1 Introduction
The reduction (or deoxygenation) of sulfoxides to the corresponding sulfides is an important reaction in organic synthesis and in biochemical reactions. A survey of the literature revealed that various methods have been reported for the reduction of sulfoxides [1–4]. However, many of these transformations are limited because of disadvantages, such as side reactions, low yields, lack of chemoselectivity, use of expensive reagents, high temperatures, difficult work-up procedures, prolonged reaction times, poor availability and harsh reaction conditions. Only a few reported methods allow rapid and mild deoxygenation of sulfoxides with inexpensive and common laboratory reagents [5,6]. Therefore, the search for alternative efficient and highly chemoselective methods for the reduction of sulfoxides are still a worthwhile goal in organic synthesis.
The reaction of N-halo compounds with triphenylphosphine as a relatively general reducing agent can lead to the formation of phosphonium intermediates. Since phosphorus has a positive charge in these intermediates, so its reaction as a strong oxophilic reagent in most cases leads to the formation of triphenylphosphine oxide, which is thermodynamically preferred [7].
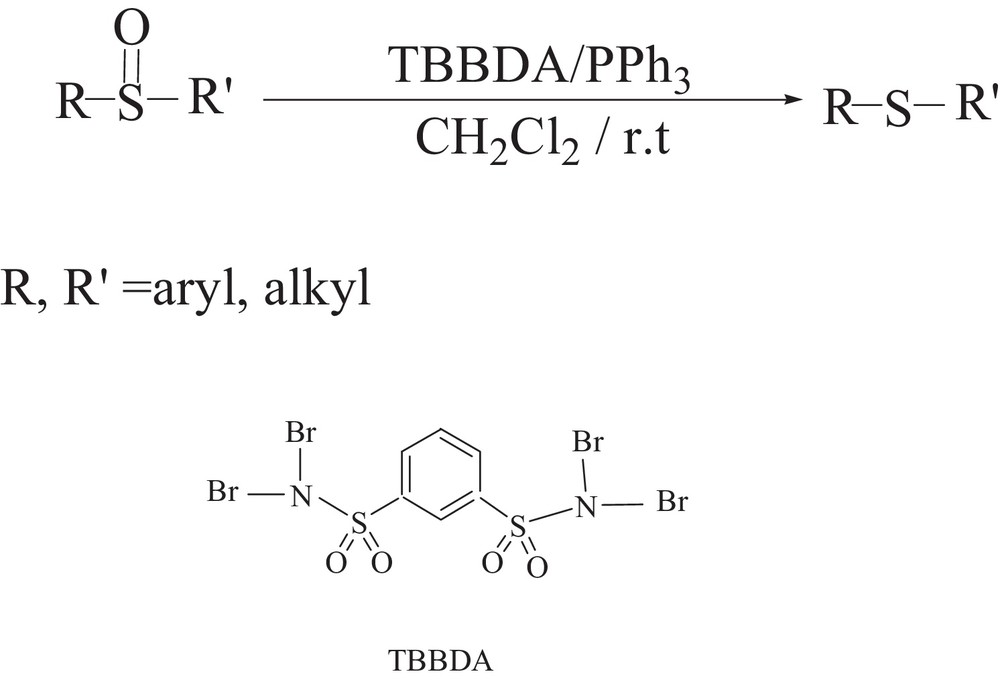
N,N,N’,N’-Tetrabromobenzene-1,3-disulfonamide [TBBDA] has been widely used in organic reactions [8]. However, up to now, it has not been studied as a reagent in the synthesis of sulfides through the reduction of sulfoxides. Therefore, herein, we investigated the successful use of the TBBDA/PPh3 system as a method of reduction of sulfoxides to the corresponding sulfides. The route for the synthesis of sulfides is shown in Scheme 1.
2 Experimental
The chemicals used in this work were purchased from Merck and Fluka Chemicals and used without purification. 1H NMR and 13C NMR spectra were measured for samples in CDCl3 with a Bruker Avance DRX-400 instrument at 400 and 100 MHz, respectively, using TMS as an internal reference. Melting points were measured on a SMPI apparatus.
2.1 General procedure for synthesis of pyrimidine
To a mixture of TBBDA (0.4 mmol) and PPh3 (2.5 mmol) in CH2Cl2 (5 mL), sulfoxide (1 mmol) was added at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction (Table 2), the solvent was evaporated. The crude product was purified by short column chromatography (packed with silica gel, using n-hexane/ethyl acetate (8:2) as the eluent) to achieve the desired sulfide with good to excellent yields.
Selective reduction of sulfoxides into sulfides.
| Entry | Producta | Yield (%)b | Time (min) | Mp (°C) | Reference |
| 1 | 95 | 1 | Oil | [9a] | |
| 2 | 94 | 1 | 43–46 | [9b] | |
| 3 | 95 | 1 | 43–47 | [9c] | |
| 4 | 92 | 1 | 58–62 | – | |
| 5 | 93 | 1 | 60–64 | – | |
| 6 | 94 | 1 | 86–89 | [9d] | |
| 7 | 90 | 1 | 44–46 | [9e] | |
| 8 | 90 | 1 | Oil | [9f] | |
| 9 | 91 | 1 | Oil | [1g] | |
| 10 | 90 | 1 | Oil | [9c] | |
| 11 | 90 | 1 | Oil | [9c] | |
| 12 | 88 | 1 | Oil | [9a] | |
| 13 | 90 | 1 | Oil | [9h] |
a Products were characterized from their physical properties, by comparison with authentic samples, and by spectroscopic methods.
b Isolated yield.
2.2 Physical and spectroscopic data
2.2.1 Benzyl phenyl sulfide
Mp = 43–46 °C; 1H NMR (400 MHz, CDCl3): δ = 4.20 (s, 2 H), 7.24–7.41 (m, 10 H); 13C NMR (100 MHz, CDCl3): δ 39.07, 126.40, 127.26, 128.58, 128.92, 129.84, 136.47, 137.52 (Table 2, entry 2).
2.2.2 Dibenzyl sulfide
Mp = 43–47 °C. 1H NMR (400 MHz, CDCl3): δ = 3.69 (s, 4 H), 7.32–7.43 (m, 10 H). 13C NMR (100 MHz, CDCl3): δ 35.66, 127.07, 128.57, 129.11, 138.24 (Table 2, entry 3).
2.2.3 4-Chlorobenzyl 4-methylphenyl sulfide
Mp = 58–62 °C; 1H NMR (400 MHz, CDCl3): δ = 2.36 (s, 3 H), 4.06 (s, 2 H), 7.11–7.29 (m, 8 H); 13C NMR (100 MHz, CDCl3): δ 21.14, 39.26, 128.59, 128.67, 129.76, 130.20, 131.15, 131.82, 132.85, 136.52, 136.98 (Table 2, entry 4).
2.2.4 Benzyl 4-bromophenyl sulfide
Mp = 60–64 °C; 1H NMR (400 MHz, CDCl3): δ = 4.13 (s, 2 H), 7.18–7.42 (m, 9 H); 13C NMR (100 MHz, CDCl3): δ 39.08, 120.35, 127.39, 128.62, 128.84, 131.47, 131.91, 135.47, 137.06 (Table 2, entry 5).
2.2.5 Benzyl 4-methylphenyl sulfide
Mp = 44–46 °C; 1H NMR (400 MHz, CDCl3): δ = 2.39–2.42 (s, 3 H), 4.15 (s, 2 H), 7.13–7.15 (d, 2 H, J = 8 Hz), 7.29–7.31 (d, 2 H, J = 8 Hz), 7.32–7.36 (m, 4 H); 13C NMR (100 MHz, CDCl3): δ 21.15, 39.82, 127.16, 128.52, 128.92, 129.70, 130.74, 132.56, 136.61, 137.86 (Table 2, entry 6).
3 Results and discussion
N,N,N’,N’-Tetrabromobenzene-1,3-disulfonamide [TBBDA] is an efficient halogenating agent. This compound is an effective catalyst and reagent for various organic transformations. Since TBBDA contains halogen atoms that are attached to nitrogen atoms, it is possible that they act in the same way as Br2. Therefore, it would be expected that the interaction of PPh3 with TBBDA generates phosphonium halides as reactive phosphonium species in our reactions.
To evaluate the solvent's effect, the reduction of diphenyl sulfoxide was carried out under similar reaction conditions using different organic solvents, such as toluene, dichloromethane, methanol, and acetonitrile. The best results with respect to yields and times were achieved using dichloromethane.
In order to optimize the reaction conditions, we first examined the effect of different molar ratios of TBBDA/PPh3 in CH2Cl2 at room temperature for the reduction of benzyl phenyl sulfoxide to benzyl phenyl sulfide as a model reaction. We found that the optimized molar ratio for the reduction of benzyl phenyl sulfoxide to benzyl phenyl sulfide was 1/0.4/2.5 (sulfoxide/TBBDA/PPh3) (Table 1).
Optimization of the reaction conditions for the reduction of benzyl phenyl sulfoxide using TBBDA and PPh3a.
| Entry | TBBDA (mmol) | PPh3 (mmol) | Yieldb (%) |
| 1 | 0.25 | 1 | 40c |
| 2 | 0.3 | 1 | 65c |
| 3 | 0.35 | 1 | 80c |
| 4 | 0.4 | 1 | 95 |
b Isolated yield.
c Reaction not complete after 60 min.
The reduction of various sulfoxides was carried out under optimized conditions. The results are shown in Table 2.
This method is general and can be easily applied to the reduction of a variety of aryl alkyl, diaryl, dialkyl, and cyclic sulfoxides to the corresponding sulfides in excellent yields (Table 2). Sulfoxides, carrying either electron-withdrawing (entry 5 and 6) or electron-donating (entries 7 and 9) substituents, gave the corresponding sulfides in excellent yields, with high purity.
To demonstrate the efficiency of the described method in comparison with formerly reported procedures in the literature, we compared the results we obtained in the reduction of dibenzyl sulfoxide (as a typical example) to dibenzyl sulfide (Table 2, entry 3) with those of other methods. The results show that this method is comparable to the previously reported ones in terms of yields, reaction times, and reaction conditions (Table 3).
Comparison of reduction of dibenzyl sulfoxide to dibenzyl sulfide (Table 2 entry3) by the Ph3P/TBBDA system with some of those reported in the literature.
| Reagent (oxidant/substrate) | Time | Yield (%) | Reference |
| Ph3P/Br2/CuBr/CH3CN/reflux | 45 min | 94 | [1l] |
| NiCI2/NaBH4/THF/0 °C (3:9) | 2 h | 81 | [1a] |
| PhSiH3/MoO2Cl2/PhCH3/reflux | 20 h | 95 | [9g] |
| 2,6-Dihydroxypyridine/CH3CN/reflux | 4 h | 98 | [2c] |
| Ph3P/TiCl4/THF/rt | 2 h | 96 | [3b] |
| TiI4/CH3CN/0 °C | 10 min | 85 | [1m] |
| BF3·Et2O/NaI/CH3CN/rt | 20 min | 98 | [1j] |
| BBr3/CH2Cl2/–23–0 °C | 40 min | 91 | [1k] |
| PhSiH3/HReO4 (5 mol%)/THF/rt | 90 min | 92 | [1n] |
| PhSiH3/ReIO2 (PPh3)(1 mol%)/THF/rt | 30 min | 83 | [1o] |
| Ph3P/TBBDA/CH2Cl2/rt | 1 min | 95 | — |
The mechanism proposed for this transformation proceeds via the activation of the triphenylphosphine by reaction with the N-halo compounds, which leads to intermediate 1. Then, the nucleophilic attack of sulfoxide 2 on intermediate 1 gives intermediate 3. Finally, the nucleophilic attack of triphenylphosphine on intermediate 3 gives the sulfide, that of triphenylphosphine on intermediate 1 and gives the oxide (Scheme 2).

Suggested mechanism.
Several attempts for the reduction of sulfoxides by poly(N-bromobenzene-1,3-disulfonylamide) (PBBS) with PPh3 have failed (Scheme 3).

4 Conclusions
In conclusion, TBBDA/PPh3 is a mild and efficient reagent system for the reduction of sulfoxides into sulfides. This procedure has interesting advantages, such as simple work-up, high yields, short reaction times, and the fact that the reactions can be carried out at room temperature.
Acknowledgements
We are thankful to Bu-Ali Sina University, Center of Excellence and Development of Chemical Methods (CEDCM) for financial support.


