1 Introduction
The addition of the 1,8-bis(trimethylsilyl)-2,6-octadiene (Bistro) 1 to various ketals afforded a large variety of 1,1-disubstituted-2,5-divinylcyclopentanes.[1] Some of them proved to be valuable building blocks for the synthesis of unnatural steroids[2] or vitamin D derivatives [3]. Scheme 1 shows a good example of this strategy [4].
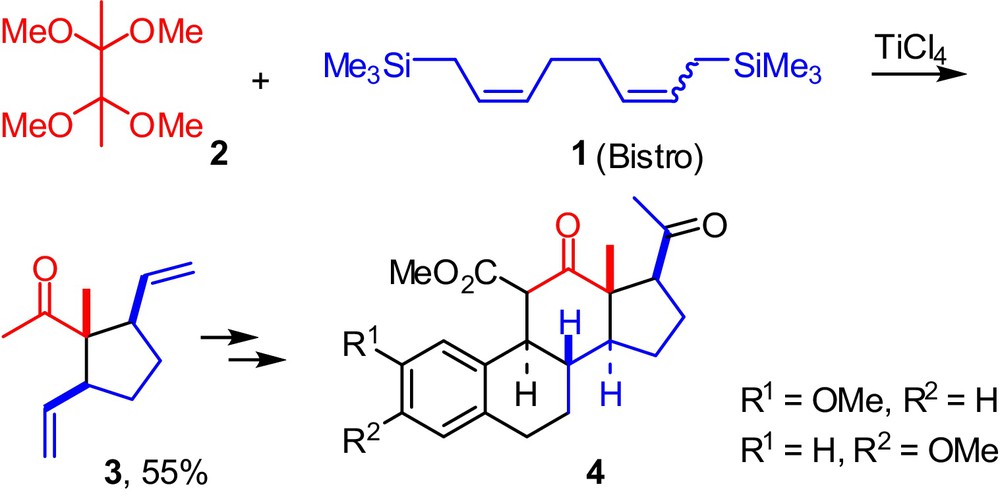
(Color online) Synthesis of unnatural steroids from Bistro 1 and bis-ketal 2.
Then, we have intended to synthesize 11-dimethylaminophenyl steroids analogous of Mifepristone® (RU 486) [5], and other similar antiprogestative steroids [6]. For this purpose, it was necessary to synthesize the 1-(4-nitrophenyl)butane-2,3-dione diketal 6 from the 1-(4-nitrophenyl)butane-2,3-dione 5 (Scheme 2).
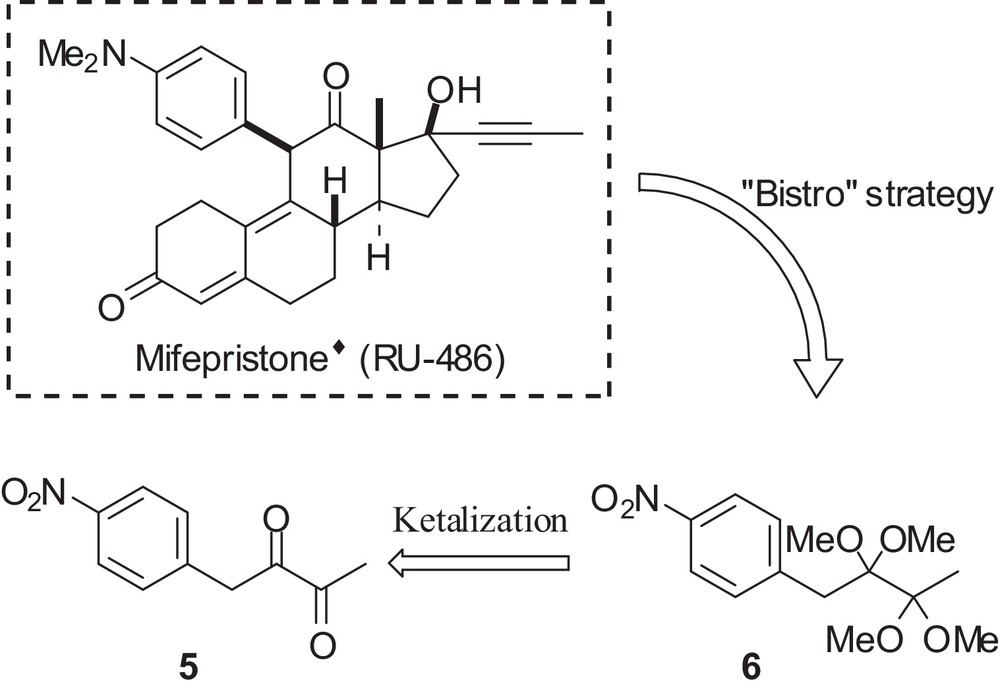
Planned synthesis of α-bis-ketal 6.
2 Synthesis of α-diketone 5
Only few methods are known to synthesize α-diketones [7]. Among them, we chose the ozonolysis of the corresponding β-diketone enolates as it appears to be straightforward. In our case, the suitable 3-(4-nitrobenzyl)pentane-2,4-dione 7 was obtained by alkylation of acetylacetone with p-nitrobenzyl bromide [8] in 70% yield [9]. Surprisingly, ozonolysis of 7 in basic medium followed by the treatment with dimethylsulfide afforded 3-hydroxy-3-(4-nitrobenzyl)pentane-2,4-dione 8 in 51% yield and not the expected α-diketone 5 [10]. To confirm the structure of 8, we also prepared it by treatment of 7 by Oxone® in basic medium (75% yield) (Scheme 3) [11,12].

Synthesis of 3-hydroxy-3-(4-nitrobenzyl)pentane-2,4-dione 8 from acetylacetone.
Ketalization of 8 with methanol in the presence of trimethyl orthoformate and a catalytic amount of sulfuric acid did not lead to the corresponding bis-ketal 9 but to the unexpected 1-(4-nitrophenyl)-2,2,3-trimethoxybutane 12 [13]. This compound resulted from a retro-Claisen like condensation [14,15] in acidic medium. Indeed, after formation of 9, its protonation allowed a fragmentation reaction [16] with formation of enol 10 and then ketalisation of the corresponding tautomeric α-methoxyketone 11 to give 12 (Scheme 4).

Preparation of 1-(4-nitrophenyl)-2,2,3-trimethoxybutane 12 from 8.
The retro-Claisen condensation is a well-known reaction in basic medium and the usual reagents are metal alkoxides and recently Lewis acid salts [17], but, to the best of our knowledge, it was never previously reported in protic medium.
3 Addition of bis(trimethylsilyl)octadiene (Bistro) to ketal 12
The addition of Bistro 1 to ketal 12 led stereoselectively to the unexpected product 13 (Scheme 5) [18]. The structure of 13 was established by spectral data, and then confirmed by a X-ray crystal structure determination (Fig. 1) which revealed two stereogenic centers with the relative configurations R* and S* and the presence of a trans-disubstituted cyclohexane.
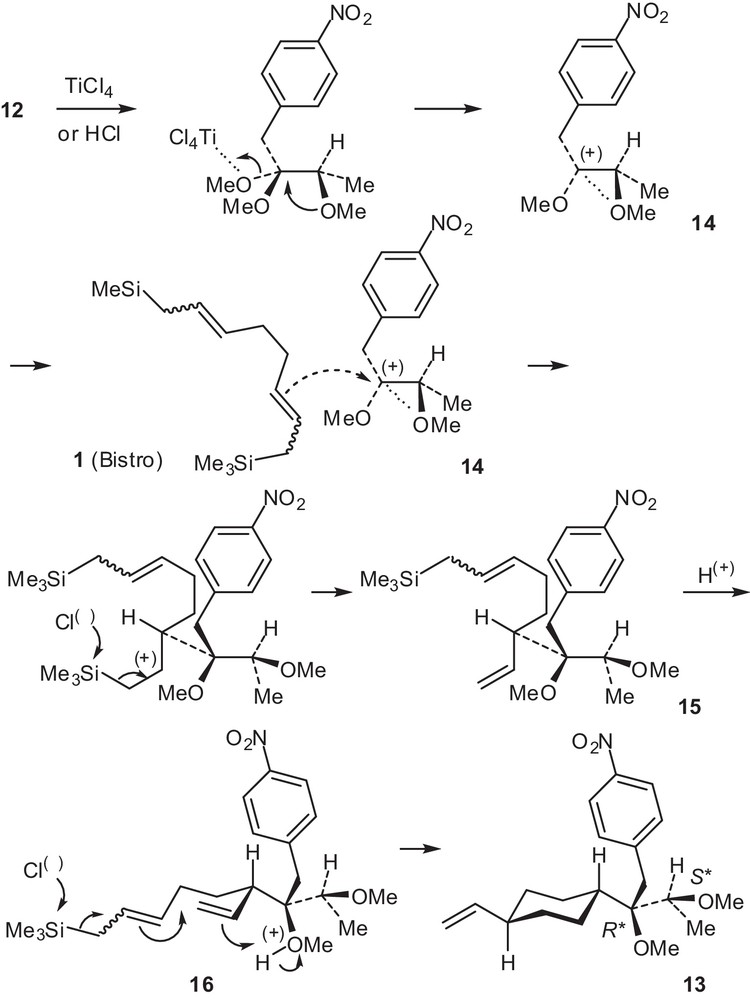
Synthesis of diether 13 from 12 and Bistro 1.

(Color online) ORTEP diagram of compound 13 [22]. The atomic displacement parameters are drawn at the 50% probability level.
The unexpected formation of 13 could be rationalized by the following mechanism. First, TiCl4, or adventitious HCl, led to the stabilized methoxycarbenium ion 14, then, a stereoselective attack of one allylsilane moiety from 1 afforded 15. Then, a protonation of the methoxy group by adventitious HCl gave 16. Finally, a cascade reaction induced by the addition of a chloride anion to the second silicon atom and only the regioselective delivery of this proton to the internal carbon atom of the vinyl group (anti-Markovnikov addition) [19] can explain the stereoselective formation of 13 (Scheme 5). In 16, the protonated methoxy group induces a polarization of the vinyl group, thus increasing the electrophilic properties of the terminal methylene group and stimulating the anti-Markovnikov nucleophilic attack from the second allylsilane moiety.
Because of this proximal effect, the primary nascent carbocation may be promptly captured by the nucleophilic double bond of the second allylsilane moiety [20].
In previous works, we have reported that the addition of Bistro 1 to 2,4-pentanedione mono-ethylene ketal or 2-acetylcyclohexanone mono-ethylene ketal [1b], or 2-methyl-1,3-cyclohexanedione mono-catechol ketal [21], gave rise to bi- or tricyclic alcohols following a similar mechanism, but with a regular Markovnikov electrophilic addition of the carbonyl group to the vinyl group.
Acknowledgments
D.M. is grateful to the French “Ministère de l’Éducation nationale” for a grant, and N.G. is grateful to the CNRS and the Région Provence-Alpes-Côte d’Azur for a grant. This work has been financially supported by the CNRS and the MEN. We thank Dr M. Giorgi (Spectropôle, Aix-Marseille Université) for X-ray structure determination of 13.


