1 Introduction
The use of additives to make food and other products more visually appealing is very common in the food and chemical industries, and so the interest in and demand for natural products have increased significantly in recent years. Due to certain requirements and consumer preferences for natural compounds, a global trend has arisen aiming to increase the use of natural products over synthetic versions. The growing demand for natural dyes is justified by their minimal or absent toxicity.
Annatto is a shrub native to the South American tropics, the natural reddish-yellow color of which is obtained from the outer coating of its seeds. The major pigments present are carotenoids, including a large amount of cis-bixin and other minor constituents, such as trans-bixin, cis-norbixin and trans-norbixin. Annatto is almost unique among the sources of carotenoids, as its pigment takes on a number of different chemical structures; the range of intense colors its compounds take include shades of red, orange and yellow. Annatto can be obtained from hydrophilic and hydrophobic extracts, and its pigments are very stable due to their interactions with protein compounds. Thus, it is an excellent candidate for a natural pigment to be used in cosmetics, pharmaceuticals and the food industry [1–3]. Unlike β-carotene, which is widely distributed in vegetables and fruit, bixin can only be found in annatto and comprises more than 80% of the total carotenoid content of its seeds [3].
Various techniques have been studied to develop clean extraction technologies with environmental benefits (so-called “green technologies”). Innovative technologies, such as ultrasound-assisted extraction [4–8], microwave-assisted extraction [9], supercritical fluid extraction [10–15] and accelerated solvent extraction [7,16,17], can be combined to develop processes that are free of the residues of organic solvents. These techniques are used to extract bioactive substances to shorten the processing time, reduce solvent consumption, increase the extraction yield and improve the quality of the extracts. These studies are needed because of the general trend of the market to identify products that generate economic, social and environmental advantages [11,18]. Extraction with organic solvents is limited by the need for a solvent that is compatible with the end use of the product. In food, the dye will be subject to serious technical restrictions on the amount of residues from potentially toxic solvents [15].
Solid-liquid extraction or solvent extraction occurs through the selective dissolution of one or more solutes from a solid matrix by a liquid solvent. This unit operation is also called leaching, decoction, elution or low-pressure solvent extraction (LPSE). Regardless of the name, this technique is one of the most widely used operations in the chemical industry [18].
For instance, a certain liquid can be pressurized and heated at pressures and temperatures below its critical point and then employed in the extraction of several compounds, based on the potentially increased solubility of the compounds to be extracted and on the acceleration of the desorption kinetics of these compounds from the vegetable matrix. The liquid extraction process at pressures higher than ambient pressure and moderate to high temperatures has various names; in this paper, we use the name “pressurized liquid extraction” (PLE).
The extraction methods used for the production of dyes from annatto seeds may produce bixin or, by aqueous hydrolysis, the simultaneous extraction of norbixin [1,19].
The most commonly used methods to extract the pigments from annatto seeds are alkaline extraction (norbixin salt), extraction with oil (bixin) and extraction with solvents, such as ethyl acetate, ethanol, chloroform and acetone, to yield products with higher purity. These dyes differ in solubility and pigmentation [1].
The great demand for annatto extracts with high quality characteristics has accentuated the deficiencies of the commonly used processes to produce dyes. Typically, these techniques require high extraction times and provide a low efficiency, even while including the risk of the thermal degradation or oxidation of the pigment extracts, which requires the use of extraction techniques at milder conditions to avoid degradation.
Ultrasonic energy has been identified as an efficient tool to improve performance in different applications of analytical chemistry, such as the extraction of organic and inorganic compounds, homogenization, and dispersion of suspensions, among other applications [4–6,18]. The improvement of the extraction efficiency for organic compounds by the use of ultrasonication is based on the phenomenon of cavitation, which is produced in the solvent by the passage of sound waves [20–22]. In general, for ultrasound techniques to be efficient, an ultrasonic probe should be used, as described by Veggi et al. [4]; however, there have been reports in the literature of improvements in the processing performance even with ultrasonic bath-assisted extractions [8]. Nonetheless, it is import to observe that the extraction efficiency is a result of the solid matrix pretreatment, solvent, and ultrasound power.
The major part of bixin in annatto seeds is located in the outside layer of the seed. Nonetheless, there is a lipid layer strongly associated to the bixin in the seed; the removal of the lipidic layer during the extraction of bixin from annatto is the most difficult step. Bixin has a very low solubility in carbon dioxide while that of the lipids is very high. Therefore, we envisioned a process in two steps: (i) removal of the lipids using supercritical carbon dioxide followed (ii) by removal of bixin. The removal of the lipids using supercritical carbon dioxide has proven to be a very efficient process [11]. Additionally, as reported in literature [23] supercritical fluid extraction is an interesting tool for solid matrix pretreatment. Therefore, in this work, we are seeking for the best process to remove bixin from defatted annatto seeds. Therefore, two process were investigated PLE and LPSE. The effects of solvent, ratio of mass of solvent to mass of solvent, temperature, pressure and ultrasound assistance were evaluated on the total yields of extract and of bixin.
2 Material and methods
2.1 Material
Annatto seeds were partially defatted as described by Albuquerque [11]. Briefly, annatto seeds of the Piave variety were defatted at 313 K and 20 MPa using supercritical CO2. The extraction was carried out in a commercial SFE unit (Thar Technologies, SFE-2 × 5LF-2-FMC, Pittsburgh, Pennsylvania, USA).
2.2 Characterization of the raw materials
The real density (ρr) of the seeds was determined following the method of helium pycnometry using a gaseous pycnometer (Quantachrome, Ultrapyc™ 1200e, Boynton Beach, Florida, USA) in the Analytical Central/Institute of Chemistry/Unicamp. Apparent density (ρa) was calculated as the ratio of the mass of raw material used to fill the extraction cell to its volume. The porosity of the bed and the particles (ɛ) was determined as (1 − (ρa/ρr)). The average diameter (dp) of the seeds was determined according to the FAO/WHO report [1], using the geometric means of the height, width and thickness of twenty randomly selected seeds, as measured by a universal pachymeter. For the analysis of their chemical composition, the samples were milled (Tecnal, model TE-631, Piracicaba, São Paulo, Brazil). The moisture [24], ash [25], lipid [26] and protein [27] contents were determined. Scanning electron microscopy analysis was performed at the Analytical Laboratory of Resources and Calibration (LRAC) at the School of Chemical Engineering (FEQ)/UNICAMP, São Paulo, Brazil. The aim was to analyze the seed surface, evaluating the effects of the extraction conditions used in this study on the structure of the pericarps of the seeds, where bixin is most commonly located. A coating of gold with a thickness of 92 Å was applied by metallic sputter coating (Polaron SC7620 sputter coater, VG Microtech, Uckfield, UK) in the presence of an inert gas, such as argon. To obtain the micrographs, a scanning electron microscope with an energy dispersive detector X-ray (Leo 440i, 6070, LEO Electron Microscopy/Oxford, Cambridge, England) was used; the accelerating voltage was equal to 20 kV, and the beam current used was 100 pA.
2.3 Chemical characterization
The bixin contents of the seeds and extracts were determined according to the methodology described in the FAO/WHO report [1] and adapted by Albuquerque and Meireles [11]. Bixin was exhaustively extracted from the seeds with acetone PA ACS (Exodus scientific Lot: A8979RA, Hortolândia, Sao Paulo, Brazil) until complete discoloration; the absorbance of this solution was read [11,19]. The obtained extracts by PLE and LPSE were diluted in acetone to the concentrations appropriate for analysis. The absorbances of the diluted samples were measured at 487 nm with a UV-vis spectrophotometer (Hach DR/4000 U Loveland, Colorado, USA), and the concentration of bixin was calculated according to the Lambert-Beer law, using [1].
2.4 Preliminary tests
Preliminary tests were performed to evaluate the efficiency of the extraction with pre-selected solvents under different conditions of temperature and pressure. These tests aimed to evaluate the extraction of bixin from the defatted seeds in extreme conditions (temperature and pressure), and the results obtained using both methods allowed for the selection of the best conditions for further studies. For the PLE method, the use of water as the solvent in conjunction with high pressures had a negative effect on the global yield and bixin recovery. Therefore, we opted to use other solvents that had been reported in the literature for the extraction of bixin from annatto, as follows: ethanol (PA, dynamics, 52990, Diadema, SP, Brazil), ethyl acetate (Merck KGaA, K40235423, Darmstadt, Germany) and a mixture of water + NaOH (pH 10 and 14). The PLE conditions tested were temperature (305, 335, 355 and 395 K) and pressure (2, 4 and 20 MPa); the assays were performed in a semi-continuous apparatus (Fig. 1) at flow rates of approximately 1.14, 1.31 and 1.45 g/min, respectively, for ethanol, ethyl acetate and sodium hydroxide solutions. LPSE were performed simultaneously with periodic agitation (every 1 minute). The extraction time was approximately 20 minutes, and three ratios of S/F for both methods were used: S/F = 6, 7 and 7.5 g (solvent)/g (seed) for the ethanol, ethyl acetate and sodium hydroxide solutions, respectively. The extractions were performed in batches at ambient conditions (approximately 0.1 MPa and 300 K).
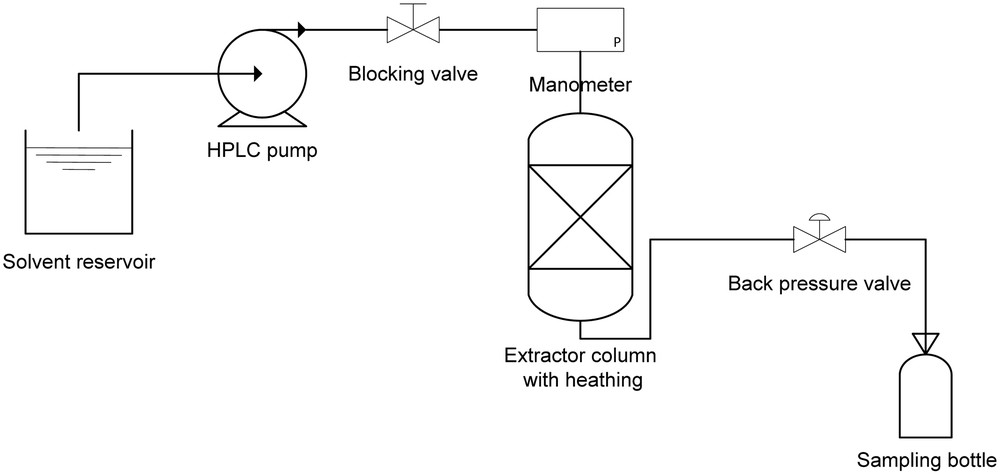
Equipment used for PLE [8].
2.5 Procedure for pressurized liquid extraction (PLE)
Fig. 1 illustrates the extraction equipment using pressurized liquid. All connections within the system are made of stainless steel tubing (1/16” and 1/8”). The extraction cell (Thar Designs, CL 1373, Pittsburg, USA) of 5 cm3 (inner diameter of 19.6 mm and height of 22.6 cm) was packed with approximately 3 g of the sample. Afterwards, the cell was attached to the heating jacket, which was already at the extraction temperature. The sample was heated over 5 minutes to ensure that the extraction cell was at the desired temperature for the subsequent procedures of filling and initially pressurizing the system with the solvent. After reaching these conditions, the blocking valve was opened, and the solvent was pumped by a HPLC pump (Thermoseparation Products, Model 3200 ConstaMetric P/F, Fremont, California, USA) into the cell, increasing the pressure to the desired value before a static extraction period of 10 minutes began. After this period, the block and back-pressure valves were opened, and the solvent was again pumped into the cell. During this step, the pressure was controlled by the back-pressure valve.
The extract was collected in pre-weighed glass bottles up to a predetermined volume. Alcoholic extracts were evaporated in a rota-evaporator (Marconi, model MA120/TH, Piracicaba, São Paulo, Brazil) under vacuum (Marconi, model MA057/1, Piracicaba, São Paulo, Brazil) at 310 K for the removal of the solvent (the same procedure was used in the preliminary tests for the removal of ethanol and ethyl acetate). The solvent-free extracts were kept at 265 K to determine the overall yields and for quantification of the total carotenoids, expressed as the bixin percentage (BY(%)).
2.6 Extraction procedure for LPSE, with and without ultrasound assistance
The LPSE equipment with or without ultrasound (US) assistance is illustrated in Fig. 2. The solvent was pumped through a peristaltic pump (Cole-Parmer Instrument Co, catalog number 7554-30, Chicago, IL, USA) into the cell extraction, which was immersed in an ultrasonic bath (frequency 40 kHz, power 135 W) (Unique, Clean Max model 1400, Indaiatuba, SP, Brazil) and maintained at the process temperature by a thermostatic bath (Marconi, model MA 127/BO, Piracicaba, SP, Brazil). The extraction cell, a glass column of 3.04 cm3 (inner diameter of 16.3 mm and height of 14.6 cm), was sealed with silicone stoppers at both ends. The assays with US assistance were performed in the same apparatus. The ethanol was removed as previously described; the water was removed by freeze-drying (LIOTOP, L101, São Carlos, SP, Brazil). The solvent-free extracts were kept at 265 K to determine the overall yields and quantifications of the total carotenoids, expressed as the bixin percentage (BY(%)).

Equipment for the low pressure extraction assisted by an ultrasound.
2.7 Experimental design
For PLE, based on the preliminary results, ethanol was chosen as the solvent. The independent variables studied were S/F (5 e 10 g/g), temperature (313, 323 e 333 K) and pressure (2, 6 e 10 MPa). The response variables were X0(%) and BY(%). The experimental design was a full factorial design (2 × 3 × 3) that was totally randomized without replication.
For LPSE, we studied the following independent variables: S/F (approximately 4 and 8 g/g), temperature (313, 323 and 333 K), ultrasound (without, intermittent and continuous use) and solvent (water and ethanol). The response variables were X0(%) and BY(%). The experimental design was a full factorial design (2 × 3 × 3 × 2) that was totally randomized without replication.
2.8 Study of the kinetics of extraction
After carrying out the extractions and determining the conditions that produce optimal (maximum) X0(%) and BY(%), kinetic experiments were performed in duplicate at these conditions. During the extraction process, the bottles for the collection of the extract were replaced by clean, pre-weighed flasks every 5 min.
The overall extraction curves (OECs) were constructed with the purpose of determining the quantity of extracted soluble material and process parameters as a function of time. For the determination of kinetic parameters, these included tCER (duration of the constant extraction rate period, [min]); MCER (mass transfer rate during the CER period, [g/s]); YCER (mass ratio of extract at the bed outlet, [g-ext/100 g solvent]) and RCER (yield during the CER period, [%]). These calculations were performed with the assistance of SAS® 9.3 software (SAS Institute Inc., version 9.2, Cary, USA), using the procedure PROC REG and PROC NLIN, as described in the literature [28].
2.9 Calculations
2.9.1 Determinations of overall yield (X0)
Global yield in dry basis, X0(%), was calculated according to Eq. (1) as the ratio of the total mass of the extract (mextract) and the initial mass of the sample (msample) on a dry basis.
| (1) |
2.9.2 Extraction yield bixin BY(%)
Bixin yield in dry basis, BY(%), was calculated according to Eq. (2), which involves X0(%), bixin in the extract, Bixinextract(%), and bixin in the seeds, Bixinseeds(%).
| (2) |
2.9.3 Statistical analysis
For both methods (PLE and LPSE), an analysis of variance (ANOVA) was performed using Minitab® 16 software and the general linear model (GLM) with a confidence interval of 95% (P-value ≤ 0.05).
3 Results and discussion
3.1 Characterization of the raw material
The chemical profile of the defatted seed (variety Piave) was (12.3 ± 0.7)% moisture, (5.8 ± 0.2)% ash, (9.7 ± 0.0)% protein, (2.7 ± 0.01)% lipids, (68.9)% carbohydrates and (2.5 ± 0.2)% bixin. The values obtained in this study are similar to those found in the literature for annatto seeds. Silva et al. [15] found approximately 2.8% bixin, 3.1% lipids, and 11.3% moisture. Albuquerque and Meireles [11] found similar values for annatto seeds of the Piave variety: (4.9 ± 0.2)% bixin, (12.3 ± 0.1)% moisture, (6.2 ± 0.1)% ash, (3.7 ± 0.0)% lipids, (12.1 ± 0.2)% protein and 65.7% carbohydrates. The mean diameter of the nondefatted seeds was 3.50 mm, and their real density, 1220 kg.m−3.
3.2 Preliminary assays
Preliminary tests were performed with the aim of evaluating the efficiency of certain pre-selected solvents, using different temperatures and pressures. The preliminary experiments were performed without a formal experimental design. Conditions were selected based in results for other raw materials worked at our laboratory. In industrial practice, bixin is extracted from annatto seeds using water at pH = 14; the main reason to use this highly alkaline solution is due to the presence of the lipid layer in the in natura seeds. Afterwards, to obtain bixin of high purity several steps are required. In the preliminary assays, we were testing the hypothesis that the use of defatted seeds would allow the extraction of bixin using pressurized water as the solvent. Nonetheless, for comparison purposes, we included the use of alkaline water at two pHs 10 and 14 and the use of two other solvents ethanol and ethyl acetate. Even so, the results shown (Table 1) that using water as solvent the bixin recovery was very low at all PLE conditions used in spite of the high total yields (X0(%)) at certain conditions. For instance, at 333 K and 2 MPa the bixin recovery was approximately 0.9%. The conclusion was that for PLE water was not a good solvent while the solvents of choice are ethyl acetate and ethanol. At 333 K and 2 MPa, the X0(%) was equal to 2.33, and the BY(%), 5.85 for ethanol. However, in ethyl acetate, the X0(%) was 6.48, and the BY(%), 22.62. Therefore, for PLE, feasible alternative solvents include ethanol and ethyl acetate. In spite of the larger X0(%) and BY(%) for ethyl acetate, ethanol has obvious advantages, such as its status as a benign solvent.
Results of preliminary tests of extraction using defatted annatto seeds.
| Conditions of extraction | |||||
| Temperature (K) | Pressure (MPa) | Solvent | X0(%) | Bixinextract (%) | BY(%) |
| Method: PLE | |||||
| 393 | 20 | Water | 14.45 | 0.03 | 0.23 |
| 393 | 2 | Water | 13.61 | 0.06 | 0.42 |
| 353 | 2 | Water | 6.20 | 0.08 | 0.26 |
| 333 | 2 | Water | 19.62 | 0.1 | 0.92 |
| 303 | 2 | Water | 3.00 | 0.06 | 0.16 |
| 303 | 4 | Water | 3.00 | 0.1 | 0.09 |
| 353 | 2 | Water (pH = 10) | 6.48 | 0.2 | 0.67 |
| 353 | 2 | Water (pH = 14) | 17.90 | 0.05 | 0.47 |
| 333 | 2 | Ethanol | 2.33 | 4.82 | 5.85 |
| 333 | 2 | Ethyl acetate | 6.48 | 6.71 | 22.62 |
| Method: manual agitation using a glass rod | |||||
| Ambienta | LPSEb | Water | 3.32 | 13.89 | 24.02 |
| Ambient | LPSE | Water (pH = 10) | 2.61 | 8.95 | 12.17 |
| Ambient | LPSE | Water (pH = 14) | 15.20 | 0.43 | 3.40 |
| Ambient | LPSE | Ethanol | 3.61 | 5.83 | 10.94 |
| Ambient | LPSE | Ethyl acetate | 4.56 | 6.96 | 16.50 |
a Approximately 300 K.
b Approximately 0.097 MPa.
Considering again PLE using water, another point worth noting is that, the color of the extracts changed substantially depending on the pressures and temperatures used (Fig. 3). At 393 K/20 MPa, the extract color was dark brown, most likely indicating the degradation of the annatto pigments, while at 303 K/2 MPa, the extract had the yellow-reddish color characteristic of bixin. The yellow-reddish color seen at 303 K/0.1 MPa was even more characteristic of annatto pigments. Consequently, the following methods were selected for further study: (i) PLE using ethanol as a solvent and (ii) LPSE, assisted or not by ultrasound, using water and ethanol as solvents.

Comparison of the extract colors from extractions using water as the solvent at different temperatures and pressures.
3.3 Pressurized liquid extraction–PLE
Table 2 presents the results for the variables studied. We observed that the X0 had maximum and minimum values, respectively, of 7.22% and 2.67%; the maximum and minimum values of BY were 9.07% and 1.75 (%).
X0 overall extraction yield (%, d.b.), the amount of bixin extract (%, d.b.), and extraction yield bixin BY (%, d.b.) in PLE using ethanol as a solvent.
| Conditions of extraction | ||||
| Temperature (K) | Pressure (MPa) | X0(%) | Bixinextract (%) | BY(%) |
| S/F = 4 | ||||
| 323 | 6 | 3.17 | 3.19 | 3.60 |
| 313 | 6 | 4.40 | 2.34 | 3.67 |
| 333 | 10 | 4.02 | 6.08 | 8.69 |
| 323 | 10 | 2.95 | 4.39 | 4.61 |
| 333 | 2 | 2.67 | 1.84 | 1.75 |
| 323 | 2 | 3.94 | 2.7 | 3.78 |
| 333 | 6 | 3.54 | 3.76 | 4.74 |
| 313 | 2 | 2.71 | 2.37 | 2.29 |
| 313 | 10 | 4.92 | 2.33 | 4.08 |
| S/F = 8 | ||||
| 333 | 10 | 7.22 | 3.53 | 9.07 |
| 333 | 2 | 4.06 | 3.47 | 5.02 |
| 323 | 6 | 3.25 | 6.38 | 7.38 |
| 323 | 10 | 4.27 | 2.99 | 4.54 |
| 313 | 6 | 4.64 | 2.15 | 3.55 |
| 323 | 2 | 3.69 | 2.83 | 3.72 |
| 333 | 6 | 3.62 | 4.48 | 5.77 |
| 313 | 10 | 4.77 | 2.69 | 4.57 |
| 313 | 2 | 3.86 | 3.46 | 4.76 |
Analyses of variance for X0(%) and BY(%) did not show any significant influences of the parameters studied; in other words, these two response variables were not significantly affected by variations in S/F or pressure. Pineiro and Palma [26] compared different types of extraction: extraction by magnetic agitation, ultrasound-assisted extraction (UAE) and PLE. In the three extraction systems, four different pure solvents were used, as follows: water, methanol, ethanol and ethyl acetate. PLE with methanol as the solvent resulted in better yields in terms of the recovery of catechin and epicatechin, notably higher than any of the other extraction conditions tested. Plaza et al. [7] evaluated the extraction of bioactive compounds from Chlorella vulgaris (carotenoids and fatty acids) using PLE and UAE (analytical scale). The authors found no significant differences among the extraction techniques; however, the PLE method resulted in higher yields for similar bioactive compositions.
3.4 Extractions at low pressures, assisted or not by ultrasound (LPSE and USA-LPSE)
Table 3 shows the experimental results obtained for the studied variables. We can observe that X0(%) has maximum and minimum values of 13.06 (ethanol; 333 K, S/F = 8 and LPSE-C) and 0.30 (water, 313 K, S/F = 8 and LPSE-C), corresponding to a BY(%) of 22.89 and 0.22, respectively. Nonetheless, the highest BY(%) was equal to 31.62 and was obtained with ethanol, 313 K, S/F = 313, LPSE-C; under these conditions, the X0(%) was 11.98. The minimum BY(%) was equal to 0.20 and was determined with water, 333 K, S/F = 4, LPSE; the X0(%) was 0.70. The analyses of variance showed that, for these extraction conditions, significant differences were observed in X0(%) for the variables solvent (P-value = 0.000) and temperature (P-value = 0.023) and the interaction between solvent and temperature (P-value = 0.034); although smaller, the interaction between S/F and temperature (P-value = 0.092) was also detected. Nonetheless, for BY(%) significant differences were observed only for the variable solvent (P-value = 0.000). Ethanol was the best solvent, most likely because bixin is lipid soluble. This behavior was expected, because, bixin is a carotenoid, therefore, insoluble in water. Ethanol is less polar than water, but more polar than acetone, a solvent in which bixin is also soluble. The values of X0(%) and BY(%) obtained by PLE were lower than those obtained by LPSE using the same solvent under the same conditions of temperature and S/F. For ethanol, a possible explanation for this is that the ethanol dielectric constant increases with pressure; thus, its polarity also increases, which reduces bixin solubility. For water, the effects of pressure were similar, as were observed in the preliminary assays (Table 1). Thus, in spite of the very strong beliefs reported in the literature that PLE performs better than LPSE, a careful analysis of the raw material shows opposite behaviors, as was observed for annatto.
Overall extraction yield, X0(%), the amount of bixin in the extract, Bixinextract (%), and bixin yield BY(%) obtained by LPSE.
| Conditions of extraction | ||||
| Temperature (K) | Method | X0(%) | Bixinextract (%) | BY(%) |
| Solvent: water; S/F = 4 | ||||
| 333 | LPSE-C | 7.97 | 1.93 | 5.48 |
| 313 | LPSE-P | 0.77 | 4.32 | 1.19 |
| 333 | LPSE | 0.70 | 0.80 | 0.20 |
| 333 | LPSE-P | 1.36 | 1.65 | 0.80 |
| 313 | LPSE | 1.30 | 5.02 | 2.31 |
| 323 | LPSE-C | 2.62 | 1.57 | 1.46 |
| 323 | LPSE-P | 7.24 | 1.09 | 2.81 |
| 323 | LPSE | 8.44 | 1.08 | 3.24 |
| 313 | LPSE-C | 7.03 | 0.32 | 0.80 |
| Solvent: water; S/F = 8 | ||||
| 313 | LPSE-P | 0.63 | 2.01 | 0.45 |
| 333 | LPSE | 8.75 | 0.97 | 3.02 |
| 323 | LPSE | 8.49 | 1.30 | 3.93 |
| 333 | LPSE-C | 6.96 | 0.91 | 2.25 |
| 323 | LPSE-P | 8.15 | 1.46 | 4.23 |
| 323 | LPSE-C | 7.68 | 3.53 | 9.65 |
| 313 | LPSE-C | 0.30 | 2.09 | 0.22 |
| 313 | LPSE | 1.08 | 0.66 | 0.25 |
| 333 | LPSE-P | 8.64 | 1.7 | 5.23 |
| Solvent: ethanol; S/F = 4 | ||||
| 313 | LPSE | 10.58 | 3.21 | 12.09 |
| 313 | LPSE-P | 9.55 | 4.7 | 15.97 |
| 333 | LPSE-P | 11.31 | 5.96 | 23.99 |
| 333 | LPSE-C | 9.73 | 5.56 | 19.25 |
| 323 | LPSE-P | 10.19 | 6.49 | 23.54 |
| 313 | LPSE-C | 11.98 | 7.42 | 31.62 |
| 323 | LPSE | 12.54 | 2.57 | 11.46 |
| 323 | LPSE-C | 10.01 | 1.41 | 5.02 |
| 333 | LPSE | 9.07 | 3.87 | 12.50 |
| Solvent: ethanol; S/F = 8 | ||||
| 313 | LPSE-P | 10.50 | 4.16 | 15.54 |
| 313 | LPSE-C | 9.51 | 5.76 | 19.49 |
| 333 | LPSE-P | 9.53 | 5.65 | 19.17 |
| 323 | LPSE-P | 10.33 | 4.13 | 15.18 |
| 323 | LPSE-C | 11.05 | 5.40 | 21.23 |
| 323 | LPSE | 10.11 | 4.48 | 16.12 |
| 313 | LPSE | 10.77 | 3.54 | 13.57 |
| 333 | LPSE-C | 13.06 | 5.14 | 23.89 |
| 333 | LPSE | 10.56 | 7.58 | 28.49 |
The analysis of variance showed that the use of ultrasound (LPSE-C and LPSE-P) did not significantly affect the response variables. The cavitation effect of the ultrasound on the surface of the seeds, where the bixin is primarily located, was expected to improve the extraction. In this work, an ultrasonic bath was used, and therefore, energy dispersion occurred. We then determined that the assistance of an ultrasound as it was used was insufficient to significantly increase X0(%) or BY(%). Most likely, the energy consumption of this technology would be justified if employing an ultrasound probe; however proving this fact would require new experimental data and an analysis of the economics of the entire process. Additionally, as will be discussed later, the use of the ultrasound bath was sufficient to alter the characteristics of the defatted annatto seeds. Therefore, it is possible that assistance of ultrasound can prove to be of importance for process setup employing large ultrasound energy.
Veggi et al. [4] studied the viability of using ultrasound to assist with obtaining extracts rich in polyphenols from jatoba bark (Hymenaea courbaril L. var stilbocarpa); these results were compared with those from a conventional agitation extraction. A statically significant (P-value < 0.05) effect was observed for the use of the ultrasound (an approximately 15% increase in total phenolic compounds content) when using the maximum power (60 W). Moreover, the three-stage and scaled-up stages of the ultrasound experiments demonstrated the efficiency of this process. DPPH analysis confirmed the higher antioxidant activity of the ultrasound extracts, and HPLC analysis demonstrated that, besides the increase in yield, ultrasound irradiation modified the composition of the extracts, supporting the suitability of ultrasounds for the preparation of antioxidant-rich plant extracts.
Pingret et al. [5] used apple pomace (Malus domestica Borkh) for an ultrasound-assisted extraction (UAE) to produce extracts rich in antioxidants. The optimized conditions obtained by response surface methodology for extracting polyphenols using water were 315 K, 40 min and 0.764 W/cm2 (555 and 420 mg of catechin equivalents per 100 g dry weight, for UAE and conventional agitated extraction, respectively). Both of the tested methods had the same extraction kinetics. In addition, the extracts obtained by UAE showed a higher antioxidant activity, which was confirmed by HPLC analysis; this confirmed that the polyphenols were not degraded under the conditions applied.
3.5 Kinetics of extraction
From the results obtained for X0(%) and BY(%), two conditions were selected for the kinetic study. The extraction conditions were selected in consideration of the levels of the independent variables that maximized the response variables, X0(%) (Kinetics I: LPSE/323 K/ethanol) and BY(%) (Kinetics II: LPSE/333 K/ethanol). A kinetic assay (Kinetics III) was established to test the condition BY(%) using nondefatted seeds in order to study the differences in BY(%) in the presence of oil in the seed, considering that the presence of oil in the seed can assist in the extraction of bixin and affect its solubility in the oil. For all kinetics experiments, the flow rate was standardized at 0.34 g/min.
Fig. 4a and b show OECs for Kinetics I, II and III. It should be noted that no significant differences in global yield were obtained by changing the temperatures in the LPSE method with defatted seed. In Fig. 4b, it can be seen that bixin extract is still being recovered, even for S/F = 60, while S/F = 50 resulted in a substantially reduced extraction rate compared to the initial values of the process. At approximately S/F = 14, which is larger than the maximum value used in the experiments to determine X0(%) (S/F = 10), we were able to recover approximately 18% (Kinetic I) and 9% (Kinetic II) of the extract. Thus, S/F = 10 was insufficient to exhaust the vegetal raw material; in spite of this, the observed behavior due to increasing S/F remained the same, as X0(%) is an intensive variable [29].
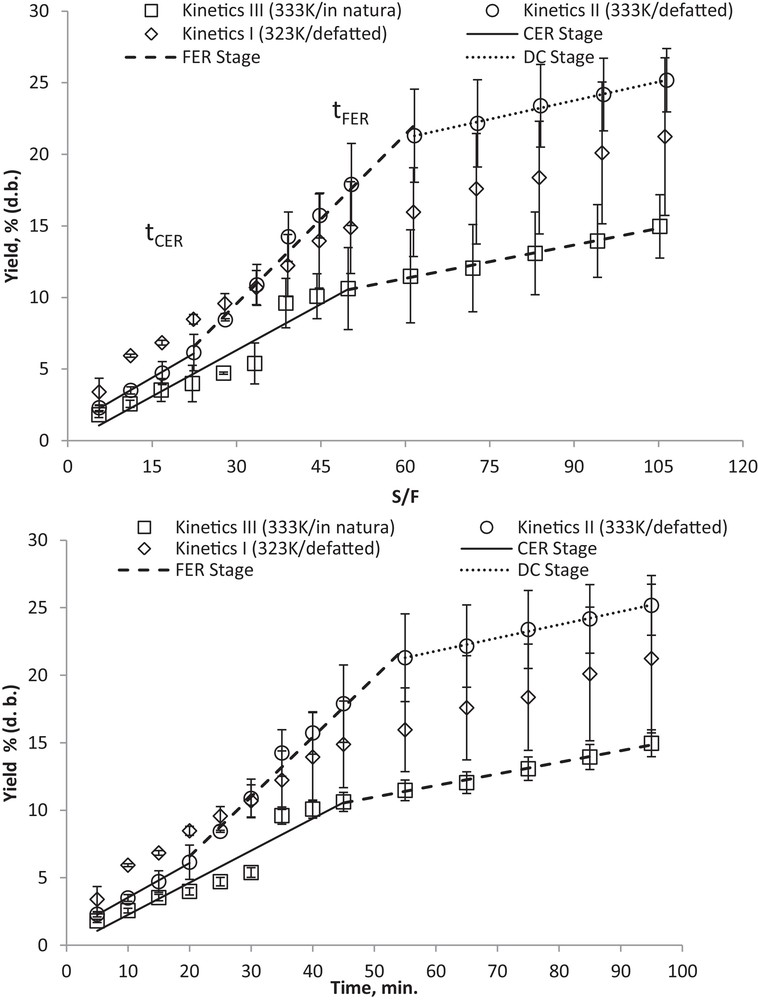
a: overall extraction curves and splines fitted to the experimental data of Kinetics II (defatted) and III (in natura) as a function of S/F; b: overall extraction curve and splines fitted to the experimental data of Kinetics of II (defatted) and III (nondefatted) as a function of time.
The exhaustive extractions for Kinetics I and II were achieved at approximately S/F = 120 over 85 minutes (Fig. 4a); during this time, 94 and 93% of the total weight of extractable compounds was recovered, respectively.
For the macela extract (Achyrocline satureioides), using fixed-bed percolation and ethanol as a solvent, S/F = 20 was sufficient to exhaust the raw material, according to Takeuchi et al. [10].
3.5.1 Kinetic calculations
The OEC provides the information necessary to calculate the kinetic parameters, including the processing time. Using straight-lines splines adjusted to the experimental data, we obtained the kinetic parameters in Table 4. The slope of the first line represents the rate of mass transfer of the CER period (MCER), and the time corresponding to the intersection between the first and second lines was identified as tCER, which represents the duration of the CER period and may be considered the minimum time for the extraction process. The concentration of the extract leaving the bed (YCER) was calculated as the ratio between MCER and the mass flow rate of the solvent. RCER is the yield obtained during the CER period [29].
Kinetic parameters.
| Kinetic parameters | Conditions of processing | ||
| Kinetics I | Kinetics II | Kinetics III | |
| tCER (min) | 7.35 | 18.74 | 44.99 |
| MCER (g.min−1) | 0.0208 | 0.00914 | 0.00713 |
| RCER (%) | 5.01 | 5.63 | 10.54 |
| YCER (g extract.g−1 ethanol) | 0.0612 | 0.0269 | 0.021 |
We observe that the tCER of Kinetic III (in natura seeds) was twice as high as that of Kinetics II obtained using defatted seeds. The presence of oil in the seeds made it difficult for the solvent to access the substrate. Comparing Kinetics I and II, significant differences can be noted in the kinetics parameters. The tCER for Kinetics I was smaller, and the value of RCER was similar to that of Kinetics II. Therefore, the method that has the lowest energy consumption and processing time should be chosen: LPSE at 323 K. Still, for a more appropriate selection of the processing conditions, it would be necessary to calculate the cost of manufacturing (COM), which is beyond the objectives of this study. In short, COM is directly related to the production rate, the cost of raw material (annatto seeds and solvents), fixed costs (costs of equipment, installation, depreciation, taxes, insurance and so on) and general expenses including administrative costs, sales expenses, research and development. Therefore, the time of extraction will affect directly the productivity of the system. Then, the choice of extraction process depends on both the quality of the extract as well its cost.
3.5.2 Extraction yield of bixin in OECs
Upon comparing the kinetics of OECs I and II, significant differences are not observed in the results for the total yield; however, the kinetic behavior differed, as tCER at 323 K was lower, and both OECs obtained an MCER of 5.01 and 5.63 g.min−1, respectively. Thus, there are advantages to using the extraction conditions of Kinetics I (Ethanol/LPSE/323 K). However, the overall yield cannot be considered as a comparative parameter alone because a higher yield does not necessarily reflect higher yields of the target substance. Instead, higher yields may represent the coextraction of compounds other than those of interest. Therefore, it is necessary to analyze the yield and chemical composition of the extract obtained in order to determine the best processing conditions to satisfy the conditions of obtaining the compound of interest [29].
The OECs obtained for BY(%) are shown in Fig. 5, which shows that a significant decrease in BY(%) can be observed when using nondefatted seeds.
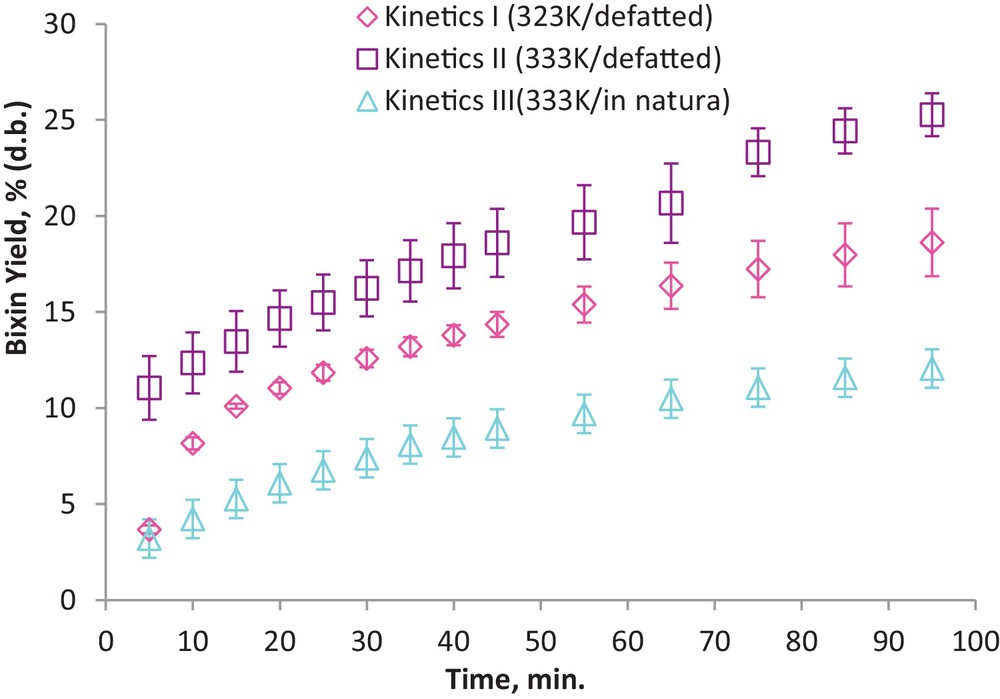
Yield curves for the extraction of bixin BY(%) from annatto seeds (Bixa orellana L.) under different processing conditions. Kinetics I: LPSE, 323 K, defatted seeds; Kinetics II: LPSE, defatted seeds and Kinetics III: LPSE, 333 K, nondefatted seeds.
Vatai et al. [30] performed extractions of phenolic compounds from elderberries and grapes in a single step with ethanol, ethyl acetate and acetone in different proportions with water, at temperatures of 293, 313 and 333 K. Extractions in two stages, combining supercritical fluid extraction (SFE) and conventional extractions, have also been used. Conventional extraction was more efficient than one-step extractions, in which mixtures of organic solvent and water were used at 333 K. For the purposes of comparison, the authors also pretreated the raw materials with supercritical CO2 (with or without ethanol as a cosolvent), and the residual material was re-extracted with a 50% ethanol–water mixture to 333 K. Results show that pretreatment with supercritical carbon dioxide improved the extraction of phenolic compounds. The phenolic yield obtained in extractions over two stages was significantly higher compared to the single-step process.
3.6 Scanning Electron Microscopy (SEM)
With the purpose of analyzing the surfaces of annatto seeds as a means of better understanding the results obtained for the different extraction methods, SEM analyses were performed in 7 seed samples: nondefatted, defatted seeds, defatted seeds extracted by PLE (333 K and 10 MPa), defatted seeds extracted by LPSE-C (333 K), defatted seeds extracted in Kinetics I, defatted seeds extracted in Kinetics II, and defatted seeds extracted in Kinetics III.
Fig. 6a and b show the nondefatted seeds with their homogeneous and intact structures. In Fig. 7a and b, the modifications caused by the supercritical fluid extraction of the oil can be observed; the surface appears to be more porous, which may have helped in the extraction of defatted bixin seeds.

a: nondefatted annatto seeds, magnified 45×; b: nondefatted annatto seeds, magnified 1000×.

a: annatto seeds after extraction with supercritical CO2 (313 K and 20 MPa), magnified 45×; b: annatto seeds after extraction with supercritical CO2 (313 K and 20 MPa), magnified 1000×.
Fig. 8a and b and Fig. 9a and b show, respectively, defatted seeds after extractions by LPSE-C (333 K) and PLE (333 K, 10 MPa), both of which were performed with ethanol. In Fig. 8a, certain highlighted structures were visualized; this may be due to the effect of the cavitation produced by the ultrasound bath. This shows that bixin is indeed present in the surface of annatto seeds. At larger magnification (Fig. 8b), the arils remained in the seeds’ surfaces. The changes in their structures can be better observed through comparison with the corresponding surfaces in Fig. 6b, taken before the extraction was performed assisted by ultrasound. A visible effect of the ultrasound appears in the resinous structure, facilitating the extraction. Fig. 9a and b show micrographs of the defatted seeds after PLE. Note the effect of the pressurized liquid on the resinous aril, which appears to have a rounded shape (Fig. 7b). After extraction with pressurized liquid, this structure was broken.

a: defatted annatto seeds after extraction with LPSE-C at 333 K using a continuous ultrasound, magnified 45×; b: defatted annatto seeds after extraction with LPSE-C at 333 K using a continuous ultrasound, magnified 1000×.

a: defatted annatto seeds after extraction with PLE at 333 K and 2 MPa, magnified at 45×; b: defatted annatto seeds after extraction with PLE at 333 K and 2 MPa, magnified 1000×.
Fig. 10a and b show micrographs of defatted seeds after the extraction of bixin for 95 minutes at 323 K/LPSE (Kinetic I). Damage to the structure can be observed; the perceived disruption and displacement of the surface may be the result of a longer exposure time of the seeds to the solvent at elevated temperatures.
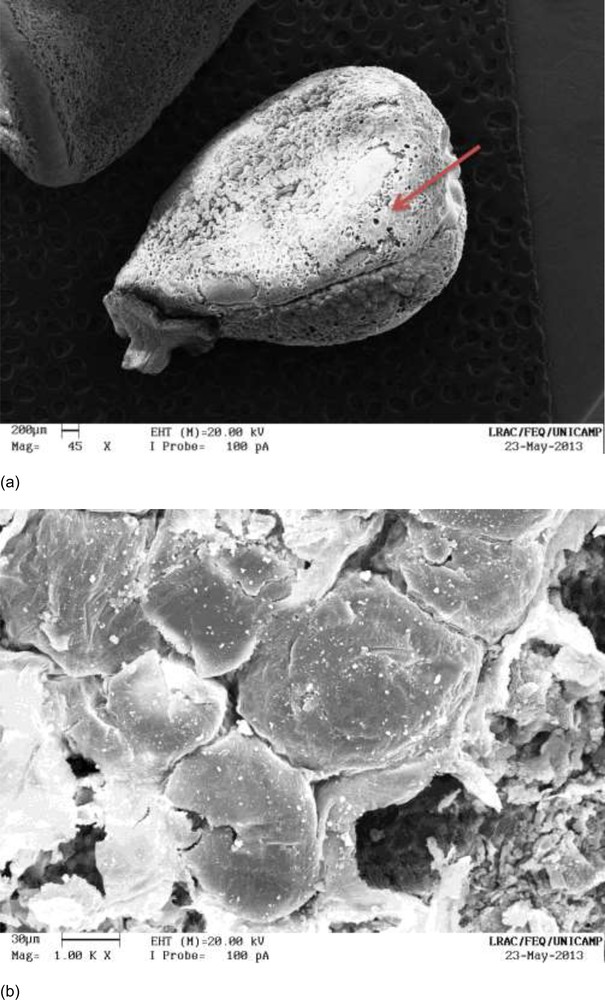
a: defatted annatto seeds after extraction with ethanol at 323 K/LPSE for 95 minutes (Kinetics I), magnified 45×; b: defatted annatto seeds after extraction with ethanol at 323 K/LPSE for 95 minutes (Kinetic I), magnified 1000×.
Fig. 11a and b are micrographs of defatted seeds after extraction for 95 minutes at 333 K/LPSE (Kinetic II). Compared with Fig. 10a and b, it is clear that temperatures influence the displacement of the seeds’ surfaces.
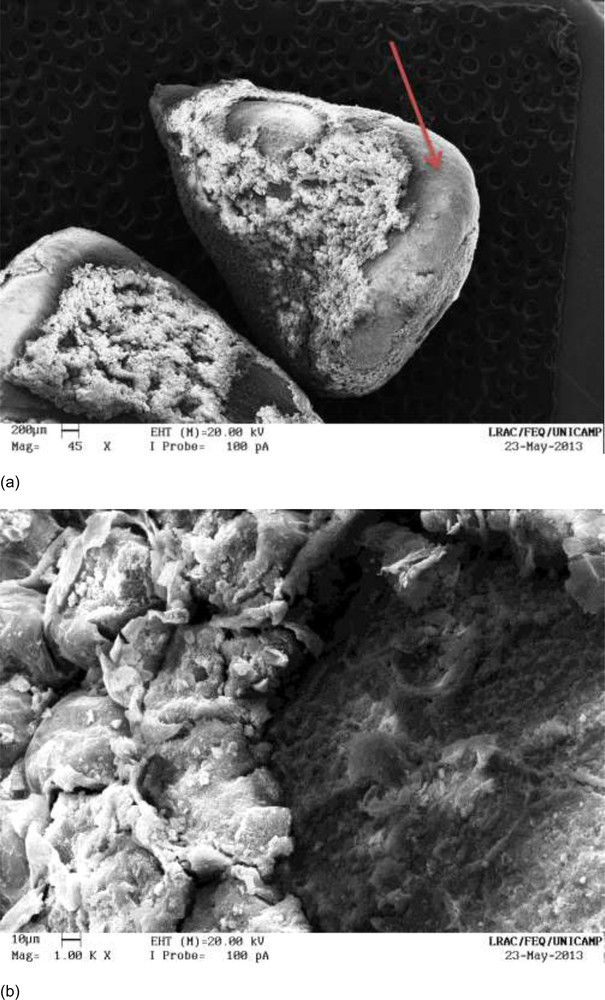
a: defatted annatto seeds after extraction with ethanol at 333 K/LPSE for 95 minutes (Kinetics II), magnified 45×; b: defatted annatto seeds after extraction with ethanol at 333 K/LPSE for 95 minutes (Kinetic II), magnified 1000×.
We were able to easily observe the differences between the seeds shown in Fig. 12a and b, which had not undergone the pretreatment (defatting step), and those shown in Fig. 11a and b, for which defatted seeds were used. In Fig. 12a and b, the surfaces of the annatto seeds were similar to those of nondefatted seeds (Fig. 6a and b), while Fig. 11a and b show a substantial removal of the surfaces.

a: defatted annatto seeds after extraction with ethanol at 333 K/LPSE for 95 minutes (Kinetics III), magnified 45×; b: defatted annatto seeds after extraction with ethanol at 333 K/LPSE for 95 minutes (Kinetic III), magnified 1000×.
Micrographs helped us to understand the results for BY(%). Some authors have claimed that bixin is in the aril of the surface of the annatto seeds. Noble et al. [31,32] stated that the yield increased without milling the seeds, possibly due to the localization of these compounds on the surface of the seed. The highest value of BY(%) was obtained under the conditions LPSE-C/333 K/defatted seeds. Observing Fig. 8a and b, one realizes that these seeds, compared to other seeds analyzed by SEM, were most affected on their surfaces; this finding explains the results of this method.
During the preliminary extractions tests (Table 1) using water at ambient conditions and manual agitation (every 1 minute for 20 minutes) had yields of approximately 24%. In the LPSE experiments for the determination of X0(%), lower yields were obtained using water, indicating that agitation is an important variable.
Barrozo et al. [2] studied the mechanical extraction of bixin seeds using a spouted bed and observed the effects of the air flow and seed mass on the bed, as well as the effect of inserting a suction tube onto the powder-rich bixin. On average, the yield of these extractions was about three times higher when using the suction pipe. The authors concluded that a 10% increase in air flow caused an average increase of 30% in mass extracted, whereas a 25% increase in the seed bed caused an average increase of 33% in the mass extracted.
The results of this study allow us to infer that the use of seeds previously defatted by supercritical CO2 as a raw material facilitates the displacement and subsequent separation of the dye layer of the seed, providing bixin extraction yields of approximately 25% (LPSE/333 K/ethanol). This yield can be obtained without the need for agitation or friction, instead using a simple system for extraction by percolation.
4 Conclusions
The best method to obtain high yields in bixin was determined to be low-pressure solvent extraction using ethanol as a solvent. While water can be used, ethanol produces higher yields and allows for the process to be coupled to a particle formation method, such as SAS (supercritical anti-solvent), to produce solid and micro-sized particles. According to the results of the OECs and micrographs, the use of pretreatment with supercritical fluid to extract the oil is able to increase the bixin yield through a low-cost methodology (LPSE). It has been demonstrated that PLE does not improve the yield using either water or ethanol. The use of an ultrasound bath had no effect on the yield, but it should not be ruled out, because effects from the ultrasound bath were observed in micrographs, showing that the cavitation surface of the ultrasound assisted in the extraction. However, the use of an ultrasonic probe is recommended. LPSE was found to be more feasible at temperatures of approximately 323 K, as there were small differences in X0(%) when the temperature was increased to 333 K. In spite of this, the literature states that the higher the temperature, the higher the yield of bixin extraction. This is because heated cis-bixin is converted to its more soluble form, trans-bixin. Still, using higher temperatures should result in a significant increase in the yield; otherwise, the increase in energy expenditure would not be justified. The use of raw materials previously defatted by supercritical CO2 here is proven to be beneficial to the extraction of bixin.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors acknowledge the financial support from CAPES (DEA/FEA/PROEX), CNPq (470916/2012-5) and FAPESP (2009/17234-9 and 2012/10685-8). L.M. Rodrigues and S.C. Alcazar-Alay thank CAPES for the MSc and Ph.D. assistantships, respectively. M.A.A. Meireles thanks CNPq for the productivity grant (301301/2010-7).


