1 Introduction
Water is essential to sustain life, and without it life becomes impossible. It is an indispensable commodity, which should be easily accessible, adequate in quantity, free of contamination, affordable and available throughout the year in order to sustain life [1]. Water is the basic need of life but tragically its quality is continuously deteriorating due to anthropogenic activities [2]. Groundwater is the major source of water supply and presently it is the most valuable natural resource for various human activities [3]. Its use in such a huge amount is due to the scarcity of potable surface water. Due to the presence of a protective soil cover, groundwater is considered purer and safer than surface water [4] and everywhere groundwater supply is developing and is important for everyday human life [5].
Whereas subterranean domains have long been considered as species-poor environments, worldwide analyses reveal an unexpectedly high diversity of living forms in groundwater [6–9]. Due to the size and diversity of habitats, groundwater hosts a very diverse assemblage of adapted taxa [7,10,11], with high rates of endemism and reduced dispersal abilities [12]. Studies and assessments show that, in spite of the severity of the underground environment [13], stygobitic communities present an unexpected richness. This richness, however, very variable in space (regions) and stations (within a region) could be considered as a water quality indicator [14–21].
In Africa, research on subterranean fauna has been progressing in many countries especially in North Africa [6,14,22–26]. However, very little is known about the Beninese stygofauna. To date there are no literature references available on that field from Benin, except recent research performed in the North-Eastern Benin [27]. The present study aims to explore whether a stygobitic community exists and can be related to the physico-chemical quality of well-water in the Pobè region.
2 Material and methods
2.1 Study area and sampling sites
The Pobè region is located in the South-East of Benin (Fig. 1). It covers 400 km2 in the lower part of the Ouémé drainage basin. The climate is sub-equatorial with two dry seasons and two rainy seasons alternately occurring during a year. Rainfall ranges from 1100 to 1200 mm per year. There are two types of soils in this region, hydromorphic soils with a limestone substratum in the northern part and ferrallitic soils in the south. The bedrock is less rich in limestone in the south. The main activity of the population in this rural area is traditional agriculture.
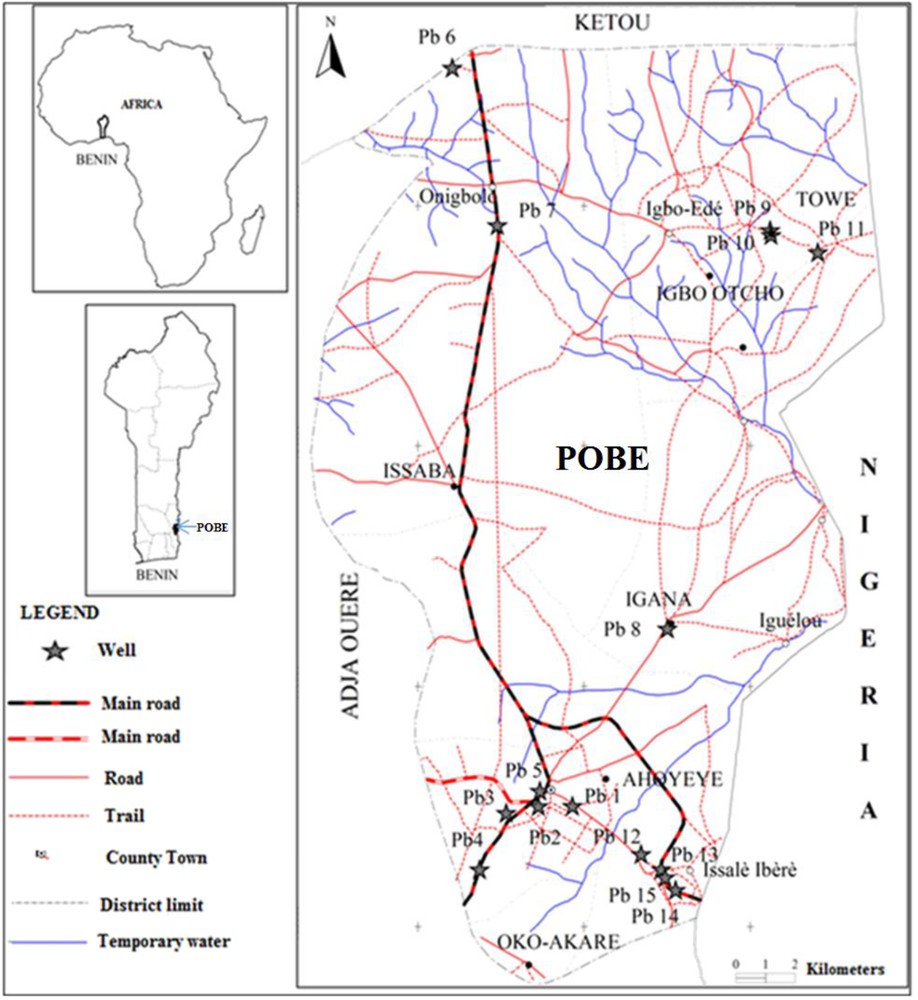
Location of the 15 studied stations in the Pobè district.
A series of 15 dug-wells has been seasonally studied for one year. Five of them (Pb1, Pb2, Pb3, Pb4 and Pb5) were selected within the urbanized district and the ten others are distributed in the rural regions (Fig. 1). Except Pb6, Pb7, Pb8 and Pb11 which are intended for domestic purposes, the others are also used for supplying drinking water. The total depth of the wells varies from 6 m for the shallower ones to a maximum of 50 m for the deepest one. Most of the studied wells have cemented walls except Pb1, Pb7 and Pb11.
2.2 Sampling and analysis methods
Sampling was carried out from June 2012 to March 2013 during the four seasons. The water pH, temperature and conductivity were recorded at the time of water sampling using a portable multi-parameter probe (HANNA, HI 991300). Samples for chemical analysis were collected in washed polyethylene bottles. Nitrate, nitrite, ammonium, sulfate, phosphate and fluoride ion concentrations were determined by using a Hach, DR 2400 spectrophotometer. Total hardness, magnesium, calcium, chlorides, and bicarbonate concentrations were measured by titrimetric methods proposed in [28].
After water collection from each well, the fauna was collected with two types of sampling equipment:
- – a plankton net or a phreatobiological net sampler [29,30];
- – a baited trap made from two mineral water bottles, following the system proposed in [31].
The annual mean of each physico-chemical descriptor is given in Table 1. The collected fauna, abundance and taxonomic richness were calculated and are given in Table 2. Two multivariate analyses using Principal Component Analysis (PCA) and Hierarchical Clustering of stations were performed for the two sets of descriptors. A PCA usually displays the groups of variables (or descriptors, either the physico-chemical characteristics of water, or the different aquatic taxa occurring in the wells) which are highly correlated, and contribute to the formation of the same factor represented by an axis, with positive (or negative) coordinates, such as in Figs. 2 and 4. Then, a hierarchical classification of the different objects (here the wells, and the stations) deduced from the same matrix displays a number of groups, mainly formed by the objects sharing the same set of correlated characteristics (such as in Figs. 3 and 5), visible in projection on the factorial plan of the first axes [32].
Mean values of physico-chemical variables of water from the 15 wells studied in the Pobè region.
| Variables | T (°C) | EC (μS/cm) | pH | F− (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | TH (mg/L) | Cl− (mg/L) | ||||||
| Wells | ||||||||||||||
| Pb1 | 27.6 | 538.2 | 6.19 | 91.7 | 0.598 | 0.388 | 26.0 | 2.84 | 0.05 | 41.76 | 12.90 | 149.0 | 67.45 | 35.07 |
| Pb2 | 28.1 | 288.5 | 5.29 | 107.0 | 0.023 | 0.009 | 8.5 | 1.46 | 0.01 | 22.34 | 18.77 | 98.5 | 50.14 | 13.72 |
| Pb3 | 28.4 | 90.7 | 5.46 | 11.9 | 0.021 | 0.032 | 1.3 | 0.46 | 0.09 | 18.71 | 11.88 | 83.5 | 20.44 | 27.45 |
| Pb4 | 28.4 | 153.1 | 5.17 | 14.9 | 0.012 | 0.009 | 1.0 | 0.80 | 0.03 | 11.35 | 9.80 | 80.0 | 36.38 | 17.53 |
| Pb5 | 28.1 | 192.0 | 5.27 | 74.0 | 0.017 | 0.406 | 6.7 | 0.58 | 0.20 | 19.00 | 12.51 | 90.0 | 29.28 | 25.92 |
| Pb6 | 28.2 | 310.0 | 6.16 | 99.8 | 0.019 | 0.088 | 3.8 | 0.36 | 0.11 | 18.43 | 13.02 | 103.0 | 30.17 | 29.73 |
| Pb7 | 28.5 | 274.2 | 6.26 | 4.3 | 0.086 | 0.053 | 17.5 | 0.22 | 0.09 | 23.61 | 18.96 | 114.0 | 33.72 | 45.75 |
| Pb8 | 27.7 | 287.7 | 6.87 | 6.8 | 0.011 | 1.087 | 17.0 | 0.65 | 0.52 | 41.46 | 20.39 | 150.0 | 31.95 | 58.71 |
| Pb9 | 28.7 | 494.7 | 6.88 | 127.0 | 0.139 | 0.048 | 4.7 | 0.62 | 0.36 | 74.29 | 11.77 | 232.0 | 34.61 | 83.87 |
| Pb10 | 28.7 | 320.5 | 6.66 | 62.1 | 0.038 | 0.126 | 8.3 | 0.94 | 0.09 | 59.31 | 5.81 | 121.0 | 25.74 | 59.47 |
| Pb11 | 28.2 | 239.7 | 6.10 | 13.3 | 0.019 | 0.519 | 1.5 | 0.77 | 0.18 | 42.01 | 6.17 | 104.0 | 22.19 | 48.80 |
| Pb12 | 28.1 | 112.5 | 5.42 | 9.0 | 0.013 | 0.023 | 0.7 | 0.47 | 0.13 | 9.89 | 10.17 | 54.0 | 23.07 | 32.02 |
| Pb13 | 28.1 | 74.8 | 5.18 | 11.7 | 0.031 | 0.074 | 1.0 | 0.31 | 0.03 | 15.20 | 14.34 | 82.0 | 31.95 | 28.97 |
| Pb14 | 27.7 | 76.0 | 5.32 | 16.9 | 0.029 | 0.026 | 0.5 | 0.35 | 0.15 | 16.55 | 15.02 | 70.5 | 17.75 | 31.26 |
| Pb15 | 27.8 | 96.7 | 5.29 | 29.1 | 0.019 | 0.019 | 0.8 | 0.43 | 0.09 | 10.15 | 22.92 | 85.5 | 21.74 | 29.73 |
| Mean | 28.2 | 236.6 | 5.83 | 45.3 | 0.072 | 0.194 | 6.6 | 0.75 | 0.14 | 28.27 | 13.63 | 107.8 | 31.77 | 37.86 |
| SD | 0.34 | 144.9 | 0.64 | 43.5 | 0.150 | 0.297 | 7.8 | 0.66 | 0.14 | 19.31 | 4.94 | 43.4 | 12.76 | 18.41 |
List and abundance of taxa collected in the fifteen wells studied in Pobè region (*Stygobionts).
| Taxa | Pb1 | Pb2 | Pb3 | Pb4 | Pb5 | Pb6 | Pb7 | Pb8 | Pb9 | Pb10 | Pb11 | Pb12 | Pb13 | Pb14 | Pb15 |
| Nematoda | 46 | ||||||||||||||
| Planaria | |||||||||||||||
| Dendrocoelidae | |||||||||||||||
| Dendrocoelum sp. * | 1 | 6 | |||||||||||||
| Oligochaeta | |||||||||||||||
| Naididae | |||||||||||||||
| Dero Aulophorus sp. | 6 | 19 | 1 | ||||||||||||
| Dero Dero sp. | 9 | ||||||||||||||
| Haplotaxidae | |||||||||||||||
| Haplotaxis sp. | 9 | 1 | 3 | ||||||||||||
| Crustacea | |||||||||||||||
| Cyclopida | |||||||||||||||
| Afrocyclops doryphorus | 1 | ||||||||||||||
| Allocyclops n. sp. 1* | 77 | 144 | 2 | 3 | 9 | 1575 | 4 | ||||||||
| Allocyclops n. sp. 2* | 29 | 89 | |||||||||||||
| Mesocyclops aspericornis | 4160 | ||||||||||||||
| Thermocyclops inopinus | 82 | 51 | 5 | ||||||||||||
| Canthocamptidae | |||||||||||||||
| Elaphoidella sp.1 | 127 | ||||||||||||||
| Elaphoidella sp.2 | 2 | ||||||||||||||
| Candonidae | |||||||||||||||
| Candoninae 1* | 170 | 153 | |||||||||||||
| Candoninae 2* | 50 | 4 | 5 | 11 | 3 | 5 | 27 | 52 | |||||||
| Cyprididae | |||||||||||||||
| Cypridopsinae | 1339 | 680 | |||||||||||||
| Cypridinae | 6 | 36 | 3 | 1024 | |||||||||||
| Cypricercinae | 78 | ||||||||||||||
| Stenasellidae | |||||||||||||||
| Metastenasellus n. sp.* | 1 | 24 | 106 | 27 | 949 | 2 | 629 | 28 | |||||||
| Acarina | 1 | 1 | 1 | 1 | 4 | ||||||||||
| Collembola | 1 | 14 | 4 | 2 | 1 | 3 | |||||||||
| Insecta | |||||||||||||||
| Ceratopogonidae | 5 | 8 | 1 | ||||||||||||
| Chironomidae | 8 | 1 | 30 | 6 | 25 | 8 | |||||||||
| Culicidae | 2 | 1 | 70 | 10 | 1 | ||||||||||
| Notonectidae | 2 | 2 | |||||||||||||
| Dytiscidae | 1 | 2 | 14 | ||||||||||||
| Coleoptera und. | 1 | 1 | |||||||||||||
| Taxa und | 9 | 1 | 7 | 3 | 3 | ||||||||||
| Total taxonomic richness | 14 | 0 | 0 | 0 | 11 | 12 | 9 | 5 | 3 | 13 | 7 | 3 | 0 | 3 | 7 |
| Stygobiotic taxa* | 3 | 0 | 0 | 0 | 3 | 5 | 5 | 1 | 3 | 3 | 3 | 3 | 0 | 0 | 0 |
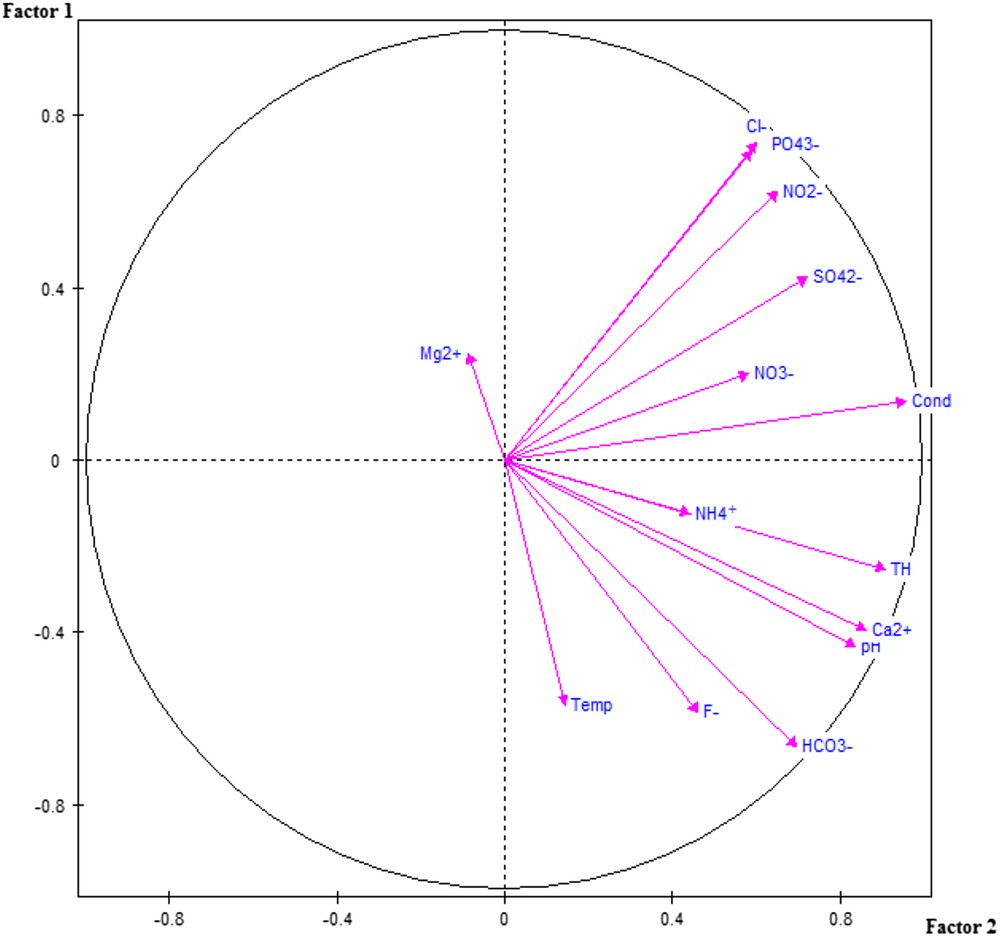
Projections of physico–chemical parameters of the waters on the plane of the two first axes of the PCA.
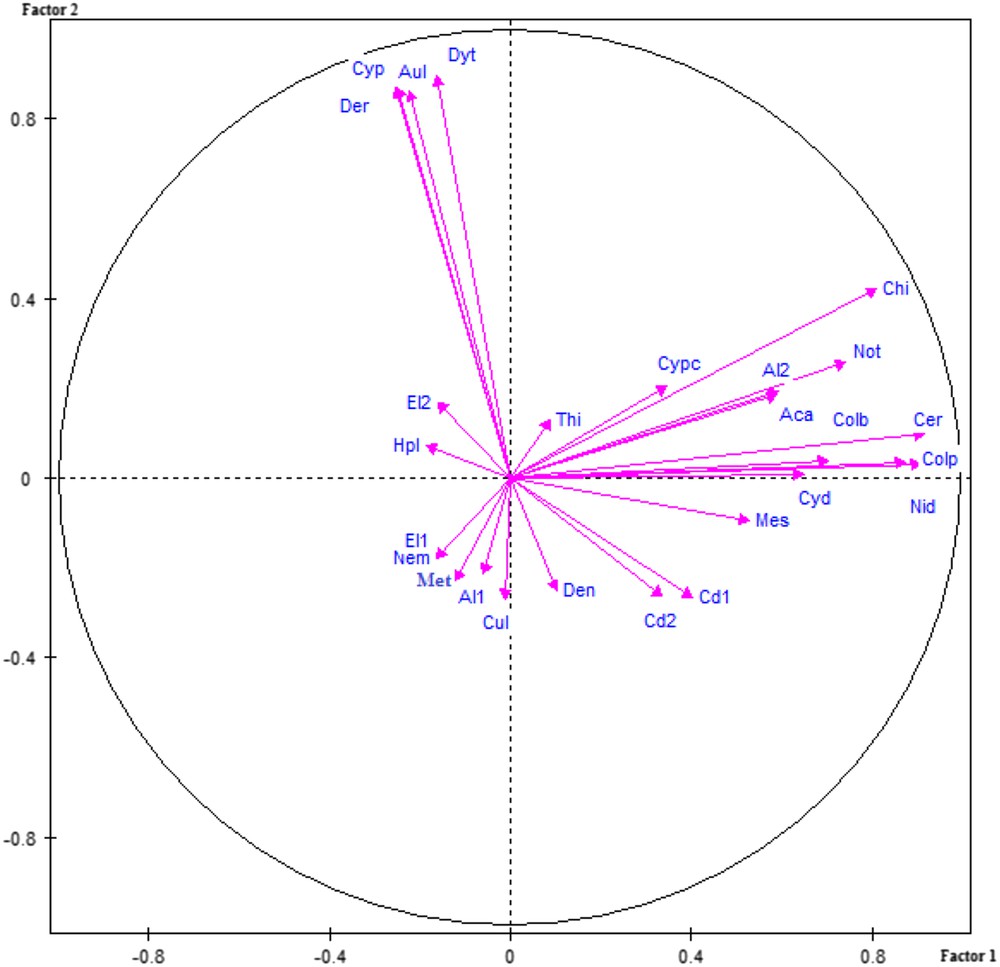
Projections of the sampled taxa from 15 wells on the plane of the first two axes of the PCA.
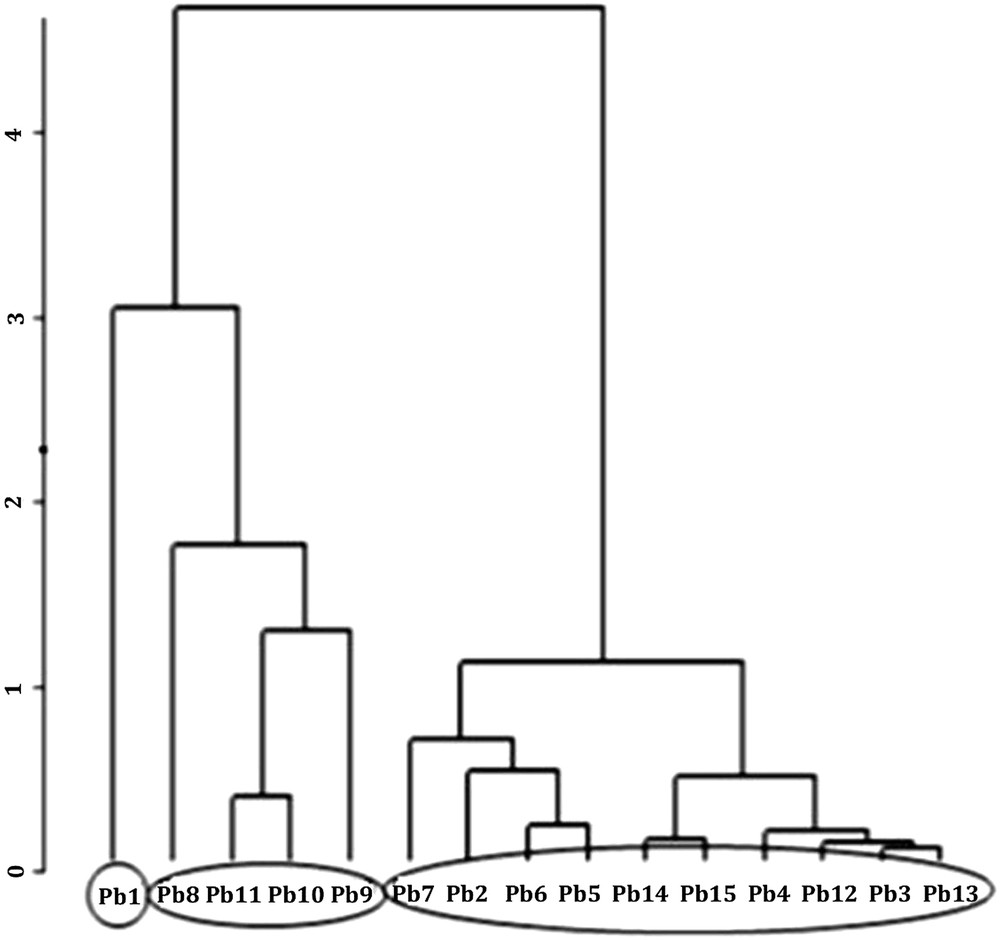
Dendrogram showing a hierarchical classification of the 15 wells obtained from the mean values of physico-chemical characteristics of water given in Table 1.
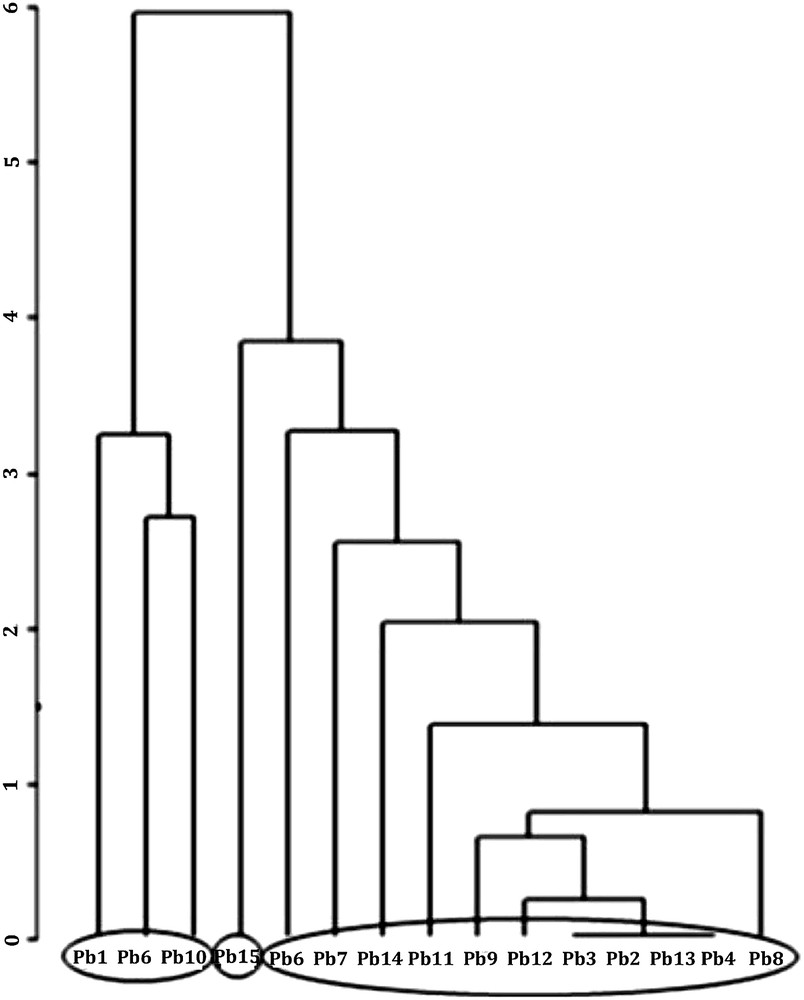
Dendrogram showing the hierarchical classification of the 15 wells described by their fauna.
3 Results
3.1 Water physico-chemical variables
The mean values of the physico-chemical characteristics of the well waters are shown in Table 1. The water temperature varies between 27.6 °C and 28.7 °C with an average of 28.2 °C. The electrical conductivity of the water varies from 75 to 538 μS/cm with an average of 237 μS/cm for all sampled wells. Water from all wells is always acidic with a mean pH that varies between 5.2 and 6.9 with an average of 5.8.
Nitrate ions vary from 4.3 to 127 mg/L with an average of 45.3, and six wells, Pb1, Pb2, Pb5, Pb6, Pb9 and Pb10, have the concentration that exceeds the acceptable drinking-water guideline limit of 50 mg/L [33]. Nitrites ranged between 0.011 mg/L in well Pb8 and 0.589 mg/L in well Pb1 with an average of 0.072 mg/L. The average content of ammonium is 0.194 mg/L and varies between 0.009 mg/L in well Pb2 and 1.087 mg/L in well Pb8. Except in Pb8 and Pb11, the ammonium concentration in well water is within the guideline levels. The following parameters have also their mean concentrations within the guideline levels with the following averages: sulphates (6.62 mg/L), phosphates (0.75 mg/L), fluorides (0.14 mg/L), calcium (28.27 mg/L), magnesium (13.63 mg/L), total hardness (107.8 mg/L), chloride (31.77 mg/L) and carbonates (37.86 mg/L).
3.2 Correlations between different physico-chemical descriptors of water and the typology of sampled wells
A principal component analysis performed on the 14 physico-chemical variables of Table 1 shows that the two first axes cumulate 67% of the total variance. The first axis explains 43% and the second axis 23% of the variance. Axis 1, horizontal in Fig. 2, is strongly correlated in the positive side with electric conductivity, pH, nitrates, nitrites, sulfates, total hardness, chlorides, bicarbonates and calcium. Thus this axis expresses both mineralization and organic pollution.
The second axis (vertical axis on Fig. 2) reveals an opposition between temperature and fluorides negatively correlated with the axis, and chlorides and phosphates positively correlated with the same axis. This factor could express, but with a lower contribution compared to that of the first factor, a part of the water mineralization which could have an anthropic origin rather than a natural geological origin.
The dendrogram representing the hierarchical classification of wells (Fig. 3) obtained from the physico-chemical variables shows, at the first level of score, three groups of stations:
- - Group 1: it contains the well Pb1 only. The electrical conductivity is the highest (538 μS/cm) like the chloride concentration (67.5 mg/L) and nitrogenous ions also occur at high concentrations, for example 92 mg/L of nitrates. Such water clearly shows signs of an important pollution.
- - Group 2: it groups together wells Pb8, Pb9, Pb10 and Pb11. The mean of the electric conductivity is 336 μS/cm and the concentration of nitrates is over 50 mg/L. Thus the water of these wells is also appreciably polluted.
- - Group 3: it brings together the majority of the studied stations and contains wells Pb2, Pb3, Pb4, Pb5, Pb6, Pb7, Pb12, Pb13, Pb14 and Pb15. The mean of electrical conductivity is 167 μS/cm and that of nitrate concentrations is 38 mg/L. Except Pb2, Pb5 and Pb6, water of these wells is globally low in mineral content and without important nitrogen pollution.
3.3 Animal biodiversity of Pobè well water
The fauna collected in Pobè was diversified and recorded 27 taxa (Table 2). Most of them (78%) were epigean. No taxon was collected in wells Pb2, Pb3, Pb4 and Pb13. Among the epigean taxa, Nematoda, the Oligochaeta Dero sp., the Copepoda Afrocyclops doryphorus, Mesocyclops aspericornis, Elaphoidella sp. and the Ostracoda Cypricercinae were rare and observed in only one well. Cypridopsinae, Notonectidae, Dytiscidae and an undetermined coleopteran were also rare but collected in two wells. The remaining taxa were moderately frequent.
Among the hypogean taxa, five were crustaceans regrouping Copepod with two new species [34], two types of Ostracoda and one of Isopoda Stenasellidae with Metastenasellus n. sp. Planaria were rare and represented by Dendrocoecum sp. which is very likely a new species presently under study (work in progress, H. Harrath, pers. comm.); it occurred in only two wells (Pb6 and Pb7). Among the collected Oligochaeta, Haplotaxis sp. was a stygophilous and considered as a possible cryptic taxon (P. Martin, pers. comm.). This genus was recorded in wells Pb5, Pb7 and Pb11. Allocyclops n sp. was collected in 8 wells with two different species in well Pb6. Candonidae and Metastenasellus n. sp. were also recorded in 8 wells. Two types of Candonidae occurred in well Pb1 and Pb7. Candonidae, Allocyclops n. sp. and Metastenasellus n sp. were frequent (more than 50% of the wells).
The taxonomic richness varied between 0 and 14 taxa with a mean of 5.8 taxa per well. Stygobionts were present in wells Pb1, Pb5, Pb6, Pb7, Pb8, Pb9, Pb10, Pb11 and Pb12. Six stygobitic taxa were recorded in Pobè wells and this richness varied from 1 (well Pb8) to 5 (wells Pb6 and Pb7). The mean in those wells was 3.22 and 1.93 taxa in all samples.
3.4 Distribution relationship between different well taxa and faunistic typology of wells
A principal component analysis performed for the 27 taxa in Table 2 shows that both first factors are cumulating only 38% of the total variance (Fig. 4). The first factor explains 23% and the second factor 15% of the variance. Factor 1 is well correlated on the positive side with Chironomidae, Notonectidae, Ceratopogonidae, Coleoptera and an undetermined taxon (larvae or juveniles Insecta). The second factor exhibits also a high positive correlation with Dytiscidae, Cypridinae, Aulophorus sp. and Dero sp. All taxa with a high positive correlation in the two first factors are epigean. The remaining taxa show low correlation with those factors. Most of the stygobionts (5) are at the opposite of the last four taxa on the second factor (Fig. 4).
The dendrogram representing the hierarchical classification of wells (Fig. 5) obtained from the fauna taxa shows, at a relatively basal level of score, three groups of stations:
- - Group 1: This group includes wells Pb1, Pb6 and Pb10; these three wells have the highest taxonomic richness: 12, 13 or 14 taxa were collected, including 3 to 5 stygobitic taxa (with an average of 3.7). Their stygofauna is among the richest of the series of wells.
- - Group 2: This group contains a single well, Pb15. This well is characterized by the complete absence of stygobitic taxa and a quite low total taxonomic richness, not exceeding seven taxa.
- - Group 3: It includes the majority of wells and contains Pb2, Pb3, Pb4, Pb5, Pb7, Pb8, Pb9 Pb11, Pb12, Pb13 and Pb14. Each well delivered less than 12 taxa (0–11), with a mean of 3.7 taxa including occasionally some stygobitic taxa varying from 0 to 5, with an average of 1.6. Compared to that of group 1, the taxonomic and stygobitic richness are lower.
4 Discussion
4.1 Water quality
The temperature recorded during this study was similar to that obtained in [35] and [36] in Equatorial Africa.
The low pH could be related to the rock nature in the African craton countries and such pH has no consequence on living organisms [6].The measured pH values in well waters are similar to those measured in [37] in Togo. Nitrates were probably the clearest indicators of water organic pollution when the water is normally oxygenated. In this study the high concentrations in wells Pb1, Pb2, Pb5, Pb6, Pb9 and Pb10, outside the guideline levels, surely indicated an anthropogenic origin, either fertilizers in cultivated regions or more probably sewage, septic tanks, animal waste, farm manures, etc. Under aerobic conditions, nitrogen is finally converted into nitrate by nitrifying bacteria [38]. A high concentration of nitrate in drinking water is toxic and may cause methaemoglobinaemia for children and gastric carcinomas [39]. Among the six wells with high nitrate values, half of them were recorded in the rural districts far from the Pobè city and the second half only within the urban district. Water in these wells is unfit for human consumption. This high concentration of nitrates is most likely related to the infiltration of sewage or the use of chemical fertilizers near these wells [40]. The total measured hardness values were relatively low. The total hardness is caused by dissolved calcium and to a lesser extent by magnesium. In wells Pb8 and Pb9, the water was slightly hard but had no major effect on human health, it can be the cause for excessive soap consumption and tartar deposits in kitchen vessels, but this is much less important than the occurrence of nitrates that may be linked with other toxic organic matters and pathogenic bacteria.
4.2 Well water fauna
Most of the fauna recorded are epigean taxa. Out of these, some can also be found in stream, ponds or lakes, for example insects, acarina, annelids or some crustaceans. Others, however, come from subterranean water. They normally live in groundwater, and can move between the sand grains and gravels of the aquifers. These are interstitial species in the strict sense. Others can move by burrowing in the sediments, by moving the grains; they are then burrowing species. Six subterranean taxa were recorded in the Pobè region: a Planaria Dendrocoelum sp., two Copepoda: Allocyclops n. sp.1 and Allocyclops n. sp.2, two Ostracoda: Candoninae1 and Candoninae 2, and an Isopoda Metastenasellus n. sp. Based on chaetotaxy and morphology of legs 4 and 5 from the genus Allocyclops, four new species have been described in [34]. Among them, A. n sp.1 and A. n sp. 2 are named. Allocyclops n. sp.1 was recorded along the Ouémé drainage basin but A. n sp.2 appears to be restricted to Pobè and the surrounding regions. Among the 8 species of Metastenasellus known to date, chaetotaxy and morphology of the male pleopod 2 significantly differ from one species to another [41] and the review of this appendix in individuals collected in Pobè indicated that it belongs to a species to be named and described. Dendrocoelidae represented by the genus Dendrocoelum sp. were recorded also in the Parakou region and their specific determination is underway (Harrath, pers. Comm.). Ostracoda Candonidae represented by two morphotypes is new in Benin. Their specific identification and description must be detailed in a forthcoming taxonomic study. The number of stygobitic taxa observed in this study was lower than those observed in Morocco which varied between 11 and 18 [15,42]. This number is greater than that observed in Cameroun and Algeria which varied between 2 and 5 [21,25]. But the same number was observed in [20] and [27]. We just provide here a first look at the Benin stygobitic richness as not much attention has been given so far to this research field.
4.3 Stygobiont occurrence and water quality
Comparing the groups of wells obtained from the two types of descriptors (Figs. 3 and 5), it is easy to make the following observations: – Well groups obtained in the two analyses are different. Well Pb1 whose water is mineralized with high nitrogen pollution (Fig. 3) is found among wells having a high taxonomic and stygobitic richness (Fig. 5). In a group formed with wells Pb8, Pb9, Pb10 and Pb11, the water was relatively polluted (Fig. 3) but the same group contained well Pb10 with a high taxonomic and stygobitic richness and well Pb8 with lower values. No stygobiont was recorded in wells Pb3, Pb4, Pb13, Pb14 and Pb15 whose water quality seemed to be relatively good (Fig. 3 and Table 2). – Wells Pb6 and Pb7 had the highest number of stygobitic taxa (5); however, in well Pb6 the nitrate concentration was high and exceeded by far the WHO guideline levels. In a similar study performed in Algeria [20], the authors clearly showed a similarity among groups of wells obtained with the two types of descriptors. The results of this study are quite different. While some authors have shown that stygobitic taxa could be used as indicators of water quality [16,17,19], others on the contrary emphasized the importance of other factors governing the presence or absence of stygobitic taxa in groundwater [42–46]. These include: interspecific relations, historical events in the regions, characteristics of the sediment forming the aquifers, etc. We observed, for example, in Pobè and also in the Parakou district [27] that Copepods of the genus Allocyclops were absent in wells containing the species M. aspericornis. It appears that this species could be a predator on other Copepods (Fiers, pers. comm). Stygobitic and stygophiles taxa (Metastenasellus, Dendrocoelum, Candoninae and Haplotaxis) often occurred where stygoxene taxa such as Naididae, Cypridinae and Dytiscidae were absent (Fig. 4). A relation of predation or competition could therefore exist between these two groups of organisms. The factors related to the geological history of a region are often responsible for the different distributions of certain thalassoid stygobionts and this was confirmed in [46]. The grain size of the sediment that houses the aquifer has a key role in many stygobiont distributions [43]. It is clear that an aquifer with good water quality and very fine sediments cannot accommodate certain stygobitic taxa. Field observations have shown that all wells in the area with a limestone substratum contain at least three stygobionts. This is the case of wells Pb6, Pb7, Pb9, Pb10 and Pb11 whose water quality is highly variable from one well to another. We also note that all the studied wells with depths greater than 27 m contain no stygobitic taxa. This is the case of wells Pb2, Pb3, Pb4, Pb13, Pb14 and Pb15. Except for well Pb15, all others are well covered by a concrete slab. The absence of stygobitic could be explained by food scarcity or by other different properties of the aquifers at a certain depth in the Pobè region. All these hypotheses remain to be re-examined in future studies.
5 Conclusions
The study of groundwater fauna and water quality performed in the Pobè region has allowed us to draw the following conclusions:
- - The recorded fauna is dominated by epigeal taxa (21). Six stygobionts are reported for the first time in this region. Among them, Allocyclops n. sp.1, Allocyclops n. sp.2 and Metastenasellus n sp. have never been identified before.
- - The water physico-chemical quality varies significantly from one well to another. This variation is related to mineralization and especially to nitrogen pollution which sometimes exceeds the World Health Organization guideline levels in some wells. The causes for such situations have also to be examined in the future and solved, as they concern a public health problem when the polluted wells are still used as a drinkable water resource.
- - Principal component analysis followed by Hierarchical classification was used for the physico-chemical parameters and for faunal data. The groups of wells obtained from the two types of descriptors (at a chosen level of partition) were quite different and did not provide a link between water quality and richness of stygobionts. Interspecific interactions, the nature and geological history of the aquifers are factors to be taken into account to explain the stygobiont distribution. And it is very likely that further similar studies, even in the same region, (where four sampling only were performed at each station) would lead to a development of our knowledge and understanding of the stygobiodiversity of Benin, and also of the complexity of the ecological research aiming at an explanation of the micro-distribution of stygobitic taxa in Beninese aquifers.
Acknowledgements
This work was supported by the commission of West African Monetary Union and the Global Taxonomic Initiative, a DGD-RBINS program. Many thanks are due to the High Ministry of Education and Scientific Research of Benin. We thank the members of the Laboratory of Water Analysis at Parakou and the numerous scientists from different African or European countries, specialists of different taxonomic groups, who provided invaluable help by identifying our material.


