1 Introduction
There is a growing interest in the study of electrical discharges and breakdowns in water. Electrical discharges in liquid can be used in very different applications like water cleaning from organic chemical impurities, inactivation of microorganisms, electrohydraulic crushing of solids, and oil-well drilling [1,2]. In particular, the technology of electrical discharges has been recently developed for enhancing extraction of biocompounds from different raw materials [3–5]. The technology of high voltage electrical discharges is a green extraction technique as it allows enhancing the rate of extracted biocompounds per initial vegetable material at low treatment energy input [3,4]. It has also been shown that this technology can reduce the required diffusion temperature, the diffusion time [6] and the ethanol content in the diffusion solvent [7] as compared to a control extraction. When compared to other physical treatments (such as pulsed electric fields, microwave and ultrasounds), the application of high voltage electrical discharges results in a higher extraction rate than that obtained with pulsed electric fields and ultrasounds [3]. Another advantage of this technique is the low temperature increase due to the treatment [3] as compared to ultrasounds and microwave. However, the use of HVED can produce very small particles with respect to the applied treatment energy and that can lead to a subsequent solid to liquid separation step more difficult [8].
High voltage electrical discharges produced directly in water (electrohydraulic discharge) initiate both chemical reactions and physical processes. It injects energy directly into an aqueous solution through a plasma channel formed by a high-current/high voltage electrical discharge between two submersed electrodes [1]. Contrarily to the electrical discharges generated in gases, the mechanisms of formation of the electrical discharge in water are insufficiently delighted. Two types of processes may lead to the establishment of a conductive channel in water [9]. The first hypothesis assumes development of a gaseous phase first, in which electronic avalanches take place. The second hypothesis posits that a gaseous phase is not required. It assumes that breakdown is governed by multiplication of the charge carriers caused by ionization of the liquid. The confrontation between the so-called bubble theory and direct impact ionization model is ongoing. The electrical discharge leads to the generation of hot, localized plasmas that strongly emit high-intensity UV light, produces shock waves, and generate hydroxyl radicals during water photodissociation.
The general issues and questions regarding the role of electrical discharge processes in extraction of biocompounds include the following: What are the mechanisms occurring during an electrical discharge in water? How are electrical discharges initiated and how do they propagate from one electrode to another? What are the main physical effects induced by electrical discharges that influence cell disruption? How can electrical discharges be applied for the extraction intensification of biocompounds from different biological materials?
Answers to the aforementioned questions are subjects of the current study. The present review seeks for placing these questions and issues within the framework of what is known about electrical discharge processes in extraction of biocompounds.
2 Principles and mechanisms
2.1 Formation and propagation of streamer and arc
With the point-plane electrode system and high pulsed voltage, the breakdown process in water is composed of two distinct phases [10,11]: a pre-breakdown phase (streamer) and a breakdown phase (electrical arc). During the pre-breakdown phase, a “streamer” is initiated at the point tip due to the very high local electric field. In liquids, streamers are composed of thin ionized vapor channels, which propagate toward the opposite electrode. Their typical propagation velocity in water is about 30 km/s (positive high voltage applied to the point electrode) [12]. The ionized gas channels created by the streamer constitute conducting path. When the streamer reaches the grounded plane electrode, an electrical arc takes place within a streamer channel (breakdown phase). Since the arc resistance decreases in a very short time (a few ns), the current rises and the voltage drops very quickly (the gap is almost short circuited). Then, the current becomes mainly limited by the external electrical circuit. A typical recording of current and voltage during one electrical discharge is shown on Fig. 1. The typical value of the total electrical energy per pulse is the sum of the energy first released when the streamer propagates plus the energy later dissipated in the arc.
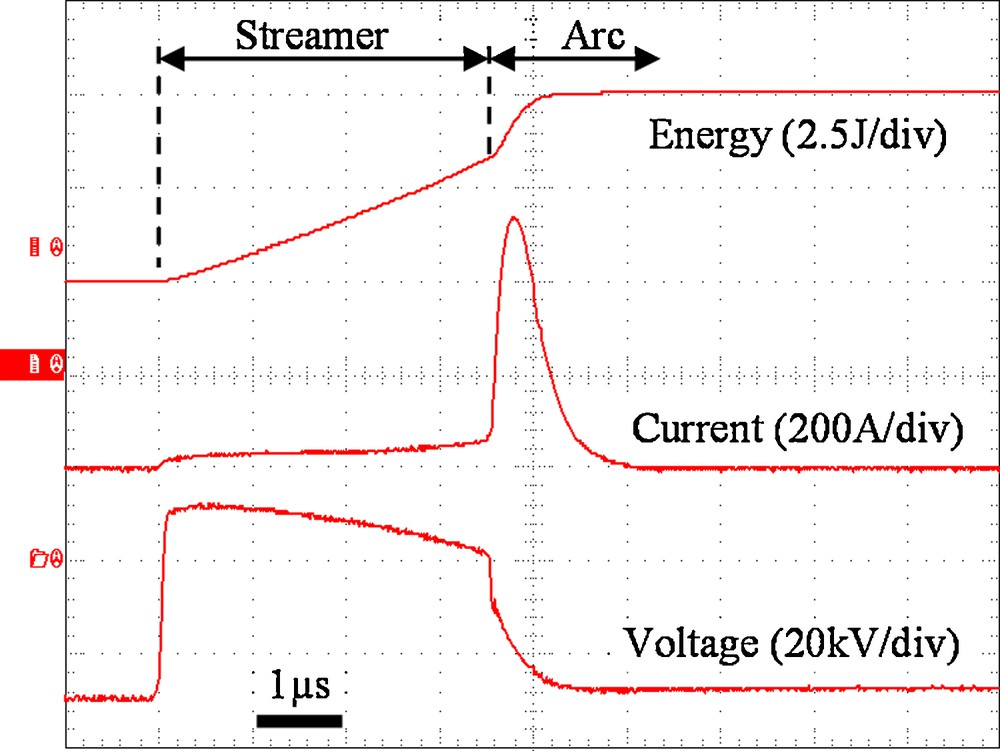
Typical voltage (lower trace), current (middle trace) and energy (upper trace) (inter-electrode space = 2 cm, electrical conductivity of water = 360 μs/cm) [9].
2.2 Formation of the vapor cavities
For both streamer and arc formation processes, a gaseous bubble appears and propagates in the inter-electrode space (Fig. 2) [12]. Four stages are observed: creation, expansion, implosion and collapse of the gaseous bubble. The maximum bubble diameters or filament diameters are about 12 mm and 2 mm respectively during arc and streamer formation. The lifetime of the bubble is longer during arc (2000 μs) than streamer processes (400 μs). The expansion of these bubbles causes the very fast displacement of a large mass of fluid surrounding the plasma channel and thus alters the solid material.
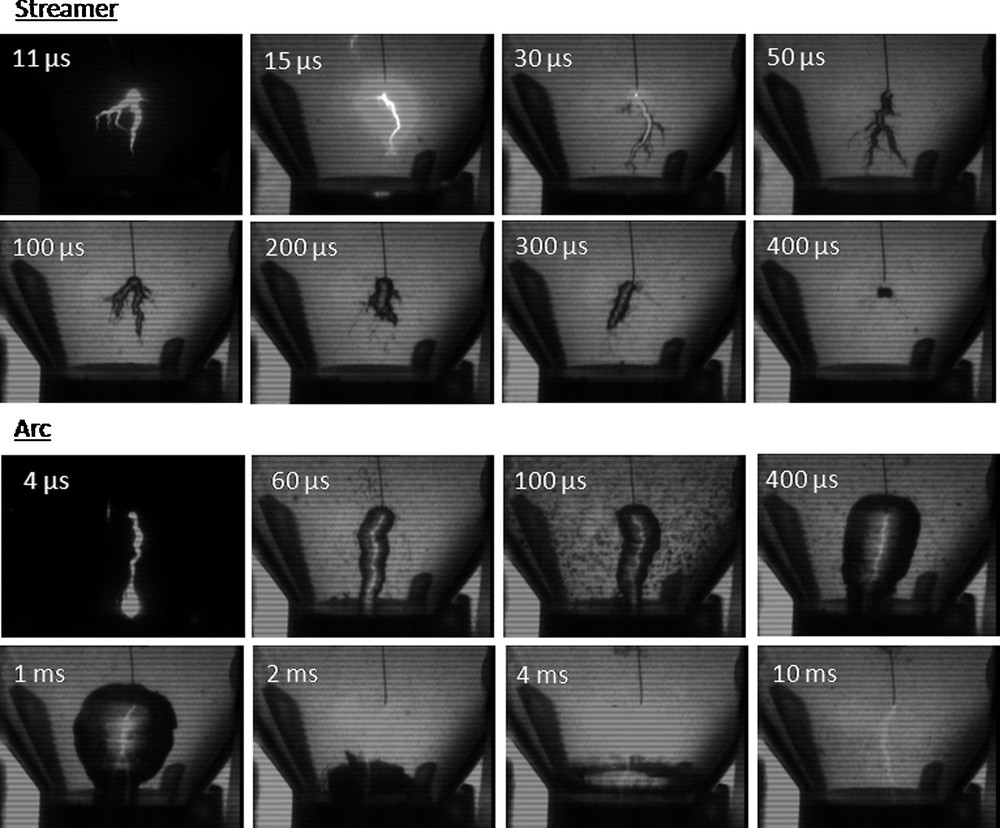
Time-resolved photographs of cavities initiated by streamer and arc phenomena in water, taken with different time delay td after voltage rise. Each photograph corresponds to a different discharge [9].
When an arc occurs as the streamer reaches the plane electrode, the energy dissipated within the filament suddenly increases (Fig. 1). This contributes to further increase the vapor volume. A shock wave is emitted at this moment, and propagates in the surrounding liquid. Its effect is clearly seen on the photograph taken at 100 μs (Fig. 2). A large number of small cavitation bubbles (100 μm in diameter), distributed in the whole liquid volume are observed. Their lifetime is short due to their small size, and most of them already disappeared at 400 μs [12].
2.3 Formation of the shock wave
Depending on the type of the discharge the generated physical and chemical processes also include overpressure shock waves and, formation of various reactive chemical species and molecular species. In the particular case of the arc formation, the electrohydraulic effects are stronger [1]. Arcs are associated with the emission of a powerful shock wave propagating radially into the water. The pressure shock wave is followed by a rarefaction wave that produces cavitations. The collapsing cavitations create strong secondary shocks with very short duration (≈ 60 ns that sometimes result in sonolumeniscence [excitation of light spikes]), and these shocks can interact with structures on the size of cells [13]. Typical pressure profile of a shock wave generated during an electrical discharge in water is shown in Fig. 3. Pressure values of about 100 bar were recorded on the wall of treatment chamber [14]. By means of this pressure profile it is then possible to determine the acoustic energy of the shock wave. The acoustic energy of the shock wave EA (J) is given by the following equation [15]:
| (1) |
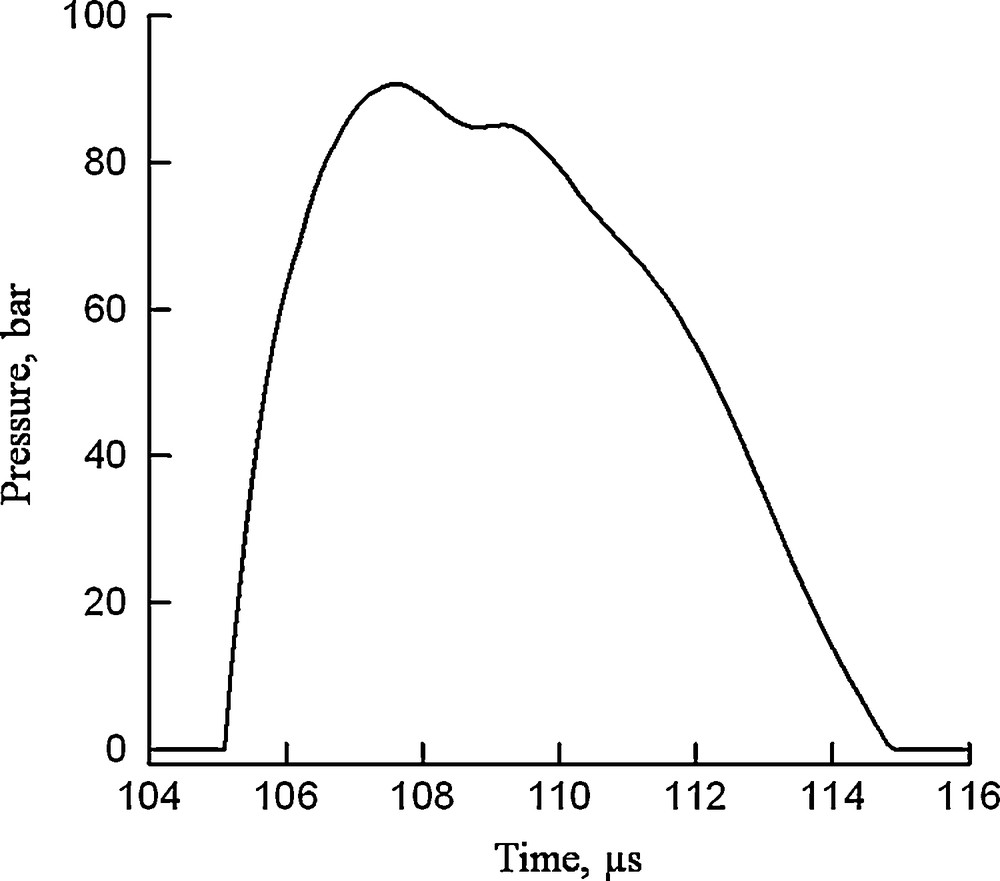
Wave pressure profile during an electrical discharge in water [11].
3 Disintegration of cell structure
3.1 Cell disruption
The degree of tissue damage can be estimated from the electrical conductivity disintegration index Z [17,18] as the electrical conductivity of suspensions is related to the extraction of ionic intracellular components from damaged cells:
| (2) |
3.2 Product fragmentation
At the macroscopic level, the application of electrical discharges on different biological materials (grape seeds, grape pomace, flaxseed cake…) results on the fragmentation of the particles due to the propagation of shock waves and explosion of cavitation bubbles [12]. For example, grape seeds treated by electrical discharges are clearly fragmented after the treatments (Fig. 4) (total specific energy input of 160 kJ/kg, specific energy input per pulse of 0.53 kJ/kg/pulse, pressure shock wave higher than 100 bar [Fig. 5]) for both laboratory and pilot scales experiments [8]. The grape seeds treated with lower specific energy input per pulse (0.02 kJ/kg/pulse), thus generating less stronger shock wave (pressures below 100 bar), were nearly intact and look like the untreated seeds. These shock waves may play an important role in the raw material fragmentation supporting biocompounds extraction. Below the threshold value of about 90–100 bars, shock waves would have a small effect on the product fragmentation (and a less efficient extraction as well) [8].
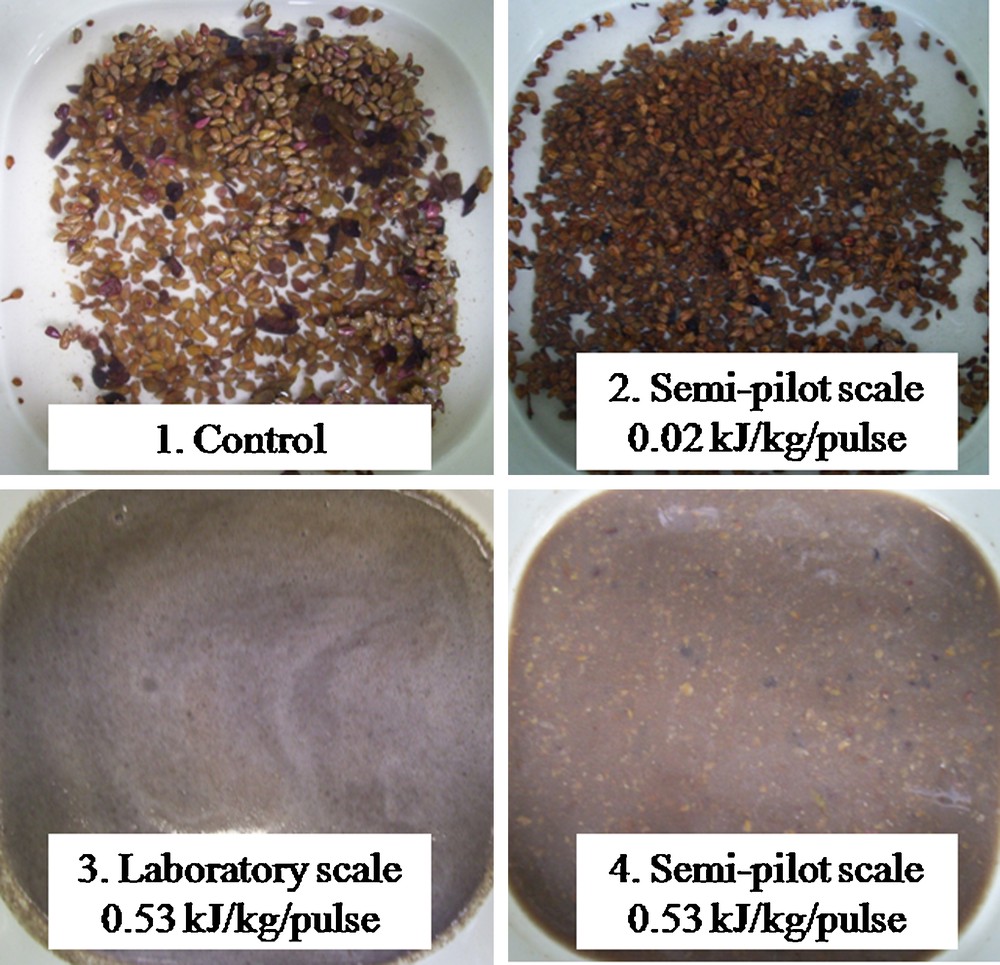
Effect of the energy input per pulse EB and the specific energy input per pulse EBm on the fragmentation of grape seeds suspensions: control samples, electrical discharges treated samples at pilot scale (EB = 0.16 kJ/pulse, EBm = 0.02 kJ/kg/pulse), electrical discharges treated samples at laboratory scale (EB = 0.16 kJ/pulse, EBm = 0.53 kJ/kg/pulse) and (4) electrical discharges treated samples at pilot scale (EB = 0.4 kJ/pulse, EBm = 0.53 kJ/kg/pulse) with a total specific energy input of 160 kJ/kg [11].
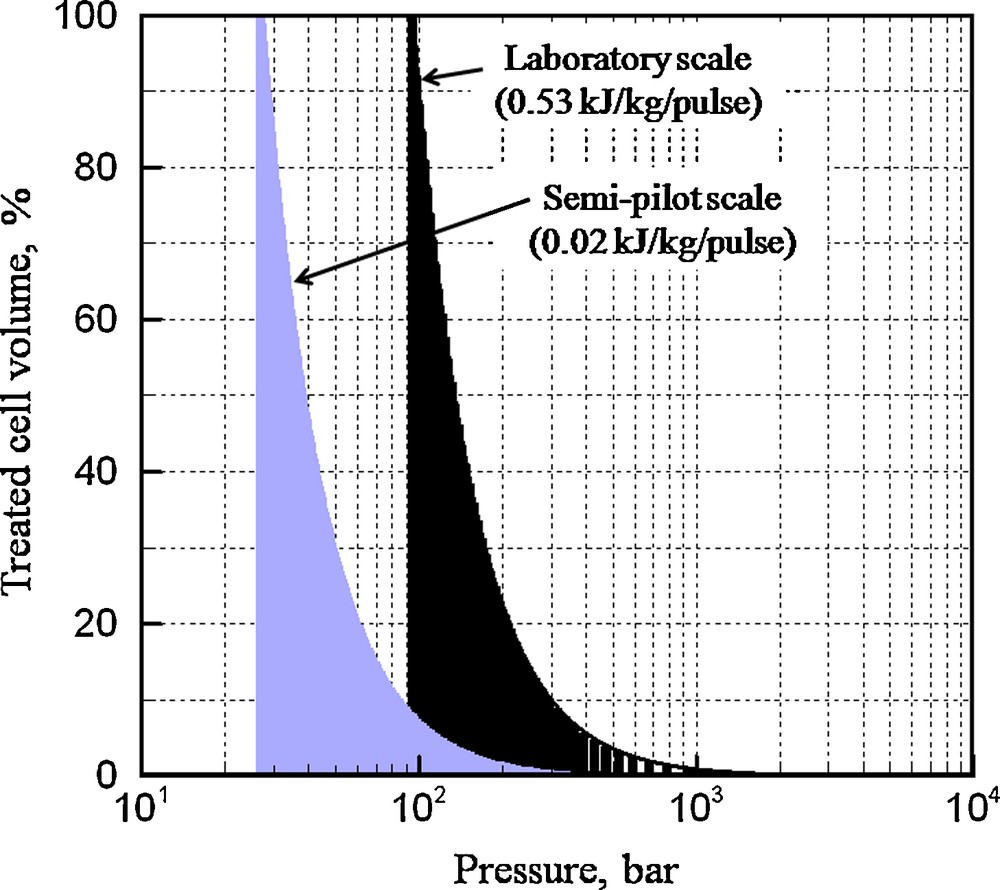
Cumulated volume of treated product as a function of the shock wave pressure (energy input per pulse EB of 0.16 kJ/pulse) [11].
Other studies have also shown that the minimum pressure required to damage biological cells is about 500 bars with shock waves induced by laser [23]. This threshold effect for mechanical damage is a well-known phenomenon concerning the fragmentation of rock [24]. Shock waves are known to mechanically rupture cell membranes [25]. At the front of the shock wave, pressure gradients of about 600 MPa can be reached after only a few micro- or nanoseconds. Cells that are located in this area are submitted to a high compression by a factor of 1.22 in a very short time (only 20.10−12 s) [13]. Taking into account the speed of these events, the effects of shock waves on tissues are located and visible at the microscopic level (cellular or subcellular level).
After effective discharge treatment (shock wave pressure higher than 100 bar), the size reduction of the particles treated by electrical discharge is rather similar to that obtained after grinding the product [8]. For example, in the case of grape seeds, the initial seed (untreated) diameter is about 4000 μm. Applying electrical discharges (53 kJ/kg) on the grape seed suspension allows reducing 20 times the grape seeds size; the seeds residues have a diameter of about 200 μm [8]. Grinding has also reduced the grape seeds size to about 400 μm of diameter. Note that a positive relationship was found between the size reduction after discharge treatment and the biocompounds extraction efficiency. The fragmentation results from the emitted shock waves, but also the development of the main cavitation bubble (Fig. 2), and generation of numerous small cavitation bubbles (Fig. 2) away from the arc. The visually observed turbulence and the intensive mixing also increase the mass transfer [12]. These are probably the main effects explaining the high extraction efficiency of arcs. Electrical discharges can thus be generated in water as a means of cell membrane disruption (and consequent intracellular compounds release).
4 Enhancement of mass transfer processes
The arc discharge leads to the generation of a hot localized plasma that strongly emits high-intensity UV light [26], produces shock waves [1,12,27], and generates hydroxyl radicals during water photodissociation [28]. UV light in the range of 200–400 nm is mutagenic to cells [29], shock waves are known to cause mechanical rupture of cell membranes [25], and hydroxyl radicals lead to oxidative cell damage. As a result, PAED can find various applications, and in particular the extraction of biocompounds [3–5]. The following sections are devoted to extraction of biocompounds from various products.
4.1 Electrical discharge assisted extraction
Electrical discharges have been applied at both laboratory (1 L) and pilot (35 L) scales for the polyphenols extraction from winemaking by-products and oilseed cake [14,21]. Optimal operating conditions for the extraction of biocompounds assisted by electrical discharges from different biological materials have been investigated. The effects of the treatment energy input, the electrodes distance gap, the liquid-to-solid ratio, the extraction temperature and duration, the solvent composition were extensively studied [6,7,22]. One of the most important parameters to consider for discharge extraction optimization is the energy input (or the pulses number). For example, Moubarik et al. [30] studied the effects of electrical discharges on the improvement of the aqueous extraction of solutes from fennel. Electrical discharges (40 kV/cm, 10 kA, 16–72 kJ/kg, 0.5 Hz, 20 °C) were applied to a suspension containing distilled water and fennel slices. The results have shown that immediately after fennel treatment at 50 kJ/kg, the yield of solutes reached about 90%. The subsequent 80 min of diffusion allowed obtaining of the final 99% yield of solutes. When the extraction was performed without pretreatment, the final yield reached only 74%. The results also showed that a higher energy input than 50 kJ/kg did not enhance extraction of solutes. Gros et al. [31] proposed a solvent-free process for mucilage extraction from linseeds by electrical discharges. Electrical discharges (40 kV/cm, 10 kA, 90 kJ/kg, 0.5 Hz) were applied to 50 g of whole linseeds (Barbara variety) immersed in 500 ml of demineralized water (20 °C). The results showed that three successive treatments of electrical discharges were effective and sufficient for almost complete extraction of mucilage. Three short (3 × 10 min) treatments allowed extraction of about twice as much mucilage as in the control experiment with long extraction (liquid-to-solid ratio of 1:10, 360 min). Another study has shown that, when increasing the energy input up to 80 kJ/kg, both total polyphenols content and antioxidant activity could be increased after discharges application from grape pomace (Fig. 6) [7]. However, above this energy value (80–800 kJ/kg), a negative effect of the electrical discharges was observed corresponding to the decrease of polyphenols content. For example, the maximum polyphenols yield reaches 1.37 ± 0.11 g GAE/100 g DM with a corresponding antioxidant activity of 23.02 ± 3.06 g TEAC/kg DM at the optimal treatment energy input (≈ 80 kJ/kg) from grape pomace. The evolution of the content of four main polyphenols from grape pomace (catechin, epicatechin, quercetin-3-O-glucoside and kaempferol-3-O-glucoside) was the same as the total polyphenols amount [7]. For all of these compounds, there also exists an optimal discharge treatment energy input. The oxidative chemical reactions that occur during streamer and arc formation may damage the extracted polyphenols [13]. In particular, for a high-energy input, the quantity of produced oxidizing species is increased and that may oxidize polyphenols.
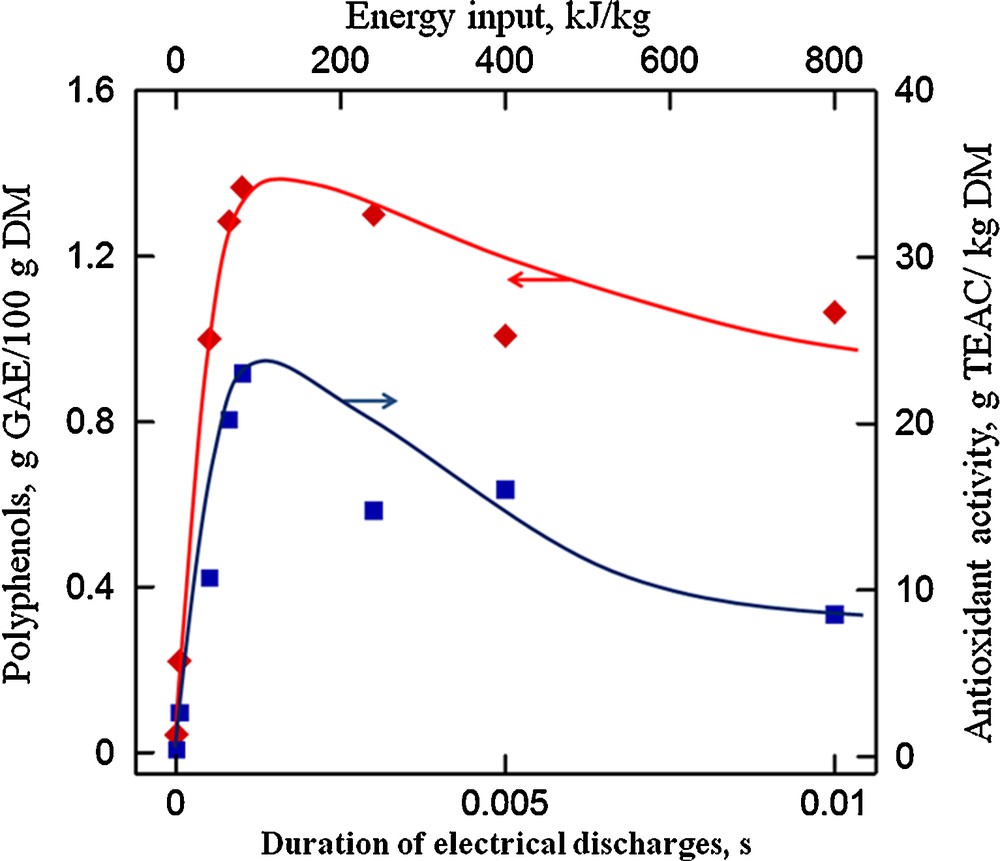
Effect of energy input on polyphenols content and antioxidant activity (grape pomace, inter-electrode distance of 5 mm, liquid-to-solid ratio of 3, solvent: water, T = 20 °C) [27].
At the optimized energy input conditions, the intensification of the extraction of total polyphenols was shown for different biological materials [6,7,14,21,8,30,32]; the extraction yields of polyphenols were increased from grape pomace, grape seeds, grape skins and grape stems treated by electrical discharges at both laboratory and pilot scales (Table 1). For example, Negm et al. [32] studied the extraction of the total soluble matter from dried stevia leaves with the application of electrical discharges. The treatment (40 kV/cm, 10 kA, 30–100 kJ/kg, 0.5 Hz) was applied to an aqueous suspension of stevia leaves (liquid-to solid ratio of 16) at 20 °C or preheated to 55 °C. Increasing the energy input resulted in improved extraction efficiency of the electrical discharges pretreatment. For example, at room temperature, the yield of solutes increased to 48%, 62%, and 68% immediately after 30, 67, and 100 kJ/kg, respectively. Compared with a simple diffusion extraction, electrical discharges applied at the optimized pulse number (100 kJ/kg) accelerated the initial rate of solute extraction by 7.9 and 1.8 times at 20 °C and 55 °C, respectively. These data clearly show that electrical discharges pretreatment can lead to higher yields of solutes at a reduced time of diffusion. Li et al. [33] studied the electrical discharges-assisted extraction of oil from linseed meal. The treatment (40 kV/cm, 10 kA, 20–1000 kJ/kg, 0.5 Hz, 20 °C) was applied to the mixture. When studying the effect of the energy input, the application of a treatment at 267 kJ/kg in water (pH = 7) at 20 °C gave an oil yield of 45% (liquid-to-solid ratio of 10). The effects of temperature (15–50 °C), pH (4–8), and liquid-to-solid ratio (6–14) were also studied for optimization of the operating parameters of electrical discharges in linseed oil extraction. Treatment in suspension at 15 °C, at pH 7, 380 kJ/kg and with a liquid-to-solid ratio of 6 provided the optimal parameters that allowed a higher yield of oil extraction (68%).
Effect of electrical discharges on the extraction of different biocompounds from various raw materials.
| Raw materials | Operating conditions | Biocompounds content (DM: dry matter; GAE: gallic acid equivalent) | Relative increasea |
| Flaxseed cake | Discharges: 181 kJ/kg; Diffusion: 25% of ethanol in water, 60 °C; 80 min | Secoisolariciresinol diglucoside (SDG): 0.3 g/100 g DM | 2.7 |
| Grape pomace | Discharges: 80 kJ/kg Diffusion: 20 °C, 60 min, 30% of ethanol in water | Total polyphenols: 2.8 g GAE/100 g DM | 11.2 |
| Grape stems | Discharges: 53 kJ/kg Diffusion: 20 °C, 10 min, water | Total polyphenols: 0.3 g GAE/100 g DM | 6 |
| Grape skins | Discharges: 53 kJ/kg Diffusion: 20 °C, 10 min, water | Total polyphenols: 0.45 g GAE/100 g DM | 18 |
| Grape seeds | Discharges: 53 kJ/kg Diffusion: 20 °C, 10 min, water | Total polyphenols: 5 g GAE/100 g DM | 150 |
| Discharges: 69 kJ/kg Diffusion: 50 °C, 60 min, 30% of ethanol in water | Total polyphenols: 9 g GAE/100 g DM | 1.5 | |
| Fennel | Discharges: 50 kJ/kg Diffusion: 20 °C, 80 min, water | Total soluble matter: yield: 90% | 1.33 |
| Stevia leaves | Discharges: 100 kJ/kg Diffusion: 55 °C, 60 min, water | Total soluble matter yield: 92% | 1.8 |
| Linseeds | Discharges: 3 × 90 kJ/kg, 20 °C, water | Mucilage yield: 99% | 3 |
| Linseed meal | Discharges: 267 kJ/kg, 20 °C, water | Oil yield: 45% | |
| Palm kernel | Discharges: 20 °C, 80 kJ/kg, water | Oil yield: 11% | |
| Fruit palm | Discharges: 20 °C, 240 kJ/kg, water | Oil yield: 36% |
a As compared to control extraction.
4.2 Biocompounds diffusivity
Biocompounds extraction can be described by Fick's second law (Eq. 3):
| (3) |
Dependencies of biocompounds diffusivity DU,HVED versus the inverse temperature 1/T in the Arrhenius form have been studied for several biocompounds:
| (4) |
5 Conclusions
Despite recent developments in high voltage electrical discharges applications and particularly their positive effect for enhancing the biocompounds extraction, several areas need further research to make this technology feasible at the commercial level: confirmation of the mechanisms of discharge establishment in water; development and evaluation of extraction kinetics models; design uniformity and processing capacity of the treatment chamber; identification and application of electrode materials that can provide longer time of operation and lower metal migration; process system design (including electric generators), evaluation, and cost reduction.


