Orange solid, yield 18%, mp 144 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.39 (s, 3H, CH3), 5.57 (s, 1H, H-3), 6.95 (m, 1H, H-6), 7.09–7.20 (m, 6H, H-2′′′,3′′′,4′′′,5′′′,6′′′,8), 7.35 (m, 1H, H-7), 7.81 (m, 3H, H-2′′,5,6′′), 8.27 (d, 2H, J = 8.1 Hz, H-3′′,5′′). 13C NMR (75 MHz, CDCl3): δC 13.9 (CH3), 100.9 (C-3), 100.9 (C-2), 116.0 (C-6), 117.6 (C-4′′′), 122.0 (C-4a), 123.5 (C-2′′′,3′′,5′′,6′′′), 124.8 (C-8), 126.1 (C-3′′′,5′′′), 128.2 (C-2′′,6′′), 128.6 (C-5), 130.0 (C-7), 137.2 (C-4), 142.0 (C-1′′), 147.0 (C-1′′′), 148.0 (C-4′′), 149.0 (C-4′), 150.0 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C23H18N3O3S2)+: 448.0790; found: 448.0794.
4.3.4 4-(Methylthio)-2′-phenyl-4′-(p-tolyl)-2′H-spiro[chromene-2,5′-[1,2,3]thiadiazole] (5d)
Yellow solid, yield 20%, mp 166 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.35 (s, 3H, CH3), 2.80 (s, 3H, CH3–Ph), 5.23 (s, 1H, H-3), 6.89–7.13 (m, 5H, H-2′′′,4′′′,6′′′,6,8), 7.32–7.63 (m, 6H, H-3′′,3′′′,5′′,5′′′,5,7), 7.94 (d, 2H, J = 8.1 Hz, H-2′′,6′′). 13C NMR (75 MHz, CDCl3): δC 14.4 (CH3), 21.3 (CH3–Ph), 100.8 (C-3), 100.8 (C-2), 116.0 (C-6), 117.6 (C-4′′′), 122.0 (C-4a), 124.8 (C-8), 126.1 (C-3′′′,5′′′), 128.2 (C-2′′′,3′′,5′′,6′′′), 128.2 (C-2′′,6′′), 128.6 (C-5), 130.0 (C-7), 137.2 (C-4), 142.0 (C-1′′), 147.0 (C-1′′′), 148.0 (C-4′′), 149.0 (C-4′), 150.0 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C24H21N2OS2)+: 417.1017; found: 417.1022.
4.3.5 4-(Methylthio)-3′,5′-diphenyl-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] (6a)
Orange solid, yield 78.5%, mp 177 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.39 (s, 3H, CH3), 5.76 (s, 1H, H-3), 6.99 (d, 1H, J = 8.1 Hz, H-6), 7.10 (m, 2H, H-5′′′,8), 7.32 (m, 3H, H-3′′′,6′′′,7), 7.41 (m, 2H, H-2′′′,7′′′), 7.56 (m, 4H, H-3′′,4′′,5′′,5), 7.73 (m, 2H, H-2′′,6′′). 13C NMR (75 MHz, CDCl3): δC 14.0 (CH3), 112.6 (C-3), 112.6 (C-2), 117.9 (C-6), 118.8 (C-2′′′,7′′′), 119.8 (C-4a), 122.6; 122.7 (C-8, 5′′′), 123.9 (C-5), 126.5 (C-3′′,5′′), 128.6 (C-2′′,6′′), 128.7 (C-3′′′,6′′′), 129.5 (C-7), 130.8 (C-1′′), 131.3 (C-4′′), 137.1 (C-4), 142.1 (C-5′), 142.2 (C-1′′′), 149.7 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C23H19N2OS2)+: 403.0939; found: 403.0949.
4.3.6 5′-(4-Chlorophenyl)-4-(methylthio)-3′-phenyl-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] (6b)
Pale yellow solid, yield 80.5%, mp 192 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.38 (s, 3H, CH3), 5.73 (s, 1H, H-3), 6.98 (d, 1H, J = 8.1 Hz, H-6), 7.10 (m, 2H, H-5′′′,8), 7.33 (m, 3H, H-3′′′,6′′′,7), 7.38 (d, 2H, J = 8.1 Hz, H-2′′,6′′), 7.54 (m, 3H, H-2′′′,5,7′′′), 7.61 (d, 2H, J = 8.1 Hz, H-3′′,5′′). 13C NMR (75 MHz, CDCl3): δC 14.0 (CH3), 112.3 (C-3), 113.0 (C-2), 117.9 (C-6), 118.8 (C-8,5′′′), 119.7 (C-4a), 122.7 (C-2′′′,7′′′), 123.0 (C-5), 127.6 (C-3′′,5′′), 128.7 (C-2′′,6′′), 128.8 (C-3′′′,6′′′), 129.9 (C-1′′), 130.1 (C-7), 135.3 (C-4′′), 137.4 (C-4), 141.0 (C-5′), 141.9 (C-1′′′), 149.5 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C23H18N2OS2Cl)+: 437.0549; found: 437.0538.
4.3.7 4-(Methylthio)-5′-(4-nitrophenyl)-3′-phenyl-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] (6c)
Orange solid, yield 92%, mp 208 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.29 (s, 3H, CH3), 6.87 (s, 1H, H-3), 6.90 (d, 1H, J = 8.1 Hz, H-6), 7.03 (m, 2H, H-5′′′,8), 7.22 (m, 1H, H-7), 7.24 (m, 2H, H-3′′′,6′′′), 7.46 (m, 3H, H-2′′′,5,7′′′), 7.73 (d, 2H, J = 8.1 Hz, H-2′′, 6′′), 8.16 (d, 2H, J = 8.1 Hz, H-3′′, 5′′). 13C NMR (75 MHz, CDCl3): δC 13.0 (CH3), 110.8 (C-3), 112.5 (C-2), 116.8 (C-6), 118.1 (C-2′′′,7′′′), 118.6 (C-4a), 122.4; 122.6 (C-8, 5′′′), 122.9 (C-3′′,5′′), 122.9 (C-5), 125.8 (C-2′′,6′′), 127.7 (C-3′′′,6′′′), 130.0 (C-7), 136.5 (C-1′′), 136.8 (C-4), 138.6 (C-5′), 140.4 (C-1′′′), 146.7 (C-4′′), 148.1 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C23H18N3O3S2)+: 448.0790; found: 448.0789.
4.3.8 4-(Methylthio)-3′-phenyl-5′-(p-tolyl)-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] (6d)
Yellow solid, yield 85%, mp 170 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 2.38 (s, 3H, CH3), 2.80 (s, 3H, CH3–Ph), 5.73 (s, 1H, H-3), 6.98 (d, 1H, J = 8.1 Hz, H-6), 7.10 (m, 2H, H-5′′′,8), 7.33–7.54 (m, 8H, H-2′′′,3′′,3′′′,5′′,5,6′′′,7,7′′′), 7.61 (d, 2H, J = 8.1 Hz, H-2′′,6′′). 13C NMR (75 MHz, CDCl3): δC 14.0 (CH3), 21.3 (CH3–Ph), 112.3 (C-3), 113.0 (C-2), 117.9 (C-6), 118.8 (C-8,5′′′), 119.7 (C-4a), 122.7 (C-2′′′,7′′′), 123.0 (C-5), 127.6 (C-3′′,5′′), 127.6 (C-2′′,6′′), 128.8 (C-3′′′,6′′′), 129.9 (C-1′′),130.1 (C-7), 135.3 (C-4′′),137.4 (C-4), 141.0 (C-5′), 141.9 (C-1′′′), 149.5 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C24H21N2OS2)+: 417.1017; found: 417.1022.
4.4 Preparation of compounds 7a–d and 9a–d (general procedure)
A mixture of 2 (1 mmol) and the appropriate hydrazonoyl chloride 4a–d (1 mmol) in anhydrous CHCl3 (20 mL) was added at room temperature to 1 equiv of triethylamine. The reaction medium was heated to 75 °C and kept under reflux for 24 h (TLC). The inorganic salts were filtered off; the filtrate was evaporated in vacuo. Chromatography of the brownish oily residue on silica gel eluting with (ether petroleum/dichloromethane; 1:1) allowed the separation of compounds 7a–d and 9a–d.
4.4.1 4-(Allylthio)-3′,5′-diphenyl-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] (7a)
Orange powder, yield 12%, mp 160 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 3.53 (m, 2H, CH2), 5.14 (dd, 1H, J = 10.0 Hz, J = 1.2 Hz, –CHCH2(a)), 5.21 (dd, 1H, J = 16.8 Hz, J = 1.2 Hz, –CHCH2(b)), 5.86 (m, 1H, –CH2–CH), 5.97 (s, 1H, H-3), 6.99 (m, 1H, H-6), 7.11 (m, 2H, H-4′′′,8), 7.28–7.35 (m, 6H, H-2′′′,3′′′,5′′′,5,6′′′,7), 7.54 (m, 3H, H-3′′,4′′,5′′), 7.63 (m, 2H, H-2′′,6′′). 13C NMR (75 MHz, CDCl3): δC 33.8 (CH2), 114.0 (C-2), 116.1 (C-3), 117.5 (C-8), 118.5 (C-2′′′,6′′′), 118.5 (–CHCH2), 119.4 (C-4a), 122.3 (C-4′′′), 122.4 (C-6), 124.0 (C-5), 127.1 (C-3′′,5′′), 128.2 (C-3′′′,5′′′), 128.3 (C-2′′,6′′), 129.4 (C-4′′), 130.3 (–CH2–CH), 131.7 (C-7), 134.0 (C-1′′), 134.8 (C-4), 140.5 (C-5′), 141.3 (C-1′′′), 149.3 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C25H21N2OS2)+: 429.1095; found: 429.1105.
4.4.2 4-(Allylthio)-5′-(4-chlorophenyl)-3′-phenyl-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] (7b)
Brown solid, yield 21%, mp 106 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 3.53 (m, 2H, CH2), 5.14 (dd, 1H, J = 9.0 Hz, J = 0.9 Hz, –CH = CH2(a)), 5.21 (dd, 1H, J = 16.8 Hz, J = 0.9 Hz, –CH = CH2(b)), 5.86 (m, 1H, –CH2–CH), 5.97 (s, 1H, H-3), 6.99 (d, 1H, J = 8.1 Hz, H-6), 7.11 (m, 2H, H-4′′′,8), 7.28–7.37 (m, 5H, H-2′′′,3′′′,5′′′,6′′′,7), 7.54 (d, 2H, J = 7.8 Hz, H-3′′, 5′′), 7.63 (m, 3H, H-2′′,5,6′′). 13C NMR (75 MHz, CDCl3): δC 33.8 (CH2), 112.2 (C-2), 116.1 (C-3), 117.5 (C-8), 118.5 (C-2′′′,6′′′), 118.5 (–CHCH2), 119.4 (C-4a), 122.3 (C-4′′′), 122.4 (C-6), 124.0 (C-5), 127.1 (C-3′′,5′′), 128.2 (C-3′′′,5′′′), 128.3 (C-2′′,6′′), 129.4 (C-1′′), 130.3 (–CH2–CH), 131.4 (C-7), 134.4 (C-4′′), 134.8 (C-4), 140.5 (C-5′), 141.3 (C-1′′′), 149.3 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C25H20N2OS2Cl)+: 463.0706; found: 463.0692.
4.4.3 4-(Allylthio)-5′-(4-nitrophenyl)-3′-phenyl-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] (7c)
Red solid, yield 14%, mp 109 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 3.42 (m, 2H, CH2), 5.03 (dd, 1H, J = 10.2 Hz, J = 1.2 Hz,–CHCH2(a)), 5.12 (dd, 1H, J = 16.8 Hz, J = 1.2 Hz,–CHCH2(b)), 5.71 (m, 1H,–CH2–CH), 5.79 (s, 1H, H-3), 6.88 (m, 1H, H-6), 7.02 (m, 2H, H-4′′′,8), 7.22 (m, 1H, H-7), 7.24 (m, 2H, H-3′′′,5′′′), 7.41 (m, 2H, H-2′′′,6′′′), 7.51 (dd, 1H, J = 8.1 Hz, J = 1.5 Hz, H-5), 7.71 (d, 2H, J = 8.1 Hz, H-2′′,6′′), 8.14 (d, 2H, J = 8.1 Hz, H-3′′,5′′). 13C NMR (75 MHz, CDCl3): δC 34.3 (CH2), 113.3 (C-2), 115.8 (C-3), 117.9 (C-8), 119.3 (–CHCH2), 119.7 (C-2′′′,6′′′), 120.3 (C-4a), 123.0 (C-4′′′), 123.6 (C-6), 123.9 (C-2′′,6′′), 124.5 (C-5), 126.8 (C-3′′,5′′), 128.8 (C-3′′′,5′′′), 131.0 (C-7), 131.8 (–CH2–CH), 135.5 (C-1′′), 137.5 (C-4), 139.6 (C-5′), 141.4 (C-1′′′), 147.8 (C-4′′), 149.4 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C25H20N3O3S2)+: 474.0946; found: 474.0936.
4.4.4 4-(Allylthio)-3′-phenyl-5′-(p-tolyl)-3′H-spiro[chromene-2,2′-[1,3,4]thiadiazole] (7d)
Yellow solid, yield 19%, mp 140 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 3.42 (m, 2H, CH2), 5.03 (dd, 1H, J = 9.0 Hz, J = 0.9 Hz,–CHCH2(a)), 5.12 (dd, 1H, J = 16.8 Hz, J = 0.9 Hz,–CHCH2(b)), 5.71 (m, 1H,–CH2–CH), 5.79 (s, 1H, H-3), 6.88 (m, 1H, H-6), 7.02 (m, 2H, H-4′′′,8), 7.20–7.51 (m, 8H, H-2′′′,3′′,3′′′,5,5′′,5′′′,6′′′,7), 7.63 (d, 2H, J = 8.1 Hz, H-2′′,6′′). 13C NMR (75 MHz, CDCl3): δC 21.3 (CH3), 34.3 (CH2), 114.0 (C-2), 115.8 (C-3), 117.9 (C-8), 119.3 (–CHCH2), 119.7 (C-2′′′,6′′′), 120.3 (C-4a), 123.0 (C-4′′′), 123.6 (C-6), 124.5 (C-5), 126.8 (C-3′′,5′′), 126.8 (C-2′′,6′′), 128.8 (C-3′′′,5′′′), 131.0 (C-7), 131.8 (–CH2–CH), 135.5 (C-1′′), 137.5 (C-4), 137.7 (C-4′′), 139.6 (C-5′), 141.4 (C-1′′′), 149.4 (C-8a). ESI–HRMS: m/z [M+H]+ calcd for (C26H23N2OS2)+: 443.1207; found: 443.1211.
4.4.5 2′-Methyl-3,5-diphenyl-2′,3′-dihydro-3H-spiro[[1,3,4]thiadiazole-2,4′-thieno[3,2,c]chromene] (9a)
Red crystals, yield 89%, mp 190 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 1.09 (d, 3H, J = 6.6 Hz, CH3a), 1.36 (d, 3H, J = 6.6 Hz, CH3b), 2.31 (dd, 1H, J = 16.5 Hz, J = 3.0 Hz, H-3a), 2.76 (dd, 1H, J = 16.2 Hz, J = 6.6 Hz, H-3b), 2.83 (dd, 1H, J = 16.5 Hz, J = 8.1 Hz, H-3b), 3.24 (dd, 1H, J = 16.5 Hz, J = 8.7 Hz, H-3a), 3.80 (m, 2H, H-2a,b), 6.82–7.21 (m, 4H, H-4′′′,6,7,8), 7.25–7.30 (m, 5H, H-2′′′,3′′′,5′′′,6′′′,9), 7.38–7.41 (m, 3H, H-3′′,4′′,5′′), 7.51 (m, 2H, H-2′′,6′′). 13C NMR (75 MHz, CDCl3): δC 22.9 (CH3a), 23.5 (CH3b), 42.9 (C-3a), 43.0 (C-3b), 44.4 (C-2), 114.4 (C-4), 116.9 (C-6), 117.5 (C-4′′′), 117.9 (C-2′′′,6′′′), 122.0 (C-3a), 122.2 (C-9a), 122.7 (C-8), 125.1 (C-9), 126.4 (C-3′′,5′′), 128.6 (C-3′′′,5′′′), 128.6 (C-2′′,6′′), 129.0 (C-4′′), 129.6 (C-1′′), 130.4 (C-7), 137.9; 138.1 (C-9b), 140.5; 140.7 (C-5′), 141.8; 142.0 (C-1′′′), 150.2 (C-5a). ESI–HRMS: m/z [M+H]+ calcd for (C25H21N2OS2)+: 429.1095; found: 429.1104.
4.4.6 5-(4-Chlorophenyl)-2′-methyl-3-phenyl-2′,3′-dihydro-3H-spiro[[1,3,4]thiadiazole-2,4′-thieno[3,2-c]chromene] (9b)
Yellow crystals, yield 90%, mp 110 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 1.07 (d, 3H, J = 6.9 Hz, CH3a), 1.36 (d, 3H, J = 6.9 Hz, CH3b), 2.31 (dd, 1H, J = 16.5 Hz, J = 3.0 Hz, H-3a), 2.72 (dd, 1H, J = 16.2 Hz, J = 6.6 Hz, H-3b), 2.84 (dd, 1H, J = 16.5 Hz, J = 8.1 Hz, H-3b), 3.21 (dd, 1H, J = 16.5 Hz, J = 8.7 Hz, H-3a), 3.80 (m, 2H, H-2a,b), 6.88 (m, 1H, H-6), 7.01 (m, 1H, H-9), 7.06 (m, 2H, H-2′′′,6′′′), 7.14 (m, 1H, H-7), 7.18 (m, 2H, H-3′′′,5′′′), 7.33 (d, 2H, J = 8.1 Hz, H-2′′,6′′), 7.42 (m, 2H, H-4′′′,8), 7.57 (d, 2H, J = 8.1 Hz, H-3′′,5′′). 13C NMR (75 MHz, CDCl3): δC 22.6 (CH3a), 23.6 (CH3b), 43.0 (C-3a), 44.3 (C-3b), 44.4 (C-2), 114.4 (C-4), 116.9 (C-6), 117.0 (C-4′′′), 117.9 (C-2′′′,6′′′), 120.4 (C-3a), 120.7 (C-9a), 122.7 (C-8), 122.9 (C-1′′), 125.3; 125.9 (C-9), 127.5 (C-3′′,5′′), 128.8 (C-2′′,6′′), 128.9 (C-3′′′,5′′′), 130.5 (C-7), 135.2; 135.3 (C-4′′), 137.9; 138.1 (C-9b), 140.5; 140.7 (C-5′), 141.6; 141.8 (C-1′′′), 150.1 (C-5a). ESI–HRMS: m/z [M+H]+ calcd for (C25H20N2OS2Cl)+: 463.0706; found: 463.0728.
4.4.7 2′-Methyl-5-(4-nitrophenyl)-3′-phenyl-2′,3′-dihydro-3H-spiro[[1,3,4]thiadiazole-2,4′-thieno[3,2-c]chromene] (9c)
Red solid, yield 75%, mp 148 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 1.06 (d, 3H, J = 6.6 Hz, CH3a), 1.37 (d, 3H, J = 6.6 Hz, CH3b), 2.28 (dd, 1H, J = 16.5 Hz, J = 3.6 Hz, H-3a), 2.76 (dd, 1H, J = 15.6 Hz, J = 6.9 Hz, H-3b), 2.83 (dd, 1H, J = 16.2 Hz, J = 8.1 Hz, H-3b), 3.21 (dd, 1H, J = 16.5 Hz, J = 8.7 Hz, H-3a), 3.81 (m, 2H, H-2a,b), 6.91 (m, 1H, H-6), 7.03 (m, 1H, H-9), 7.06 (m, 2H, H-2′′′,6′′′), 7.18 (m, 1H, H-7), 7.24 (m, 2H, H-3′′′,5′′′), 7.39 (m, 2H, H-4′′′,8), 7.98 (d, 2H, J = 8.1 Hz, H-2′′,6′′), 8.17 (d, 2H, J = 8.1 Hz, H-3′′,5′′). 13C NMR (75 MHz, CDCl3): δC 22.5 (CH3a), 23.5 (CH3b), 44.0 (C-3a), 44.3 (C-3b), 44.4 (C-2), 113.8 (C-4), 116.9 (C-6), 117.9 (C-4′′′), 118.4 (C-2′′′,6′′′), 119.7 (C-3a), 120.2 (C-9a), 122.9 (C-8), 123.9 (C-3′′,5′′), 125.3; 125.4 (C-9), 126.8 (C-2′′,6′′), 128.9; 129.0 (C-3′′′,5′′′), 130.7 (C-7), 137.5; 137.6 (C-1′′), 138.6; 138.7 (C-9b), 139.2; 139.3 (C-5′), 141.2; 141.4 (C-1′′′), 147.8 (C-4′′), 149.8 (C-5a). ESI–HRMS: m/z [M+H]+ calcd for (C25H20N3O3S2)+: 474.0946; found: 474.0934.
4.4.8 2′-Methyl-3-phenyl-5-(p-tolyl)-2′,3′-dihydro-3H-spiro[[1,3,4]thiadiazole-2,4′-thieno[3,2-c]chromene] (9d)
Yellow solid, yield 70%, mp 156 °C (CH2Cl2/EP, 2:8). 1H NMR (300 MHz, CDCl3): δH 1.07 (d, 3H, J = 6.6 Hz, CH3a), 1.36 (d, 3H, J = 6.6 Hz, CH3b), 2.34 (s, 3H, CH3), 2.31 (dd, 1H, J = 16.5 Hz, J = 3.6 Hz, H-3a), 2.71 (dd, 1H, J = 15.6 Hz, J = 6.9 Hz, H-3b), 2.84 (dd, 1H, J = 16.2 Hz, J = 8.1 Hz, H-3b), 3.21 (dd, 1H, J = 16.5 Hz, J = 8.7 Hz, H-3a), 3.80 (m, 2H, H-2a,b), 6.88 (m, 1H, H-6), 7.01 (m, 1H, H-9), 7.06 (m, 2H, H-2′′′,6′′′), 7.14 (m, 1H, H-7), 7.18 (m, 2H, H-3′′′,5′′′), 7.39 (m, 2H, H-4′′′,8), 7.30 (d, 2H, J = 8.1 Hz, H-3′′,5′′), 7.85 (d, 2H, J = 8.1 Hz, H-2′′,6′′). 13C NMR (75 MHz, CDCl3): δC 21.3 (CH3), 22.6 (CH3a), 23.6 (CH3b), 43.0 (C-3a), 44.3 (C-3b), 44.4 (C-2), 114.4 (C-4), 116.9 (C-6), 117.0 (C-4′′′), 117.9 (C-2′′′,6′′′), 120.4 (C-3a), 120.7 (C-9a), 122.7 (C-8), 125.3; 125.4 (C-9), 129.0 (C-3′′,5′′), 129.0 (C-2′′,6′′), 129.4 (C-3′′′,5′′′), 130.5 (C-7), 135.2; 135.3 (C-1′′), 137.9; 138.1 (C-9b), 140.7 (C-4′′), 140.5;140.7 (C-5′), 141.6; 141.8 (C-1′′′), 150.1 (C-5a). ESI–HRMS: m/z [M+H]+ calcd for (C26H23N2OS2)+: 443.1207; found: 443.1211.
4.5 Synthesis of compound 13 (general procedure)
Compound 3 (1 mmol) was dissolved in anhydrous CHCl3 (20 mL) and boiled under reflux for 24 h. The solvent was then removed under reduced pressure. The resulting residue was purified by silica gel column chromatography (EP/AcOEt, 8:2) to give compound 13.
4.5.1 2-Methyl-2H-thieno[3,2-c]chromene-4(3H)-thione (13)
Orange solid, yield 75%, mp 160 °C (EP/AcOEt, 8:2). 1H NMR (300 MHz, CDCl3): δH 1.45 (s, 3H, CH3), 2.97 (dd, 1H, J = 16.5 Hz, J = 5.4 Hz, H-3a), 3.42 (dd, 1H, J = 16.5 Hz, J = 8.7 Hz, H-3b), 4.09 (m, 1H, H-2), 7.15–7.31 (m, 3H, H-6,8,9), 7.43 (m, 1H, H-7). 13C NMR (75 MHz, CDCl3): δC 23.0 (CH3), 41.4 (C-3), 45.7 (C-2), 116.7 (C-3a), 116.9 (C-6), 119.3 (C-9a), 124.2 (C-8), 125.8 (C-9), 131.7 (C-7), 157.4 (C-5a), 160.1 (C-9b), 193.2 (C-4). ES–MS m/z 235 [M+H]+.


