1 Introduction
Thyroid carcinomas are rare and represent 1% of malignant tumors, women being more likely to have thyroid cancer, at a ratio of three to one. Its incidence had a twofold increase during the last decade to a better detection of small papillary cancers by ultrasonography [1,2]. In some cases, the presence of nodule or calcifications [3–10] in thyroid can be noticed through thyroid ultrasonography (US). Epidemiologic studies suggest that nodular thyroid disease is a common clinical problem, with a prevalence of nodules in 4%–7% of the adult population. Even if most nodules are benign, less than 5% of them being malignant, a recent study shows that thyroid calcifications found using preoperative computed tomography (CT) may represent an increased risk for thyroid malignancy [11].
US is widely used to differentiate between benign and malignant nodules. US features predictive of malignancy include hypoechogenicity, intranodular vascularity or microcalcifications and finally ill-defined–irregular edge (or absence) or breaking of the peripheral halo [12–14]. When these criteria are present, a histological diagnosis should be performed by a fine-needle aspiration. Of note, in some particular cases, US may lead to false-positive results [15]. Moreover, as discussed by Mac Henry et al. [16], the nodule size is not always considered as an independent predictor of thyroid malignancy and a nodule smaller than 5 mm could be malignant. Kwak et al. [17] have stated that US can be satisfactory for diagnosis of thyroid carcinomas without a mass that manifest as microcalcifications on sonography. Such histological diagnosis is based on coloration and optical microscopy. In some cases, other techniques such as fluorescence may be useful [18]. All these techniques are not able to determine the chemical composition of the pathological calcification present in thyroid. The fact that the chemical composition as well as the morphology constitute key parameters to establish a significant relationship between pathological calcifications [19–30] and the disease as it is the case for other calcifications has motivated this study.
The aim of this work is to highlight the chemical diversity of calcifications present in thyroid. Here, we used classical characterization techniques such as, last generation Field Effect Scanning Electron Microscopy (FE-SEM) [31] and FTIR [32] to describe, respectively, their structural characteristics at the micrometer scale as well as their chemical composition [33].
2 Experimental
A set of samples including 34 milimeter scale calcifications extracted from 34 thyroids removed during surgical procedures coming from the Service de Chirurgie digestive, Générale et Endocrinienne, CHU Dupuytren (Limoges, France) and the Department of Thoracic Surgery of Geneva have been considered. Of the 34 patients, two had Graves’ disease, 6 had papillary cancer, 4 had benign nodules and finally 22 had multinodular goiter. For 6 patients, the thyroid disease was associated with primary hyperparathyroidism (HPT). All patient-derived tissues were collected and archived at the Tumorotheque of Limoges University Hospital, under protocols approved by the Institutional Review Board (AC N 2007-34, DC 2008-604 and 72-2011-18). Written informed consent was obtained by all subjects of this study. Each sample was only named by a study number, without indication of the name of the patient or potential identification data.
All the calcifications were investigated with a Zeiss SUPRA55-VP FE-SEM in order to describe their morphology at the micrometer scale. To maintain the integrity of the samples, measurements were performed at low voltage (1.4 keV) and without the usual deposits of carbon at the surface of the sample.
All calcifications were characterized using μFTIR spectrometry (Vector 22; Brucker Spectrospin, Wissembourg, France) as previously described [30]. Data were collected in the absorption mode between 4000 and 400 cm−1, with a resolution of 4 cm−1. The different compounds were identified by comparing them to the reference spectra [34].
3 Results
μFTIR data collected for pathological calcifications are listed in Table 1. A careful quantitative analysis of the absorption spectrum and its second derivative revealed some features specific to the presence of different absorption bands of the calcium phosphate apatite [Ca5(PO4)3(OH)] and calcium oxalate groups [35–40]. The ν1 and ν3 P–O stretching vibration modes are measured, respectively, at 960–962 cm−1 and at 1035–1045 cm−1 while the O–P–O ν4 bending mode corresponds to the doublet at 602–563 cm−1 (Fig. 1). A key point in the analysis is linked to the presence or absence of a shoulder in ν3 absorption band which can be used as a fingerprint for the presence of amorphous carbonated calcium phosphate compound [41–43]. Of note, apatite contained also carbonate ions as observed in other biomaterials like bone or tooth and also in kidney stones. Carbonate ions are detected by their ν3 C–O stretching vibration mode around 1420 cm−1 and the ν2 C–O bending mode at 875 cm−1.
Chemical composition of calcifications as given by FTIR.
| Samples | Prot. | Trig. | CA | Polysac. | Chol. | CaOx | Ass. HPT | Pathology |
| 1 | 70% | 5% | 10% | 8% | 0% | <5% | No | Graves' disease |
| 2 | 40% | 3% | 45%a | 3% | 5% | 0% | No | Graves' disease |
| 3 | 40% | 5% | >45%a | 5% | 0% | <5% | No | Papillary carcinoma |
| 4 | 65% | 5% | 15% | 10% | 0% | 5% | No | Papillary carcinoma |
| 5 | 40% | 1% | 35% | 20% | <5% | 0% | Yes | Papillary carcinoma |
| 6 | 25% | 10% | 50%a | 10% | <5% | 0% | Yes | Papillary carcinoma |
| 7 | 90% | 0% | 10% | 0% | 0% | 0% | No | Papillary carcinoma |
| 8 | 45% | 15% | 40% | 0% | 0% | 0% | No | Papillary carcinoma |
| 9 | >53% | 15% | 12% | 15% | 0% | <5% | Yes | Benign nodule |
| 10 | 60% | 30% | 0% | 10% | 0% | 0% | No | Benign nodule |
| 11 | 45% | 50% | 0% | 5% | 0% | 0% | Yes | Benign nodule |
| 12 | 73% | 2% | 15% | 5% | 5% | 0% | No | Benign nodule |
| 13 | >57% | 10% | <20% | 8% | 0% | <5% | No | Multinodular goiter |
| 14 | 80% | 2% | 5% | 8% | 0% | >5% | No | Multinodular goiter |
| 15 | >62% | 3% | 20% | 10% | 0% | <5% | Yes | Multinodular goiter |
| 16 | 25% | 5% | 65% | 0% | 0% | 0% | Yes | Multinodular goiter |
| 17 | 30% | 0% | 70% | 0% | 0% | 0% | No | Multinodular goiter |
| 18 | 50% | 3% | 45%a | 0% | 0% | 0% | No | Multinodular goiter |
| 19 | 50% | 2% | 33%a | 10% | 10% | 0% | No | Multinodular goiter |
| 20 | 73% | 2% | 10% | 15% | 0% | 0% | No | Multinodular goiter |
| 21 | 25% | 1% | 70% | 4% | 0% | 0% | No | Multinodular goiter |
| 22 | 65% | 2% | 0% | 15% | 10% | 0% | No | Multinodular goiter |
| 23 | 40% | 2% | 45% | 0% | 10% | 0% | No | Multinodular goiter |
| 24 | 30% | 10% | 55% | 0% | 5% | 0% | No | Multinodular goiter |
| 25 | 75% | 5% | 5% | 10% | 0% | 5% | No | Multinodular goiter |
| 26 | 80% | 0% | 20% | 0% | 0% | 0% | No | Multinodular goiter |
| 27 | 75% | 5% | 10% | 0% | 10% | 0% | No | Multinodular goiter |
| 28 | 60% | 5% | 35% | 0% | 0% | 0% | No | Multinodular goiter |
| 29 | 80% | 0% | 15% | 0% | 0% | 5% | No | Multinodular goiter |
| 30 | 75% | 0% | 25% | 0% | 0% | 0% | No | Multinodular goiter |
| 31 | 90% | 0% | 0% | 0% | 10% | 0% | No | Multinodular goiter |
| 32 | 80% | 5% | 15% | 0% | 0% | 0% | No | Multinodular goiter |
| 33 | 80% | 0% | 20% | 0% | 0% | 0% | No | Multinodular goiter |
| 34 | 80% | 0% | 20% | 0% | 0% | 0% | No | Multinodular goiter |
a Presence of amorphous carbonated calcium phosphate, Prot.: Proteins, Trig.: Triglycéride, Polysac.: Polysaccharides, Chol.: Cholesterol, CA: carbonated calcium apatite, CaOx: weddellite and/or whewellite, Ass. HPT: Associated HPT.
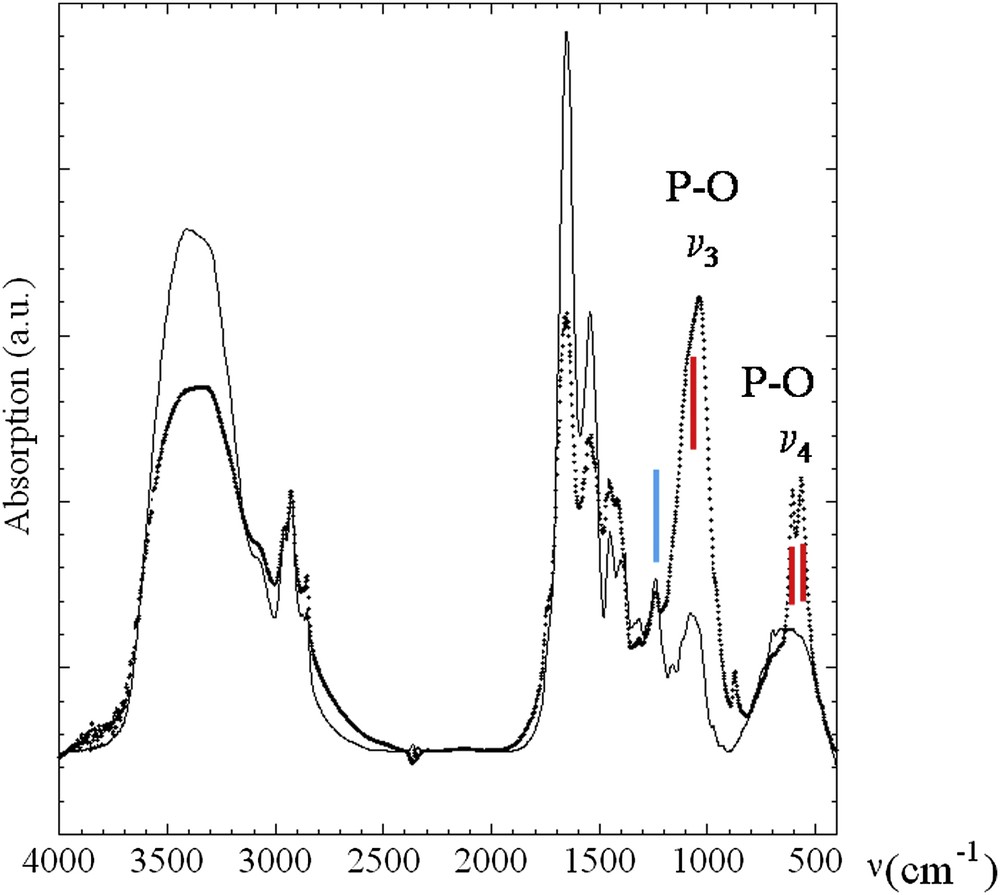
FTIR absorption spectra collected for sample 5 (dots) and sample 6 (line). Red lines correspond to ν3 P–O stretching vibration modes measured at 1035–1045 cm−1 and O–P–O ν4 bending mode (doublet) at 602–563 cm−1. The blue line corresponds to absorption peaks 1315 and 1318 cm−1 and at 780 cm−1 and is used to assess the presence of calcium oxalate in several samples, as weddellite [CaC2O4–2H2O] and/or whewellite [CaC2O4–H2O] species.
Moreover, absorption peaks and peaks on the second derivative spectra between 1315 and 1318 cm−1 and at 780 cm−1 were used to assess the presence of calcium oxalate in several samples, as weddellite [CaC2O4–2H2O] and/or whewellite [CaC2O4–H2O] species (Fig. 1). Finally, the presence of cholesterol has been underlined in the case of several patients through the observations of vibration mode around 1052 cm−1. In four patients (10, 11, 22 and 31), no crystalline phases were detected in the biopsies, but high rates of triglycerides or cholesterol were found. All had a benign thyroid disease. Then, six patients had hyperparathyroidism (HPT) associated with thyroid nodules. These nodules contained more carbonate apatite (33% vs 25% without HPT).
SEM images allow an accurate observation at the micrometer scale of the crystallite morphologies present in pathological calcifications. Fig. 2 shows small spheres probably made of Ca apatite [43,44] while in the case of weddellite (Fig. 3), we can see a crystallite with a bipyramidal structure [45–49]. Regarding FTIR data, it is interesting to underline that while apatite is detected in almost every samples, a high percentage of apatite is observed in the case of papillary carcinoma.

SEM image of spherical apatite crystallites.

SEM image of calcium oxalate dihydrate (weddellite) crystallite.
Finally, we studied the possibility of analyzing the nature of thyroid calcifications on smears or histological sections (Figs. 4 and 5). A microscopic examination of the thyroid tissues removed was systematically done during the operation to detect a carcinoma. The pathology was done on fresh nodules containing calcifications which were cutted and apposed on an appropriate microscope slide to perform a smear by an input apposition, coupled with a frozen section. The thyroid calcifications were noticed by microscopic analysis at different scales (Fig. 4(a) and (b) and Fig. 5(a) and (b)). FTIR spectroscopy was performed on the calcifications localized by examining their spatial distribution and visualized (Fig. 4(c) and Fig. 5(c)) and revealed the presence of apatite (Fig. 4(d) and Fig. 5(d)). Smears and frozen sections were used to determine the nature of thyroid calcifications.
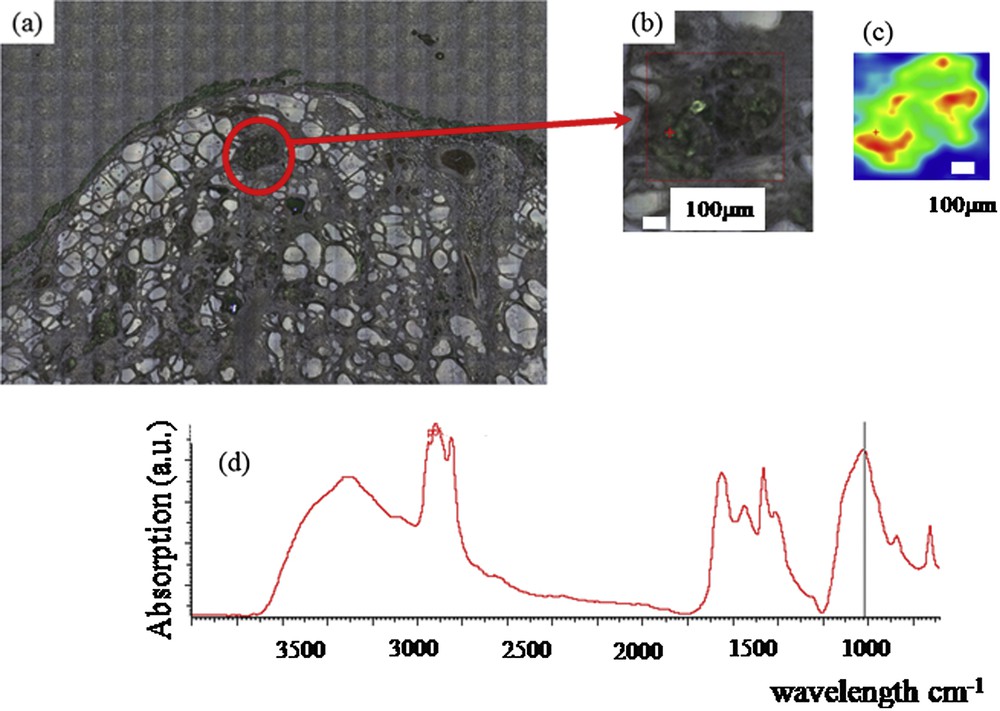
Thyroid tissue (a) and (b) Optical photography at different scales, (c) spatial distribution of phosphate apatite (d), FTIR spectra showing an absorption peak indicating the presence of apatite.
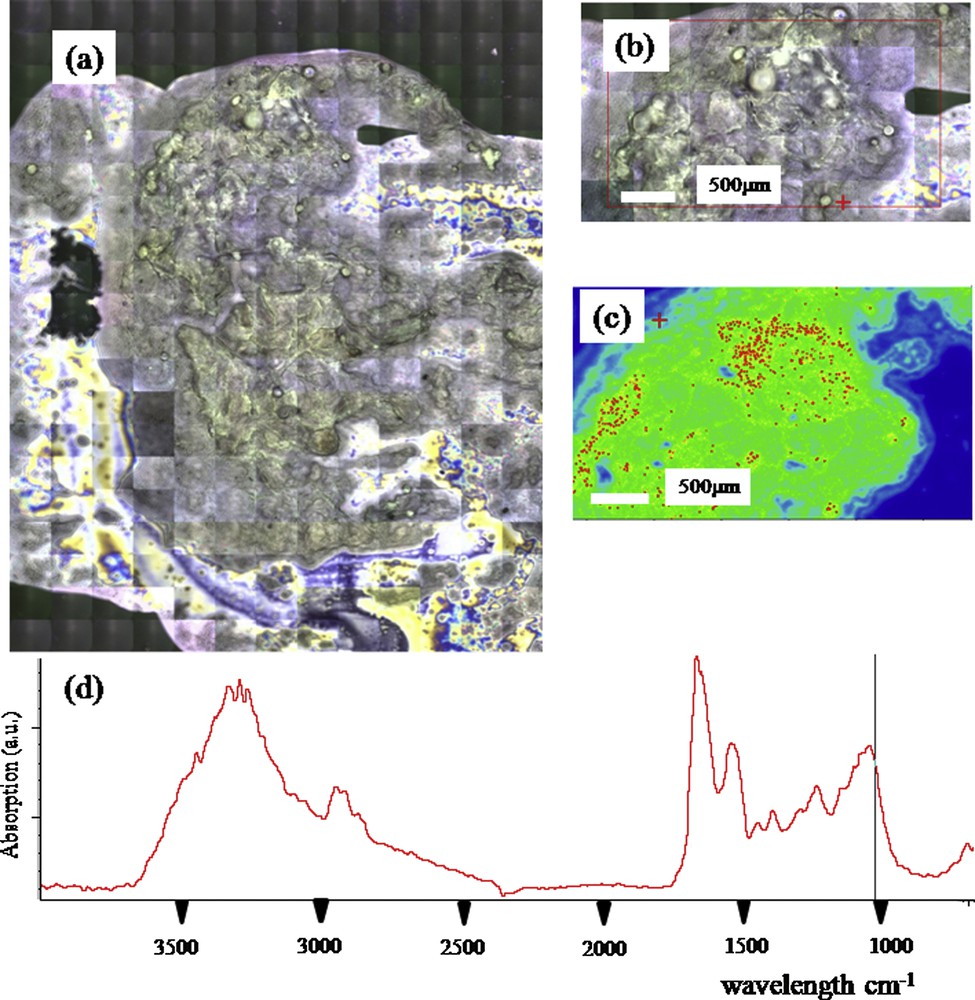
Thyroid smear (a) and (b) Optical photography at different scales, (c) Spatial distribution of phosphate apatite (d) FTIR spectra showing an absorption peak indicating the presence of apatite.
4 Discussion
Pathological calcifications may occur in various parts of the body. For organs producing or in contact with biological fluids such as salivary glands, pancreas, and testis, the presence of mineral or organic deposits has been already underlined [50–52]. More precisely, different investigations have pointed out the chemical diversity of such pathological calcifications present in breast [53,54], kidney [55–57], cartilage [58] and prostate [59,60]. Such chemical diversity reflects the fact that these entities are related to very different diseases including genetic disorders, acquired diseases, eating disorders, infection or cancer.
Establishing a significant relationship between the physicochemistry of pathological calcification and the disease or other factors such as diet or environment, needs at least a two step process. First, experimental data have to show precisely their chemical diversity, with physical techniques capable of distinguishing the different types of calcium phosphate (carbonated calcium phosphate apatite or carbapatite, hydroxyapatite, amorphous carbonated calcium phosphate apatite, octacalcium phosphate pentahydrate, and brushite), different types of calcium oxalate (whewellite, weddellite, caoxite) as well as the possible presence of organic compounds such as anhydrous and hydrated uric acid [61]. Secondly, a large study has to be conducted in order to establish a statistically significant link between the different chemical phases and the various pathologies which affect the organ.
Regarding calcifications in thyroids, as already mentioned [62], any type of sonographically detected calcifications represent a risk of malignancy. Different patterns of intrathyroidal calcifications have been described in ultrasonography. These included « egg-shell » or rimlike peripheral calcification, coarse dense nodular calcification and fine stippled calcifications or microcalcification. The latter represents psammoma bodies that are characteristic of papillary carcinoma. But, other pathologic processes like dense fibrosis or condensed colloid can mimic microcalcification on ultrasonography [63]. The two formers are thought to be dystrophic in nature, occurring in both benign and malignant thyroid lesion. As underlined by Khoo et al. [4], when calcification is noted within a solitary thyroid nodule, the risk of malignancy is very high. Also, calcification of goiter increases steadily with advancing age and is more common in multinodular than solitary thyroid nodules [64].
In this investigation, we have shown a chemical diversity of pathological calcifications present in thyroid. Calcifications made of Ca phosphate (amorphous carbonated calcium phosphate and carbonated calcium apatite), as well as Ca oxalate (weddellite and/or whewellite) have been detected. Of note, the presence of cholesterol has also been underlined. More precisely, among the 34 calcifications, most of them contain Ca phosphate apatite while only nine calcifications contain Ca oxalate. The fact that most calcifications contain Ca phosphate apatite, together with a recent study [65] showing that, of 383 patients undergoing thyroid operations, 135 (35.2%) had intrathyroidal calcifications identified using computed tomography, indicates that most of the thyroid calcifications are made of Ca phosphate apatite.
Also, we tried in this study to establish a relationship between the chemical composition of the calcification and the disease. Such a relationship is not easy to assess. In a recent study, Chen et al. [66] underline the fact that thyroid carcinoma, especially microcarcinoma, often coexists with benign thyroid disease. In this study, Ca phosphate apatite as well as CaOx species has been detected in each pathological condition, namely Graves' disease, papillary carcinoma, benign nodule and multinodular goiter. Regarding associated HPT, CaOx species were present for only 2 patients with HPT and 6 patients without HPT. Taking into account the complete set of data, it seems that there is not a clear relationship between the chemical composition of the calcification and the disease.
5 Conclusion and perspectives
In conclusion, we show through a last generation FE-SEM and a μFTIR experimental device a chemical diversity of millimetre scale calcifications present in the thyroid glands. The complete set of data seems to indicate that there is not a clear relationship between the chemical composition of the calcification and the disease. Nevertheless, from a biochemical point of view, the presence of the various types of crystals is a marker of very different biological conditions.
Note that in the case of calcifications, the study of trace elements can lead to interesting results [67–73]. For example, regarding osteoarthritis cartilage, pathological calcifications may significantly alter the spatial distribution of trace elements such Zn. A part of the Zn may be trapped in the calcification and may modify the associated biological function of Zn metalloproteins [74]. In the case of thyroid, it is possible that apatite calcification well known for its capacity to trap trace elements [75,76] can change the spatial distribution of Iode [77–79]. Moreover, the characterization technique specific to synchrotron radiation such as X-ray absorption spectroscopy can describe precisely the electronic state and the local environment of one specific elements [80–87]. Interesting results have been already obtained in the case of thyroid regarding the status of I [88–90]. All these investigations underline that major breakthroughs can be obtained through an approach combining physics, chemistry and medicine [25,33,91].
Finally, we have started an investigation on ectopic microcalcifications in thyroid following an approach we have developed for ectopic calcifications present in kidney [92]. A set of experiments has been performed either on an in-lab facility or on the beamline SMIS dedicated to FTIR spectroscopy [92]. Fig. 4 presents preliminary results showing the presence of microcalcifications of apatite. Note that the same approach can be performed on intra-operative smears performed on the interest part of the removed thyroid (Fig. 5).
Smears could be obtained intra-operatively from an input apposition or preoperatively with a fine-needle aspiration (FNA). The former increases the diagnosis accuracy of the extemporaneous analysis. Chehrei et al. [93] found that the sensitivity and specificity of intra-operative smears are 78.6% and 98%, respectively, increasing to 100% and 97.6% when a frozen section was coupled. In the preoperative evaluation, FNA is considered to be the most accurate and cost-effective method to detect malignancy. Ten guidelines were published between 2000 and 2013. All advocated US to consider thyroid features such as echogenicity, calcification or margin, and FNA as the procedure of choice in the evaluation of solid thyroid nodules [94,95]. The rate of indeterminate cytology is about 30%, despite the use of the Bethesda system [96]. More recently, L. Yip et al. [97] had proposed to improve the accuracy of preoperative FNA using molecular analysis. He claimed that analysis of BRAF, RAS, PAX8-PPARy and RET-PTC in the FNA issued from thyroid could impact the definitive management of patients decreasing dramatically the rate of indeterminate histology [70]. Despite these results, molecular analysis is not still performed routinely. Thyroid calcifications remain an obstacle to the FNA while they often sign the malignity. These preliminary data constitute thus an exciting new research axis.
Fundings
This research was supported by the physics and chemistry institutes of CNRS (Centre National de la Recherche Scientifique) and by an Agence Nationale de la Recherche contract (grant number: ANR-09-BLAN-0120-02 and ANR-09-BLAN-0120-0). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All the supporters have no commercial interests.
Acknowledgments
We acknowledge SOLEIL synchrotron for provision of synchrotron radiation facilities at beamline SMIS (proposal number 20100566).


