1 Introduction
Structurally novel and diverse heterocycles are very useful in drug discovery and related fields [1]. Among the large varieties of heterocycles, 1,5-benzodiazepines have been integral parts of many drugs, therapeutic leads, and bioactive naturally occurring substances [2]. In fact, compounds possessing the 1,5-benzodiazepine scaffold show a broad range of biological activities including antioxidant and anthelmintic, anti-Alzheimer, and antiproliferative activity against some cancer cells [3–5]. Importantly, these diazepinic structures are also known to act on the central nervous system with anxiolytic and hypnotic activities, it is also the most widely prescribed minor tranquilizer in current use [6].
Moreover, triazoles and isoxazoles are an important class of five-membered ring system, displaying a wide variety of biological properties including antiviral [7], antidepressant [8], and anti-inflammatory activities [9,10]. Particularly, 1,2,3-triazole derivatives have been reported as antibacterial, antifungal, antitubercular, anticancer, antihypertensive, anticholinergic, and anti-inflammatory agents [11–14]. They are of great importance in medicinal chemistry because they can act as pharmacophores and linkers between two or more substances of interest in molecular hybridization approaches [15]. Most of articles report biologically active benzodiazepine conjugates whose two pharmacophores are linked together through a 1,2,3-triazole moiety specifically positioned on the phenyl moiety of the fused ring system [16–19]. In view of the biological importance of the benzodiazepine scaffold and to increase the breadth of accessible library members of this family, it was of considerable interest to develop novel structures incorporating both the diazepinic structures and five-membered ring linkers at position 2′, hoping that such a combination could provide more biologically effective 1,5-benzodiazepines [20]. It is well known that the conventional route to triazole and isoxazole relies on [3+2] cycloaddition between alkynes and organic azides or nitrile oxides, respectively [21,22]. We describe in this article an efficient synthesis of a series of novel 1,4-disubstituted 1,2,3-triazoles and 3,5-disubstituted isoxazoles linked to the diazepine ring via Cu(I)-catalyzed 1,3-dipolar alkyne–azide coupling reaction of the 4-(2-O-prop-2-yn)N-alkyl-1,5-benzodiazepin-2-ones 3a–c with aromatic azides 4a–e or arylnitrile oxides 5a–e, respectively.
2 Results and discussion
In a previous work, our research team has reported the preparation of the 1,5-benzodiazepinone 1, which manifested very interesting anticonvulsant and hypnotic activities (Scheme 1) [23]. Moreover, we have also recently reported the preparation and subsequent determination of photoluminescence properties of an underexplored family of 1,5-benzodiazepin-2-one derivatives [24]. In continuation of these efforts and as a part of our ongoing interest in the synthesis of readily derivatizable benzodiazepines in particular in the context of multitarget drug approach, we investigated herein the synthetic viability of introducing five-membered ring linkers at the position 2′ of the benzodiazepine scaffold (Scheme 1). First, with the aim of establishing a convenient divergent synthesis of a library of readily functionalizable benzodiazepines modified on the phenyl ring system, we investigated whether the regioselective hydroxyl functionalization of the key intermediate 1 was possible in the presence of the primary amide. Under strictly the same reaction conditions (base, solvent, and temperature), we observed that methylation occurred specifically on the amide moiety, whereas the acylation took place only on the hydroxyl group, an interesting results, which could presumably be interpreted in terms of the hard and soft acids and bases principle. Indeed, whatever the alkyl halide involved in the reaction (methyl-, ethyl iodide, or benzyl bromide) only one product was formed, which was identified on the basis of their spectral data as the N-substituted-1,5-benzodiazpin-2-ones 2a–c. The presence of the deshielded phenolic hydrogen singlet observed at 13.90 ppm and the disappearance of the amide-NH proton signal from the 1H NMR spectrum of compound 2a–c confirm the obtention of the N-substituted-1,5-benzodiazepin-2-ones 2a–c (Scheme 1).

Regioselective synthesis of compounds 2a–d.
Moreover, the 1H NMR spectrum of compound 2c recorded at 300 MHz in CDCl3 revealed two doublets at 3.15 and 4.36 ppm assigned to the methylene protons H-3a and H-3b, respectively, and a singlet at 5.13 ppm corresponding to the methylene H-1″. In addition, the intensity of the signal in the aromatic region (δH 6.99–7.93 ppm), which corresponds to the aromatic protons of the starting material 1, has accordingly increased with those introduced by the phenyl moiety of compound 2c. On the other hand, using acylating groups such as acetyl chloride under similar reaction conditions (1 equiv of NaH in THF at 0 °C), we unexpectedly obtained only the product of O-alkylation, that is, the 2′-O-acetylphenyl-1,5-benzodiazepin-2-one 2d, whose structure was determined by NMR spectroscopy and unambiguously confirmed by X-ray diffraction analysis. Crystals for X-ray analysis were obtained by slow evaporation of a methylene chloride (DCM)/MeOH solution. The crystal structure has been determined from single crystal X-ray diffraction (Fig. 1).
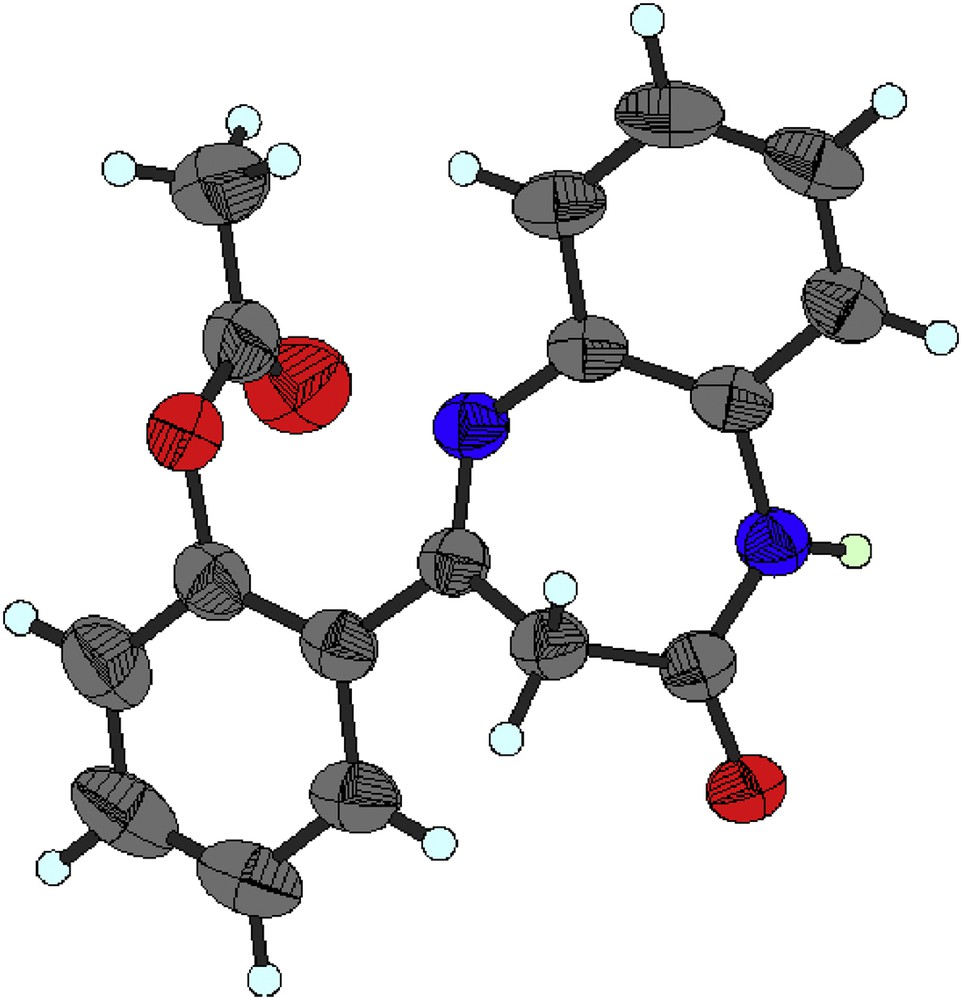
Crystal structure of compound 2d: thermal ellipsoidal representation (50% of probability).
However, phenyl ester derivatives such as 2d could be readily hydrolyzed under physiological conditions, which are required for potential biological applications. To form a nonhydrolyzable ether bond from the hydroxyl group, the amide was first derivatized before functionalization of the hydroxyl group (Scheme 2). Accordingly, the propargylation of the hydroxyl group of the diazepine 2a–c was achieved in acetonitrile in the presence of K2CO3 and provided the corresponding 4-(O-prop-2-yn)N-alkyl-1,5-benzodiazepin-2-ones 3a–c in excellent yield (89–95%). Thus, as an illustration example, the 1H NMR spectrum of compound 3a showed a doublet at 4.82 ppm (J = 3 Hz) corresponding to the methylene group at C-7′, which is coupled with the acetylenic proton H-9′, the latter proton that appears as a triplet at 2.53 ppm (J = 3 Hz). The absence of the deshielded phenolic hydrogen singlet observed at 13.90 ppm confirms the obtention of the O-prop-2-yn-N-alkyl-1,5-benzodiazepin-2-one derivatives 3a–c (Scheme 1).
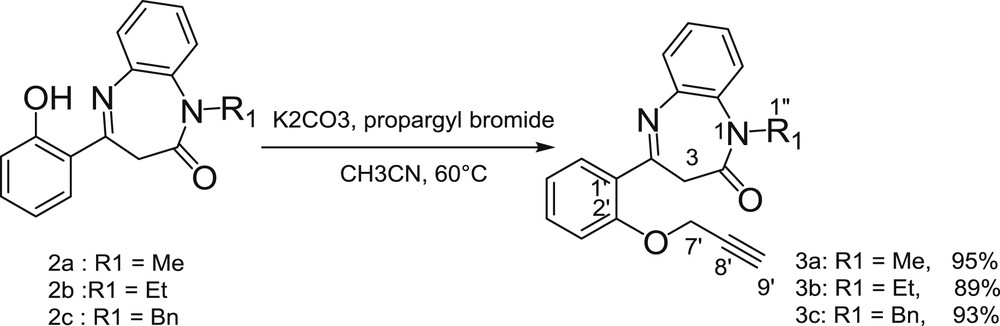
Synthesis of compounds 3a–c.
Having established a suitable access to key intermediates 3a–c, their subsequent use in the 1,3-dipolar cycloaddition with azide partners was investigated. Using phenylazide (4c, R = OCH3) as a model azide, the most suitable conditions to carry out the cyclization with alkyne 3a were explored (Table 1).
Optimization of Cu(I)-catalyzed 1,3-dipolar cyclization for the synthesis of 1,2,3-triazoles.a
| Entry | CuI (equiv)b | Solvent | Et3N (equiv) | Condition | Time (h) | Yield (%)d |
| 1 | 0.1 | DMF | 2 | Room temperature (rt) | 4 | 45 |
| 2 | 0.1 | DMF | 2 | 100 °C | 4 | 65 |
| 3 | 0.1 | DMF | 2 | MWc | 0.1 | 76 |
| 4 | 0.1 | t-BuOH/H2O | 2 | rt | 4 | Traces |
| 5 | 0.1 | t-BuOH/H2O | 2 | MWc | 0.1 | 82 |
| 6 | 0.1 | DCM | 2 | rt | 2 | 92 |
| 7 | 0.05 | DCM | 2 | rt | 2 | 90 |
| 8 | 0.05 | DCM | 0 | rt | 2 | 43 |
| 9 | 0.03 | DCM | 2 | rt | 2 | 40 |
| 10 | 0 | DCM | 2 | rt | 2 | 0 |
a Alkyne 3a (1 mmol) and two equivalents of phenylazide 4c.
b Referred to the starting alkyne 3a.
c The reaction was performed at 700 W.
d Isolated yield after column chromatography based on the starting dipolarophile 3a.
Different reaction conditions (solvent, temperature, and amount of catalyst) were screened. Although under strictly thermal conditions, as expected a mixture of 1,4- and 1,5-triazole derivatives was obtained, the reaction carried out in DMF in the presence of CuI (0.1 equiv) at rt afforded the corresponding 1,4-triazole exclusively, but in a low 45% yield. A marked improvement was observed running the reaction either at 100 °C or under microwave conditions with the formation of the product in 6 and 76% yield, respectively (entries 2–3). Comparatively, better results were obtained running the reactions in a binary mixture under microwave conditions (82% yield, entry 5). However, the best yield (90%, entry 6) was obtained in DCM, at rt. Interestingly, the amount of catalyst could be reduced to 0.05 equiv without noticeable decrease in product yield (entry 7).
With the optimal conditions (entry 7), the scope of this approach was subsequently explored with various aromatic azides 4a–e. The precursors 4a–e were synthesized from the appropriate anilines using a two-step procedure: (1) a diazotization conducted with sodium nitrite under acidic conditions; and (2) a diazonium displacement carried out with sodium azide. The desired aromatic azides 4a–e were obtained in high yields ranging from 85 to 98% [25]. Besides, the regiospecific formation of 1,4-substituted triazolyl derivatives 6a–o was observed in excellent yields, thus demonstrating the robustness of this approach (Scheme 3) (Table 2). The 1H NMR spectra of compounds 6a–o showed a singlet at δH 8.00–8.10 ppm attributable to the proton H-9′ of the triazole moiety and signals in the aromatic region (δH 6.95–7.57 ppm) corresponding to the aromatic protons introduced by the phenyl ring of azide derivatives. These structures were further supported by 13C, heteronuclear single quantum coherence spectroscopy (HSQC), and HMBC NMR spectra, which showed all the expected carbon signals corresponding to benzodiazepine–triazolyl derivatives, essentially the aromatic carbons resonating at δC 114.2–159.3 ppm introduced by the azide moiety. In case of the 1.5-disubstituted triazole one should expect a peak at ∼133 ppm corresponding to C-4 of the 1.5-disubstituted triazolic ring [26]. However, the presence in the 13C NMR spectra of a peak at δC ˜120.6–121.7, which is corresponding to the C-9′ carbon (C-5 of the 1.4-disubstituted triazolic ring), came comforting the obtention of the 1.4-regioisomers [27].
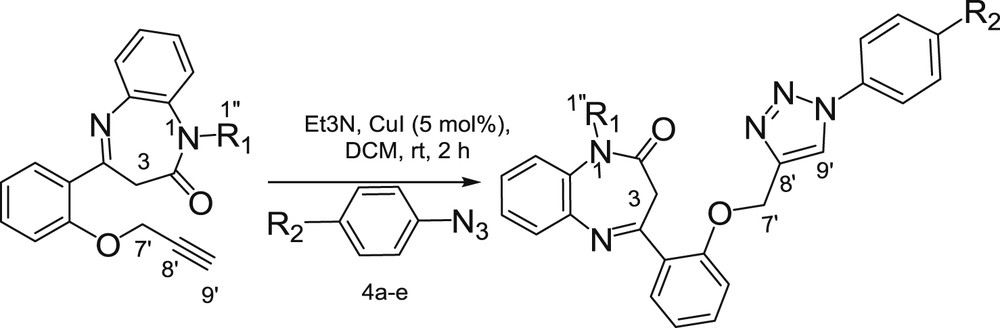
Synthesis of O-triazolo-N-alkyl-1,5-benzodiazepine 6a–o.
One-pot synthesis of 1,4-disubstituted 1,2,3-triazole 6a–o.
| Entry | Compound | R1 | R2 | Time (h) | Yield of 6 (%) |
| 1 | 6a | CH3 | H | 2 | 89 |
| 2 | 6b | CH3 | CH3 | 2 | 91 |
| 3 | 6c | CH3 | OCH3 | 2 | 90 |
| 4 | 6d | CH3 | Cl | 2 | 86 |
| 5 | 6e | CH3 | NO2 | 2 | 82 |
| 6 | 6f | Et | H | 2 | 83 |
| 7 | 6g | Et | CH3 | 2 | 85 |
| 8 | 6h | Et | OCH3 | 2 | 90 |
| 9 | 6i | Et | Cl | 2 | 79 |
| 10 | 6j | Et | NO2 | 2 | 75 |
| 11 | 6k | CH2-ph | H | 2 | 86 |
| 12 | 6l | CH2-ph | CH3 | 2 | 87 |
| 13 | 6m | CH2-ph | OCH3 | 2 | 92 |
| 14 | 6n | CH2-ph | Cl | 2 | 88 |
| 15 | 6o | CH2-ph | NO2 | 2 | 81 |
Encouraged by these results, we next turned our attention to new classes of pentacyclic compounds namely the O-isoxazolo-N-substituted-1,5-benzodiazepin-2-ones 7a–o. Literature reports that the intermolecular [3 + 2] cycloaddition reaction of arylnitrile oxides with various alkynes represents an efficient and convergent method for the construction of such compounds [28].
Whatever the solvent involved (DMF, t-BuOH/H2O, and DCM), the reaction did occur; however, DCM proved by far to be the most suitable solvent at rt (entries 1, 4 and 6). The best results were obtained in DCM under gentle heating (entry 7) in the presence of 0.1 equiv of CuI. However, changing the catalyst loading from 0.1 to 0.05 significantly altered the course of the reaction (decrease from 70% to 48%, entries 7–8). As observed with azide derivatives, the presence of triethylamine also proved to be important for the outcome of the reaction because no product was formed without the organic base (Tables 2–4).
Optimization of Cu(I)-catalyzed 1,3-dipolar cyclization for the synthesis of 3,5-disubstituted isoxazoles.a
| Entry | CuI (equiv)b | Solvent | Et3N (equiv) | Condition | Time (h) | Yield (%)d |
| 1 | 0.1 | DMF | 2 | rt | 12 | 16 |
| 2 | 0.1 | DMF | 2 | 80 °C | 6 | 65 |
| 3 | 0.1 | DMF | 2 | MWc | 0.1 | 62 |
| 4 | 0.1 | t-BuOH/H2O | 2 | rt | 4 | Traces |
| 5 | 0.1 | t-BuOH/H2O | 2 | MWc | 0.1 | 49 |
| 6 | 0.1 | DCM | 2 | rt | 10 | 46 |
| 7 | 0.1 | DCM | 2 | 40 °C | 6 | 70 |
| 8 | 0.05 | DCM | 2 | 40 °C | 6 | 48 |
| 9 | 0.1 | DCM | 0 | 40 °C | 6 | No reaction |
a Alkyne 3a (1 mmol) and 2 equiv of arylnitrile oxides 5c.
b Referred to the starting alkyne 3a.
c The reaction was performed at 700 W.
d Isolated yield after column chromatography based on the starting dipolarophile 3a.
One-pot synthesis of 3,5-disubstituted isoxazoles 7a–o.
| Entry | Compound | R1 | R2 | Time (h) | Yield of 7 (%) |
| 1 | 7a | CH3 | H | 6 | 69 |
| 2 | 7b | CH3 | CH3 | 6 | 71 |
| 3 | 7c | CH3 | OCH3 | 6 | 75 |
| 4 | 7d | CH3 | Cl | 6 | 68 |
| 5 | 7e | CH3 | NO2 | 6 | 66 |
| 6 | 7f | Et | H | 6 | 70 |
| 7 | 7g | Et | CH3 | 6 | 71 |
| 8 | 7h | Et | OCH3 | 6 | 72 |
| 9 | 7i | Et | Cl | 6 | 69 |
| 10 | 7j | Et | NO2 | 6 | 66 |
| 11 | 7k | CH2-ph | H | 6 | 69 |
| 12 | 7l | CH2-ph | CH3 | 6 | 71 |
| 13 | 7m | CH2-ph | OCH3 | 6 | 73 |
| 14 | 7n | CH2-ph | Cl | 6 | 67 |
| 15 | 7o | CH2-ph | NO2 | 6 | 65 |
Then, dipolarophiles 3a–c were treated with various arylnitrile oxides 5a–e generated in situ from aromatic oxime precursors under identical reaction conditions, to furnish the desired 4-(O-isoxazolo)-N-alkyl-1,5-benzodiazepin-2-ones in high yields (65–75%) (Scheme 4) (Table 4).

Synthesis of O-isoxazolo-N-alkyl-1,5-benzodiazepines 7a–o.
The analytical and spectroscopic data are in agreement with the proposed structures illustrated in Scheme 4. The regiochemistry of the adduct was deduced from the 1H NMR, which displays the resonance of the isoxazole proton (H-9′) as a singlet at δ = 6.60 ppm, as well as from 13C spectrum displaying two signals at 160 and 102 ppm for the two characteristic isoxazole carbons C8′ and C9′, respectively. These chemical shift values clearly confirm the regiochemistry of the cycloaddition and are in line with the values reported by Fokin et al. [29,30]. In the case of the hypothetical reverse regioisomer 7c, one should expect a chemical shift value for the H-9′ proton between 6 and 7 ppm because of the proximity of the isoxazolinic oxygen. The H-7″ protons appear as a singlet at δ = 5.31 ppm. The set of two doublets at 3.12 (d, 1H, J = 12.00 Hz) and 4.17 ppm (d, 1H, J = 12.00 Hz) is attributed to the methylenic protons H-3, which are nonequivalent because of the nonplanarity of the diazepine ring. Particularly characteristic are the 13C NMR isoxazole ring resonances with three peaks at δ = 101.6, δ = 162.3, and δ = 167.8 ppm for the isoxazole carbons C-9′, C-8′, and C-10′. The two secondary carbons C-3 and C-7′ are observed at δ = 43.2 and 61.9 ppm. Mass spectrometry analysis is also in accordance with the proposed structure for the compound 7c.
A further proof of our structural assignment stems from an X-ray structure determination performed on 7d, confirming unambiguously the stereochemistry of the product. Crystals for X-ray analysis were obtained by slow evaporation of a DCM/MeOH solution. The crystal structure has been determined from single crystal X-ray diffraction (Fig. 2).

Crystal structure of compound 7d: thermal ellipsoidal representation (50% of probability).
3 Conclusion
This work reports a novel synthetic access to readily conjugatable benzodiazepinones specifically modified at position 2′ by an alkyne chemical handle. Besides, optimization studies of subsequent 1,3-dipolar cycloaddition reaction of benzodiazepines with azides or nitrile oxides were carried out successfully, thus offering an effective strategy for the preparation of benzodiazepinones conjugates suitable for an array of aforementioned biological applications.
4 Experimental section
4.1 General
All solvents were dried following standard procedures: toluene and DCM were obtained from MB SPS-800 apparatus from MBRAUN. THF: distillation over Na/benzophenone. Cyclohexane, ethyl acetate (EtOAc), acetonitrile (CH3CN), and diethyl ether (OEt2) were purchased as ACS grade quality and used without further purification, unless otherwise stated. Commercially available reagents were used without further purification, unless otherwise stated. All reactions involving air- and moisture-sensitive reagents were performed under argon using syringe–septum cap technique. Column chromatography purifications were performed on silica gel (40–63 μm) carried out on Merck DC Kiesel gel 60F-254 aluminum sheets. Thin-layer chromatography analyses were carried out on Merck DC Kiesel gel 60F-254 aluminum sheets. The spots were visualized through illumination with UV lamp (λ = 254 nm) and/or staining with KMnO4.
4.2 Instruments and methods
1H and 13C NMR spectra were recorded on a 300 MHz spectrometer. Chemical shifts are expressed in parts per million (ppm) from the residual nondeuterated solvent signal: DMSO-d6 (δΗ = 2.50, δC = 39.52), Multiplicities are described as s (singlet), d (doublet), dd, ddd, and so forth (doublet of doublets, doublet of doublets of doublets, and so forth), t (triplet), dt (doublet of triplets), td (triplet of doublets), m (multiplet), br s (broad singlet). Coupling constants, J values, are reported in hertz. Melting points were determined on a Buchi 510 apparatus using capillary tubes. Elemental analysis was recorded on a Perkin–Elmer 240B microanalyzer. The microwave was a Biotage AB Initiator EXP EU with a maximum power of 800 W (2450 MHz). High-resolution mass spectra (HRMS) were obtained using an orthogonal acceleration time-of-flight mass spectrometer equipped with an electrospray source and in the positive and negative modes (ESI±).
4.3 General procedure for the synthesis of compounds 2a–c
To a solution of 4-(2′-hydroxypheny1)-1,5-benzodiazepin-2-one 1 (1.01 g, 4 mmol) in anhydrous THF (50 mL) at 0 °C was added NaH (60% in mineral oil, 0.176 g, 4.4 mmol, 1.1 equiv) and placed under nitrogen. The mixture was stirred for 10–15 min before the addition appropriate derivative (R1X), 1.2 equiv. The reaction mixture was maintained at rt for 2 h, before removing the solvent under reduced pressure. The crude material was poured into distilled water and extracted with dichloromethane. The organic layers were combined and dried over anhydrous MgSO4, then filtered, and the filtrate concentrated at reduced pressure. The crude material was purified by flash column chromatography on silica gel (cyclohexane/EtOAc from 100:0 to 60:40).
4.3.1 1-Methyl-4-(2-hydroxyphenyl)-1,5-benzodiazepin-2-one (2a) [24]
.
4.3.2 1-Ethyl-4-(2-hydroxyphenyl)-1,5-benzodiazepin-2-one (2b)
Yield: 963 mg (86%); yellow solid; mp 98–100 °C; 1H NMR (300 MHz, CDCl3) δ = 13.88 (s, 1H, OH), 7.89 (d, J = 9 Hz, 1H, ArH), 7.26–7.44 (m, 5H, ArH), 6.95–7.07 (m, 2H, ArH), 4.24 (d, J = 12 Hz, 1H, CH2), 4.10–4.17 (m, 1H, NCH2), 3.77–3.87 (m, 1H, NCH2), 2.99 (d, J = 12 Hz, 1H, CH2), 1.15 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3): δ = 165 (C2), 164.9 (C4), 162.3 (C2′), 139.5, 134.8, 134.2, 129.7, 127.4, 127.2, 125.8, 122.5, 119.3, 118.3, 118.3, 43.1 (C2″), 38.8(C3), 13.7 (C2″); Anal. Calcd for C17H16N2O2 (280.12): C, 72.84; H, 5.75; N, 9.99; found: C, 72.79; H, 5.73; N, 9.87.
4.3.3 1-Benzyl-4-(2-hydroxyphenyl)-1,5-benzodiazepin-2-one (2c)
Yield: 1.2 g (88%); yellow solid; mp 108–110 °C; 1H NMR (300 MHz, CDCl3) δ = 13.98 (s, 1H, OH), 7.90–7.93 (dd, J = 6 Hz, J = 3 Hz, 1H, ArH), 6.99–7.44 (m, 12H, ArH), 5.13 (s, 2H, NCH2), 4.36 (d, J = 12 Hz, 1H, CH2), 3.15 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 165.4 (C2), 164.8(C4), 162.4(C2′), 139.2, 137.0, 135.0, 134.3, 129.7, 128.8, 127.4, 127.3, 127.1, 126.8, 126.0, 122.6, 119.3, 118.4, 118.2, 51.5 (C2″), 38.4(C3); Anal. Calcd for C22H18N2O2 (242.14): C, 77.17; H, 5.30; N, 8.18; found: C, 77.02; H, 5.27; N, 8.16.
4.3.4 4-(Phenyl acetate)-1,5-benzodiazepin-2-one (2d)
To a solution of 4-(2′-hydroxypheny1)-1,5-benzodiazepin-2-one 1 (0.057 g, 2 mmol) in anhydrous THF (25 mL) at 0 °C, was added NaH (60% in mineral oil, 0.088 g, 2.2 mmol, 1.1 equiv.) and placed under nitrogen. The mixture was stirred for 10–15 min before the addition of acetyl chloride (0.162 mL, 2.4 mmol, 1.2 equiv). The reaction mixture was maintained at rt for 2 h, before removing the solvent under reduced pressure. The crude material was poured into distilled water and extracted with dichloromethane. The organic layers were combined and dried over anhydrous MgSO4, then filtered, and the filtrate concentrated at reduced pressure. The crude material was purified by flash column chromatography on silica gel (cyclohexane/EtOAc from 100:0 to 60:40); Yield: 517 mg (88%); white solid; mp 188–190 °C; 1H NMR (300 MHz, CDCl3) δ = 9.63 (s, 1H, NH), 7.84–7.87 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.43–7.47 (m, 2H, ArH), 7.34 (t, J = 9 Hz, 1H, ArH), 7.23–7.27 (m, 2H, ArH), 7.13–7.18 (m, 2H, ArH), 3.51 (s, 2H, CH2), 2.34 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 169.7 (C2), 168.5 (C4), 158.0 (C3′), 148.6 (C2′), 139.3, 132.1, 131.4, 130.1, 129.2, 128.5, 127.1, 126.3, 125.1, 123.6, 42.5 (C2), 21.5 (C4′); HRMS (ESI+): calcd for C17H15N2O3 [M + H]+ 295.1086; found 295.1083.
4.4 General procedure for the synthesis of compounds 3a–c [31]
To a stirred acetonitrile solution (30 mL) of 2a–c (1 mmol) at 50 °C was added 3-bromopropyne (0.163 ml, 1.1 mmol) and K2CO3 (0.276 g, 2 mmol). The solution was stirred at 50 °C for 24 h. The reaction mixture was filtered through Celite before removing the solvent under reduced pressure. The crude material was purified by flash column chromatography on silica gel (Cyclohexane/EtOAc from 100:0 to 70:30).
4.4.1 1-Methyl-4-(2-(prop-2-yn-1-yloxy)phenyl)-1,5-benzodiazepin-2-one (3a)
Yield: 288 mg (95%); white solid; mp 102–104 °C; 1H NMR (300 MHz, CDCl3) δ = 7.58–7.61 (dd, J = 6 Hz, J = 3 Hz, 1H, ArH), 7.41–7.49 (m, 2H, ArH), 7.25–7.32 (m, 3H, ArH), 7.05–7.13 (m, 2H, ArH), 4.82 (d, J = 3 Hz, 2H, CH2), 4.15 (d, J = 9 Hz, 1H, CH2), 3.43 (s, 3H, CH3), 3.12 (d, J = 9 Hz, 1H, CH2), 2.53 (t, J = 3 Hz, 1H, CH); 13C NMR (75 MHz, CDCl3) δ = 167.6 (C2), 163.8 (C4), 156.2 (C2′), 141.3, 135.2, 131.7, 130.8, 129.6, 127.8, 126.4, 125.1, 122, 121.7, 113.1, 78.4 (C8′), 76.1 (C9′), 56.4 (C7′), 43.1 (C3), 35.4 (C1″); Anal. Calcd for C19H16N2O2 (304.12): C, 74.98; H, 5.30; N, 9.20 found: C, 74.90; H, 5.27; N, 9.18.
4.4.2 1-Ethyl-4-(2-(prop-2-yn-1-yloxy)phenyl)-1,5-benzodiazepin-2-one (3b)
Yield: 283 mg (89%); white solid; mp 98–100 °C; 1H NMR (300 MHz, CDCl3) δ = 7.51–7.47 (dd, J = 6 Hz, J = 3 Hz, 1H, ArH), 7.37–7.43 (m, 3H, ArH), 7.25–7.29 (m, 2H, ArH), 7.04–7.12 (m, 2H, ArH), 4.81 (d, J = 3 Hz, 2H, CH2), 4.19–4.26 (m, 1H, CH2), 4.13 (d, J = 12 Hz, 1H, CH2), 3.73–3.84 (m, 1H, CH2), 3.12 (d, J = 12 Hz, 1H, CH2), 2.53 (t, J = 3 Hz, 1H, CH), 1.17 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 166.2 (C2), 164.1(C4), 156.1(C2′), 142.4, 133.6, 131.7, 130.7, 129.4, 127.7, 126.4, 125.3, 122.3, 122, 113.0, 78.3(C8′), 76.1(C9′), 56.3 (C7′), 43.4(C1″), 42.9 (C3), 13.8 (C2″); Anal. Calcd for C20H18N2O2 (318.14): C, 75.45; H, 5.70; N, 8.80; found: C, 75.39; H, 5.74; N, 8.76.
4.4.3 1-Benzyl-4-(2-(prop-2-yn-1-yloxy)phenyl)-1,5-benzodiazepin-2-one (3c)
Yield: 353 mg (93%); white solid; mp 108–110 °C; 1H NMR (300 MHz, CDCl3) δ = 7.50–7.53 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.06–7.43 (m, 12H, ArH), 5.32 (d, J = 15 Hz, 1H, CH2), 5.05 (d, J = 15 Hz, 1H, CH2), 4.8 (d, J = 3 Hz, 2H, CH2), 4.25 (d, J = 12 Hz, 1H, CH2), 3.28 (d, J = 12 Hz, 1H, CH2), 2.53 (t, J = 3 Hz, 1H, CH); 13C NMR (75 MHz, CDCl3) δ = 166.7 (C2), 163.8 (C4), 156.1(C2′), 142.0, 137.4, 133.8, 131.7, 130.7, 129.4, 128.7, 127.7, 127.3, 126.9, 126.4, 125.5, 122.4, 122.0, 113.0, 78.3, 76.2, 56.2 (C7′), 51.3(C1″), 43.2 (C3); Anal. Calcd for C25H20N2O2 (380.15): C, 78.93; H, 5.30; N, 7.36; found: C, 78.85; H, 5.28; N, 7.31.
4.5 General procedure for the synthesis of compounds (6a–o)
To a mixture of compounds 3a–c (0.5 mmol, 1 equiv) and Et3N (2 equiv, 134 μL, 1 mmol) in DCM (10 mL) was added CuI (5 mg, 0.025 mmol, 5 mol%) and the appropriate derivative of phenylazide 4a–e (1 mmol, 2 equiv). The reaction mixture was stirred at rt for 2 h. The crude reaction was filtered through Celite and the filtrate was concentrated under reduced pressure. The crude material was purified by flash column chromatography on silica gel (cyclohexane/EtOAc from 100:0 to 70:30), to give pure 6a–o in 75–90% yields.
4.5.1 1-Methyl-4-(2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6a)
Yield: 188 mg (89%); white solid; mp 143–145 °C; 1H NMR (300 MHz, CDCl3) δ = 8.20 (s, 1H, CHtriazole), 7.70 (d, J = 9 Hz, 2H, ArH), 7.57–7.60 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.37–7.50 (m, 5H, ArH), 7.18–7.27 (m, 4H, ArH), 7.02 (t, J = 6 Hz, 1H, ArH), 5.42 (s, 2H, CH2), 4.17 (d, J = 12 Hz, 1H, CH2), 3.38 (s, 3H, CH3), 3.06 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 167.6 (C2), 163.5 (C4), 156.6 (C2′), 144.7, 141.2, 137.1, 135.1, 132.0, 130.8, 129.8, 129.1, 128.9, 127.8, 126.4, 125.1, 121.6, 121.6 (C9′), 120.6, 113.1, 62.4 (C7′), 43.0 (C3), 35.4 (C1″); Anal. Calcd for C25H21N5O2 (423.17): C, 70.91; H, 5.00; N, 16.54; found: C, 70.85; H, 4.98; N, 16.49; HRMS (ESI+): calcd for C25H22N5O2[M + H]+: 424.1771; found: 424.1773.
4.5.2 1-Methyl-4-(2-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6b)
Yield: 198 mg (91%); white solid; mp 120–122 °C; 1H NMR (300 MHz, CDCl3) δ = 8.07 (s, 1H, CHtriazole), 7.51 (d, 2H, ArH), 7.31–7.37 (m, 2H, ArH), 7.11–7.22 (m, 6H, ArH), 6.93–6.98 (td, J = 9 Hz, J = 3 Hz, 1H, ArH), 5.35 (s, 2H, CH2), 4.10 (d, J = 12 Hz, 1H, CH2), 3.32 (s, 3H, CH3), 2.99 (d, J = 12 Hz, 1H, CH2), 2.32 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 167.7 (C2), 163.6 (C4), 156.6 (C2′), 144.5, 141.3, 139.1, 135.1, 134.8, 132.0, 130.8, 129.1, 127.8, 126.4, 125.2, 121.7, 121.5 (C9′), 120.6, 113.0, 62.4 (C7′), 43.0 (C3), 35.4 (C1″), 21.2 (C15′); Anal. Calcd for C26H23N5O2 (437.19): C, 71.38; H, 5.30; N, 16.01; found: C, 71.33; H, 5.26; N, 15.97.
4.5.3 4-(2-((1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-methyl-1,5-benzodiazepin-2-one (6c)
Yield: 215 mg (95%); yellow solid; mp 153–155 °C; 1H NMR (300 MHz, CDCl3) δ = 8.02 (s, 1H, CHtriazole), 7.49–7.55 (m, 3H, ArH), 7.31–7.38 (m, 2H, ArH), 7.11–7.22 (m, 4H, ArH), 6.9–6.98 (m, 3H, ArH), 5.35 (s, 2H, CH2), 4.10 (d, J = 12 Hz, 1H, CH2), 3.77 (s, 3H, OCH3), 3.32 (s, 3H, CH3), 2.99 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 167.7 (C2), 163.6 (C4), 160.0 (C14′), 156.6 (C2′), 144.5, 141.3, 135.1, 132.0, 130.8, 130.6, 129.1, 127.8, 126.4, 125.2, 122.3, 121.7, 121.7 (C9′), 114.9, 113.0, 62.4 (C7′), 55.8 (C15′), 43.0 (C3), 35.4 (C1″); Anal. Calcd for C26H23N5O3 (453.18): C, 68.86; H, 5.11; N, 15.44; found: C, 68.73; H, 5.09; N, 15.37; HRMS (ESI+): calcd for C26H24N5O3 [M + H]+: 454.1879; found: 454.1879.
4.5.4 4-(2-((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1-methyl-1,5-benzodiazepin-2-one (6d)
Yield: 196 mg (86%); yellow solid; mp 168–170 °C; 1H NMR (300 MHz, CDCl3) δ = 8.25 (s, 1H, CHtriazole), 7.62–7.72 (m, 3H, ArH), 7.19–7.49 (m, 8H, ArH), 7.05 (t, J = 6 Hz, 1H, ArH), 5.45 (s, 2H, CH2), 4.19 (d, J = 12 Hz, 1H, CH2), 3.41 (s, 3H, CH3), 3.09 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 167.7 (C2), 163.4 (C4), 156.5 (C2′), 141.3, 135.8, 135.1, 134.7, 132, 130.9, 130.1, 129.2, 127.8, 126.5, 125.2, 121.8, 121.8, 121.7, 121.6 (C9′), 113.1, 62.4 (C7′), 43 (C2), 35.4 (C1″); Anal. Calcd for C25H20ClN5O2 (457.13): C, 65.57; H, 4.40; N, 15.29; found: C, 65.46; H, 4.38; N, 15.16.
4.5.5 1-Methyl-4-(2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6e)
Yield: 191 mg (82%); yellow solid; mp 188–190 °C; 1H NMR (300 MHz, CDCl3) δ = 8.50 (s, 1H, CHtriazole), 7.38 (d, J = 9 Hz, 1H, ArH), 7.99 (d, J = 9 Hz, 2H, ArH), 7.68 (d, J = 6 Hz, 1H, ArH), 7.16–7.46 (m, 6H, ArH), 7.06 (t, J = 6 Hz, 1H, ArH), 5.43 (s, 2H, CH2), 4.20 (sb, 1H, CH2), 3.40 (s, 3H, CH3), 3.10 (sb, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 167.8 (C2), 163.1 (C4), 156.5 (C2′), 147.5, 145.8, 141.4, 135.2, 132.1, 131.2, 129.1, 127.9, 126.6, 125.7, 125.3, 121.9, 121.8, 121.7 (C9′), 120.7, 113.2, 62.4 (C7′), 42.9 (C3), 35.4 (C1″); Anal. Calcd for C25H20N6O4 (468.15): C, 64.10; H, 4.30; N, 17.94; found: C, 63.98; H, 4.27; N, 17.90.
4.5.6 1-Ethyl-4-(2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6f)
Yield: 181 mg (83%); yellow solid; mp 87–89 °C; 1H NMR (300 MHz, CDCl3) δ = 8.19 (s, 1H, CHtriazole), 7.71 (d, J = 9 Hz, 2H, ArH), 7.34–7.55 (m, 7H, ArH), 7.16–7.28 (m, 3H, ArH), 7.03 (t, J = 9 Hz, 1H, ArH), 5.42 (s, 2H, CH2), 4.08–4.23 (m, 2H, CH2), 3.69–3.81 (m, 1H, CH2), 3.08 (d, J = 12 Hz, 1H, CH2), 1.12 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 166.2 (C2), 164.0 (C4), 156.6 (C2′), 144.7, 142.2, 137.1, 133.6, 132.1, 130.8, 129.9, 128.9, 127.7, 126.5, 125.4, 122.3, 121.7, 121.5 (C9′), 120.7, 113.0, 62.5 (C7′), 43.4 (C1″), 43,0 (C3), 13.6 (C2″); Anal. Calcd for C26H23N5O2 (468.15): C, 71.38; H, 5.30; N, 16.01; found: C, 71.24; H, 5.35; N, 15.97.
4.5.7 1-Ethyl-4-(2-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6g)
Yield: 191 mg (85%); yellow solid; mp 146–148 °C; 1H NMR (300 MHz, CDCl3) δ = 8.15 (s, 1H, CHtriazole), 7.54–7.61 (m, 3H, ArH), 7.18–7.46 (m, 8H, ArH), 7.04 (t, J = 9 Hz, 1H, ArH), 5.43 (s, 2H, CH2), 4.16–4.24 (m, 2H, CH2), 3.74–3.80 (m, 1H, CH2), 3.10 (d, J = 12 Hz, 1H, CH2), 2.40 (s, 3H, CH3), 1.14 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 165.5 (C2), 163.3 (C4), 156 (C2′), 143.9, 138.4, 134.2, 133.0, 131.4, 130.2, 129.7, 128.4, 127.1, 125.8, 124.8, 121.7, 121 (C9′), 120.8, 119.9, 112.4, 61.8 (C7′), 42.7 (C1″), 42.4 (C3), 20.6 (C15′), 13 (C2″); Calcd for C27H25N5O2 (451.53): C, 71.82; H, 5.58; N, 15.51; found: C, 71.74; H, 5.62; N, 15.34.
4.5.8 1-Ethyl-4-(2-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6h)
Yield: 210 mg (90%); yellow solid; mp 155–157 °C; 1H NMR (300 MHz, CDCl3) δ = 8.07 (s, 1H, CHtriazole), 7.60 (d, J = 9 Hz, 2H, ArH), 7.51–7.54 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.34–7.44 (m, 3H, ArH), 7.16–7.28 (m, 3H, ArH), 6.97–7.05 (m, 3H, ArH), 5.41 (s, 2H, CH2), 4.14–4.23 (m, 2H, CH2), 3.85 (s, 1H, OCH3), 3.72–3.81 (m, 1H, CH2), 3.08 (d, J = 12 Hz, 1H, CH2), 1.13 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 166.3 (C2), 164 (C4), 160 (C14′), 156.6 (C2′), 144.5, 142.4, 133.6, 132.0, 130.8, 130.6, 129.1, 127.8, 126.4, 125.4, 122.3, 121.7, 121.6 (C9′), 114.9, 113.0, 62.5 (C7′), 55.8 (C15′), 43.4 (C1″), 43 (C3), 13 (C2″); Anal. Calcd for C27H25N5O3 (467.20): C, 69.36; H, 5.39; N, 14.98; found: C, 69.34; H, 5.41; N, 14.74.
4.5.9 1-Ethyl-4-(2-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6i)
Yield: 186 mg (79%); white solid; mp 150–152 °C; 1H NMR (300 MHz, CDCl3) δ = 8.22 (s, 1H, CHtriazole), 7.68 (d, J = 9 Hz, 2H, ArH), 7.55 (d, J = 6 Hz, 1H, ArH), 7.24–7.44 (m, 7H, ArH), 7.14 (d, J = 9 Hz, 1H, ArH), 7.03 (t, J = 6 Hz, 1H, ArH), 5.42 (s, 2H, CH2), 3.71–3.80 (m, 1H, CH2), 3.08 (d, J = 12 Hz, 1H, CH2), 1.12 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 166.2 (C2), 163.8 (C4), 156.5 (C2′), 145.0, 142.2, 135.6, 134.7, 133.6, 132.1, 130.9, 130.0, 128.9, 127.7, 126.5, 125.4, 122.3, 121.8, 121.5 (C9′), 113, 62.4 (C7′), 43.3 (C1″), 43 (C3), 13.6 (C2″); Anal. Calcd for C26H22ClN5O2 (471.15): C, 66.17; H, 4.70; N, 14.84; found: C, 66.01; H, 4.61; N, 14.78.
4.5.10 1-Ethyl-4-(2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one(6j)
Yield: 180 mg (75%); yellow solid; mp 188–190 °C; 1H NMR (300 MHz, CDCl3) δ = 8.49 (s, 1H, CHtriazole), 7.98 (d, J = 9 Hz, 2H, ArH), 7.57–7.60 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.24–7.45 (m, 5H, ArH), 7.14 (d, J = 9 Hz, 1H, ArH), 7.03 (t, J = 6 Hz, 1H, ArH), 5.44 (s, 2H, CH2), 4.17–4.2 (m, 2H, CH2), 3.72–3.79 (m, 1H, CH2), 3.08 (d, J = 12 Hz, 1H, CH2), 1.12 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 166.2 (C2), 163.4 (C4), 156.3 (C2′), 147.3, 145.6, 142.2, 141.2, 133.5, 132.1, 131.1, 128.8, 127.7, 126.5, 125.6, 125.5, 122.3, 121.9, 121.7, 120.6 (C9′), 112.9, 62.2 (C7′), 43.2 (C1″), 43 (C3), 13.6 (C2″); Anal. Calcd for C26H22N6O4 (482.17): C, 64.72; H, 4.60; N, 17.42; found: C, 64.84; H, 4.56; N, 17.35.
4.5.11 1-Benzyl-4-(2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6k)
Yield: 214 mg (86%); yellow solid; mp 80–82 °C; 1H NMR (300 MHz, CDCl3) δ = 8.21 (s, 1H, CHtriazole), 7.73–7.75 (m, 2H, ArH), 7.61–7.64 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH, ArH), 7.38–7.56 (m, 6H, ArH), 7.10–7.34 (m, 9H, ArH), 5.48 (d, J = 3 Hz, 2H, CH2), 5.37 (d, J = 15 Hz, 1H, CH2), 5.08 (d, J = 15 Hz, 1H, CH2), 4.33 (d, J = 12 Hz, 1H, CH2), 3.30 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 166.8 (C2), 163.6 (C4), 156.7 (C2′), 144.6, 142.0, 137.3, 137.1, 133.8, 132.0, 130.8, 129.8, 129, 128.9, 128.7, 127.7, 127.3, 126.9, 126.4, 125.6, 122.4, 121.7, 121.6 (C9′), 120.6, 113, 62.4 (C7′), 51.2 (C1″), 43.1 (C3); Anal. Calcd for C31H25N5O2 (499.2): C, 74.53; H, 5.04; N, 14.02; found: C, 74.45; H, 5.10; N, 13.97.
4.5.12 1-Benzyl-4-(2-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6l)
Yield: 223 mg (87%); yellow solid; mp 84–86 °C; 1H NMR (300 MHz, CDCl3) δ = 8.07 (s, 1H, CHtriazole), 7.50–7.55 (m, 3H, ArH), 7.39, 7.45 (m, 2H, ArH), 7.16, 7.33 (m, 9H, ArH), 7.01–7.10 (m, 3H, ArH), 5.40 (d, J = 9 Hz, 2H, CH2), 5.30 (d, J = 15 Hz, 1H, CH2), 4.99 (d, J = 15 Hz, 1H, CH2), 4.24 (d, J = 12 Hz, 1H, CH2), 3.22 (d, J = 12 Hz, 1H, CH2), 2.40 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 166.9 (C2), 163.6 (C4), 156.7 (C2′), 144.5, 142.1, 139.1, 137.3, 134.8, 133.8, 132.0, 130.8, 130.3, 129.1, 128.7, 127.8, 127.3, 126.9, 126.4, 125.6, 122.4, 121.7, 121.6 (C9′), 120.6, 113.0, 62.4 (C7′), 51.2 (C1″), 43.2 (C3), 21.3 (C15′); Anal. Calcd for C32H27N5O2 (513.22): C, 74.83; H, 5.30; N, 13.64; found: C, 75.01; H, 5.27; N, 13.64.
4.5.13 1-Benzyl-4-(2-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6m)
Yield: 243 mg (92%); yellow solid; mp 78–80 °C; 1H NMR (300 MHz, CDCl3) δ = 8.03 (s, 1H, CHtriazole), 7.51–7.57 (m, 3H, ArH), 7.38–7.45 (m, 2H, ArH), 37.6–7.33 (m, 7H, ArH), 7.01–7.10 (m, 3H, ArH), 6.95 (d, J = 9 Hz, 2H, ArH), 5.39 (d, J = 6 Hz, 2H, CH2), 5.30 (d, J = 15 Hz, 1H, CH2), 5.00 (d, J = 15 Hz, 1H, CH2), 4.25 (d, J = 12 Hz, 1H, CH2), 3.84 (s, 3H, OCH3), 3.22 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 166.9 (C2), 163.6 (C4), 160 (C14′), 156.7 (C2′), 144.4, 142.1, 137.3, 133.8, 132, 130.8, 130.5, 129.1, 128.7, 127.3, 126.9, 126.4, 125.6, 122.4, 122.3, 121.7, 121.7 (C9′), 114.9, 113, 62.4 (C7′), 55.8 (C15′), 51.2 (C1′), 43.1 (C3); Anal. Calcd for C32H27N5O3 (529.21): C, 72.57; H, 5.14; N, 13.22; found: C, 72.64; H, 5.18; N, 13.12.
4.5.14 1-Benzyl-4-(2-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6n)
Yield: 234 mg (88%); yellow solid; mp 94–96 °C; 1H NMR (300 MHz, CDCl3) δ = 8.15 (s, 1H, CHtriazole), 7.55–7.61 (m, 3H, ArH), 7.31–7.41 (m, 5H, ArH), 7.02–7.26 (m, 9H, ArH), 5.39 (d, J = 6 Hz, 2H, CH2), 5.31 (d, J = 15 Hz, 1H, CH2), 4.98 (d, J = 15 Hz, 1H, CH2), 4.24 (d, J = 12 Hz, 1H, CH2), 3.22 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 166.8 (C2), 163.4 (C4), 156.6 (C2′), 144.9, 141.9, 137.3, 135.5, 133.8, 132.1, 130.9, 129.9, 128.7, 127.7, 127.3, 126.8, 126.5, 125.6, 122.4, 121.7, 121.7, 121.6 (C9′), 113, 62.3 (C7′), 51.2 (C1″), 43.1 (C3); Anal. Calcd for C31H24ClN5O2 (533.16): C, 69.72; H, 4.53; N, 13.11; found: C, 69.70; H, 4.51; N, 13.12.
4.5.15 1-Benzyl-4-(2-((1-(4-nitroxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (6o)
Yield: 220 mg (81%); yellow solid; mp 108–110 °C; 1H NMR (300 MHz, CDCl3) δ = 8.39 (s, 1H, CHtriazole), 8.30 (d, J = 9 Hz, 2H, ArH), 7.85 (d, J = 9 Hz, 2H, ArH), 7.62–7.65 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.33–7.46 (m, 3H, ArH), 7.15–7.26 (m, 6H, ArH), 7.05–7.10 (m, 3H, ArH), 5.42 (d, J = 9 Hz, 2H, CH2), 5.32 (d, J = 15 Hz, 1H, CH2), 4.99 (d, J = 15 Hz, 1H, CH2), 4.27 (d, J = 12 Hz, 1H, CH2), 3.23 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 167.0 (C2), 163.0 (C4), 156.4 (C2′), 147.3, 145.6, 142.0, 141.2, 133.8, 132.1, 131.1, 128.9, 128.7, 127.8, 127.4, 126.8, 126.6, 125.7, 125.6, 122.4, 121.9, 121.9 (C9′), 120.5, 113, 62.3 (C7′), 51.2 (C1″), 43.0 (C3); Anal. Calcd for C31H24N6O4 (544.19): C, 68.37; H, 4.44; N, 15.43; found: C, 68.42; H, 4.43; N, 15.41.
4.6 General procedure for the synthesis of compounds (7a–o)
To a mixture of compounds 3a–c (0.5 mmol, 1 equiv) and Et3N (2 equiv, 134 μL, 1 mmol) in 10 mL DCM was added CuI (5 mg, 0.025 mmol, 5 mol%) and the appropriate derivative of arylnitrile oxide (1 mmol) 5a–e (1 mmol, 2 equiv). The reaction mixture was stirred at 50 °C for 6 h. The crude mixture was extracted with DCM (2 × 25 mL) and the combined organic layer was dried over sodium sulfate, concentrated under reduced pressure, and purified through a column chromatography on silica gel (cyclohexane/EtOAc from 100:0 to 70:30), to give pure 7a–o in 55–75% yields.
4.6.1 1-Methyl-4-(2-((3-phenylisoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7a)
(145 mg, 0.35 mmol; 69%); white solid; mp 144–146 °C; 1H NMR (300 MHz, CDCl3) δ = 7.75–7.78 (m, 2H, ArH), 7.57–7.60 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.22–7.32 (m, 3H, ArH), 7.40–7.46 (m, 5H, ArH), 7.03–7.11 (m, 2H, ArH), 6.66 (s, 1H, CH-isoxazole), 5.33 (s, 2H, CH2), 4.17 (d, J = 12 Hz, 1H, CH2), 3.41 (s, 3H, CH3), 3.12 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 168.1 (C10′),167.4 (C2), 163.3 (C4), 162.6 (C8′), 156.2 (C2′), 141.2, 135.1, 132, 131, 130.3, 129.4, 129, 128.8, 127.8, 127, 126.6, 125.2, 122.3, 121.7, 112.8, 101.9 (C9′), 61.8 (C7′), 43.1 (C3), 35.4 (C1″); Anal Calcd for C26H21N3O3 (423.16): C, 73.74; H, 5.00; N, 9.92; found: C, 73.68; H, 5.06; N, 9.89.
4.6.2 1-Methyl-4-(2-((3-(p-tolyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7b)
Yield: 155 mg (71%); white solid; mp 118–120 °C; 1H NMR (300 MHz, CDCl3) δ = 7.68 (d, J = 9 Hz, 2H, ArH), 7.58–7.62 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.41–7.43 (m, 2H, ArH), 7.23–7.31 (m, 5H, ArH), 7.05–7.12 (m, 2H, ArH), 6.64 (s, 1H, CH-isoxazole), 5.33 (s, 2H, CH2), 4.19 (d, J = 12 Hz, 1H, CH2), 3.42 (s, 3H, CH3), 3.14 (d, J = 12 Hz, 1H, CH2), 2.39 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 167.9 (C10′), 167.4 (C2), 163.3 (C4), 162.6 (C8′), 156.2 (C2′), 141.3, 140.4, 135.1, 131.9, 130.9, 129.7, 129.5, 127.8, 126.9, 126.5, 125.9, 125.2, 122.3, 121.7, 112.7, 101.8 (C9′), 61.9 (C7′), 43.2 (C3), 35.4 (C1″), 21.6 (C15′); Anal. Calcd for C27H23N3O3 (437.17): C, 74.13; H, 5.30; N, 9.60; found: C, 74.06; H, 5.28; N, 9.69.
4.6.3 4-(2-((3-(4-Methoxyphenyl)isoxazol-5-yl)methoxy)phenyl)-1-methyl-1,5-benzodiazepin-2-one (7c)
Yield 170 mg (75%); yellow solid; mp 112–114 °C; 1H NMR (300 MHz, CDCl3) δ = 7.56–7.71 (m, 2H, ArH), 7.39–7.49 (m, 2H, ArH), 7.21–7.32 (m, 4H, ArH), 6.91–7.10 (m, 4H, ArH), 6.60 (s, 1H, CH-isoxazole), 5.31 (s, 2H, CH2), 4.17 (d, J = 12 Hz, 1H, CH2), 3.83 (s, 3H, OCH3), 3.41 (s, 3H, CH3), 3.12 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 167.8 (C10′), 167.4 (C2), 163.3 (C4), 162.3 (C8′), 161.2 (C14′), 156.3 (C2′), 141.3, 135.1, 131.9, 131, 129.5, 128.4, 127.8, 126.6, 125.2, 122.3, 121.7, 121.3, 114.5, 112.7, 101.6 (C9′), 61.9 (C7′), 55.5 (C15′), 43.2 (C3), 35.5 (C1″); Anal. Calcd for C27H23N3O4 (453.17): C, 71.51; H, 5.11; N, 9.27; found: C, 71.42; H, 5.13; N, 9.25; HRMS (ESI+): calcd for C26H22N3O3[M + H]+·454.1751; found 454.1767.
4.6.4 4-(2-((3-(4-Chlorophenyl)isoxazol-5-yl)methoxy)phenyl)-1-methyl-1,5-benzodiazepin-2-one (7d)
(155 mg, 0.34 mmol, 68%); isolated as a yellow solid; mp 143–145 °C; 1H NMR (300 MHz, CDCl3) δ = 7.69 (d, J = 9 Hz, 2H, ArH), 7.59 (d, J = 9 Hz, 1H, ArH), 7.26–7.46 (m, 7H, ArH), 7.02–7.11 (m, 2H, ArH), 6.64 (s, 1H, CH-isoxazole), 5.32 (s, 2H, CH2), 4.15 (d, J = 12 Hz, 1H, CH2), 3.40 (s, 3H, CH3), 3.11 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 168.5 (C10′), 167.4 (C2), 163.2 (C8′), 161.7(C4), 156.2 (C2′), 141.2, 136.3, 135.1, 132, 131.1, 129.4, 129.4, 128.3, 127.8, 127.3, 126.6, 125.2122.4, 121.7, 112.7, 101.8 (C9′), 61.8 (C7′), 43.2 (C3), 35.5 (C1″); Anal. Calcd for C26H20ClN3O3 (457.12): C, 68.20; H, 4.40; N, 9.18; found: C, 68.13; H, 4.46; N, 9.01; HRMS (ESI+): calcd for C26H20ClN3O3[M + H]+ 458.1260; found 458.1271.
4.6.5 4-(2-((3-(4-Nitrophenyl)isoxazol-5-yl)methoxy)phenyl)-1-methyl-1,5-benzodiazepin-2-one (7e)
(154 mg, 0.33 mmol, 66%); isolated as a yellow solid; mp 196–198 °C; 1H NMR (300 MHz, CDCl3) δ = 8.28 (d, J = 9 Hz, 2H, ArH), 7.95 (d, J = 9 Hz, 2H, ArH), 7.60–7.63 (dd, J = 6 Hz, J = 3 Hz, 1H, ArH), 7.40–7.46 (m, 2H, ArH), 7.22–7.31 (m, 3H, ArH), 7.03–7.12 (m, 2H, ArH), 6.77 (s, 1H, CHtriazole), 5.36 (s, 2H, CH2), 4.15 (d, J = 12 Hz, 1H, CH2), 3.40 (s, 3H, CH3), 3.12 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 169.3 (C10′), 167.4 (C2), 163(C8′), 160.9 (C4), 156.1(C2′), 148.9, 141.1, 135.1, 134.9, 132, 131.1, 129.4, 127.9, 127.8, 126.7, 125.2, 124.3, 122.5, 121.7, 112.7, 102 (C9′), 61.7 (C7′), 43.1(C3), 35.4 (C1″); Anal. Calcd for C26H20N4O5 (468.14): C, 66.66; H, 4.30; N, 11.96; found: C, 66.79; H, 4.29; N, 11.82.
4.6.6 1-Ethyl-4-(2-((3-phenylisoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7f)
(152 mg, 0.35 mmol, 70%); isolated as a white solid; mp 114–116 °C; 1H NMR (300 MHz, CDCl3) δ = 7.67–7.70 (m, 2H, ArH), 7.43–7.47(dd, J = 6 Hz, J = 3 Hz, 1H, ArH), 7.16–7.38 (m, 8H, ArH), 6.94–7.02 (m, 2H, ArH), 6.57 (s, 1H, CH-isoxzole), 5.23 (s, 2H, CH2), 4.02–4.14 (m, 2H, CH2), 3.65–3.72 (m, 1H, CH2), 3.06 (d, J = 12 Hz, 1H, CH2), 1.06 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 168 (C10′), 166 (C2), 163.6 (C4), 162.6 (C8′), 156.2 (C2′), 142.4, 133.5, 131.9, 130.9, 130.3, 129.4, 129, 128.8, 128.5, 127.7, 127, 126.5, 125.4, 122.3, 112.7, 101.8 (C9′), 61.8 (C7′), 43.5 (C1″), 43(C3), 13.6 (C2″); Anal. Calcd for C27H23N3O3 (437.17): C, 74.13; H, 5.30; N, 9.60; found: C, 73.98; H, 5.21; N, 9.51.
4.6.7 1-Ethyl-4-(2-((3-(p-tolyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7g)
(160 mg, 0.35 mmol, 71%); isolated as a yellow solid; mp 118–120 °C; 1H NMR (300 MHz, CDCl3) δ = 7.58 (d, J = 9 Hz, 2H), 7.14–7.46 (m, 8H), 6.94–7.02 (m, 2H), 6.54 (s, 1H), 5.22 (s, 2H), 4.06–4.17 (m, 2H), 3.63–3.75 (m, 1H), 3.05 (d, J = 12 Hz, 1H), 2.30 (s, 3H), 1.07 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 166.1 (C10′), 163.8 (C2), 162.6 (C4), 156.2 (C2′), 142.4, 140.4, 133.6, 131.9, 130.9, 130.3, 129.8, 129.3, 127.7, 126.9, 126.5, 125.9, 125.5, 122.4, 112.7, 101.8 (C9′), 61.9 (C7′), 43.5 (C1″), 43.1 (C3), 21.6 (CH3), 13.6(CH3); Anal. Calcd for C28H25N3O3 (451.19): C, 74.48; H, 5.58; N, 9.31; found: C, 74.37; H, 5.58; N, 9.19.
4.6.8 1-Ethyl-4-(2-((3-(4-methoxyphenyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7h)
(168 mg, 0.36 mmol, 72%); isolated as a white solid; mp 138–140 °C; 1H NMR (300 MHz, CDCl3) δ = 7.70 (d, J = 9 Hz, 1H, ArH), 7.51–7.54 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.22–7.46 (m, 6H, ArH), 6.92–7.10 (m, 4H, ArH), 6.59 (s, 1H, CH-isoxazole), 5.30 (s, 2H, CH2), 4.12–4.24 (m, 2H, CH2), 3.71–3.83 (m, 4H, CH2, CH3), 3.14 (d, J = 12 Hz, 1H, CH2), 1.14 (t, J = 6 Hz, 3H, CH2); 13C NMR (75 MHz, CDCl3) δ = 167.8 (C10′), 166 (C2), 163.7 (C4), 162.3 (C8′), 161.2 (C14′), 156.2 (C2′), 142.4, 133.6, 131.9, 130.9, 129.7, 129.4, 128.4, 127.7, 126.5, 125.5, 122.4, 121.3, 114.5, 112.7, 101.6 (C9′), 61.9 (C7′), 55.5 (C15′), 43.5 (C1″),43(C3), 13.6 (C1″); Anal. Calcd for C28H25N3O4 (467.18): C, 71.93; H, 5.39; N, 8.99; found: C, 71.79; H, 5.43; N, 8.92.
4.6.9 1-Ethyl-4-(2-((3-(4-chlorophenyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7i)
(162 mg, 0.34 mmol, 69%); isolated as a yellow solid; mp 142–144 °C; 1H NMR (300 MHz, CDCl3) δ = 7.68–7.71 (m, 2H, ArH), 7.53 (d, J = 6 Hz, 1H, ArH), 7.36–7.45 (m, 5H, ArH), 7.22–7.30 (m, 2H, ArH), 7.01–710 (m, 2H, ArH), 6.63 (s, 1H, CH-isoxazole), 5.30 (s, 2H, CH2), 4.10–4.25 (m, 2H, CH2), 3.71–3.82 (m, 1H, CH2), 3.12 (d, J = 12 Hz, 1H, CH2), 1.13 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 168.3(C10′), 165.9 (C2), 163.5 (C4), 161.6 (C8′), 156 (C2′), 142.2, 136.2, 133.5, 131.9, 130.9, 129.3, 129.2, 128.2, 127.6, 127.2, 126.5, 125.4, 122.3, 122.3, 112.5, 101.6 (C9′), 61.7 (C7′), 43.4 (C1″), 43(C3), 13.6 (C2″); Anal. Calcd for C27H22ClN3O3 (471.13): C, 68.72; H, 4.70; N, 8.90; found: C, 68.62; H, 4.64; N, 8.84.
4.6.10 1-Ethyl-4-(2-((3-(4-nitrophenyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7j)
(159 mg, 0.33 mmol, 66%); isolated as a yellow solid; mp 158–160 °C; 1H NMR (300 MHz, CDCl3) δ = 8.29 (d, J = 9 Hz, 2H, ArH), 7.95 (d, J = 9 Hz, 2H, ArH), 7.54–7.57 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.26–7.45 (m, 5H, ArH), 7.02–7.12 (m, 2H, ArH), 6.77 (s, 1H, CH-isoxazole), 5.38 (s, 2H, CH2), 4.11–4.24 (m, 2H, CH2), 3.72–3.80 (m, 1H, CH2), 3.14 (d, J = 12 Hz, 1H, CH2), 1.14 (t, J = 6 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 169.3 (C10′), 166 (C2), 163.4 (C4), 160.9 (C8′), 156 (C2′), 146.9, 142.3, 134.9, 133.6, 132, 131.1, 129.4, 127.9, 127.7, 126.6, 125.5, 124.4, 122.6, 122.4, 112.6, 102 (C9′), 61.7 (C7′), 43.5 (C1″), 43.1 (C2), 13.6 (C2″); Anal. Calcd for C27H22N4O5 (482.16): C, 67.21; H, 4.60; N, 11.61; found: C, 67.07; H, 4.58; N, 11.57.
4.6.11 1-Benzyl-4-(2-((3-phenylisoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7k)
(172 mg, 0.34 mmol, 69%); isolated as a yellow solid; mp 70–72 °C; 1H NMR (300 MHz, CDCl3) δ = 7.66–7.69 (m, 2H, ArH), 7.41–7.44 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.07–7.37 (m, 12H, ArH), 6.95–7.03 (m, 3H, ArH), 6.55 (s, 1H, CH-isoxazole), 5.18–5.23 (m, 3H, CH2), 4.94 (d, J = 15 Hz, 1H, CH2), 4.16 (d, J = 12 Hz, 1H, CH2), 3.19 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 168 (C10′), 166.6 (C2), 163.4(C4), 162.7(C8′), 156.2 (C2′), 142.1, 137.3, 133.8, 132, 130.9, 130.3, 129.4, 129.1, 128.8, 128.7, 127.7, 127.3, 127, 127, 126.5, 125.6, 122.4, 122.4, 112.6, 101.9 (C9′), 61.8 (C7′), 51.3 (C1″), 43.3(C3); Anal. Calcd for C32H25N3O3(499.19): C, 76.94; H, 5.04; N, 8.41; found: C, 76.72; H, 5.12; N, 8.37.
4.6.12 1-Benzyl-4-(2-((3-(p-tolyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7l)
(182 mg, 0.36 mmol, 71%); isolated as a white solid; mp 82–84 °C; 1H NMR (300 MHz, CDCl3) δ = 7.64 (d, J = 9 Hz, 2H, ArH), 7.51–7.44 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.17, 7.44 (m, 10 H, ArH), 7.04–7.11 (m, 4H, ArH), 6.60 (s, 1H, CH-isoxazole), 5.29–5.33 (m, 3H, CH2), 5.02 (d, J = 15 Hz, 1H, CH2), 4.24 (d, J = 12 Hz, 1H, CH2), 3.27 (d, J = 12 Hz, 1H, CH2), 2.39 (s, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ = 167.9 (C10′), 166.7 (C2), 163.5(C4), 162.7 (C8′), 156.4 (C2′), 142.1, 140.4, 137.4, 133.9, 132, 131, 130.3, 129.8, 129.3, 128.7, 127.8, 127.4, 127, 126.5, 127.1, 125.6, 122.5, 122.4, 112.9, 101.9 (C9′), 62 (C7′), 51.3 (C1″), 43.3 (C3), 21.5 (C15′); Anal. Calcd for C33H27N3O3 (513.21): C, 77.17; H, 5.30; N, 8.18; found: C, 76.97; H, 5.41; N, 8.16.
4.6.13 1-Benzyl-4-(2-((3-(4-methoxyphenyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7m)
(193 mg, 0.37 mmol, 73%); isolated as a white solid; mp 136–138 °C; 1H NMR (300 MHz, CDCl3) δ = 7.69 (d, J = 9 Hz, 1H, ArH), 7.17–7.51 (m, 9H, ArH), 7.02–7.11 (m, 4H, ArH), 6.93 (d, J = 9 Hz, 2H, ArH), 6.57 (s, 1H, CH-isoxazole), 5.28–5.32 (m, 2H, CH2), 5.02 (d, J = 15 Hz, 1H, CH2), 4.24 (d, J = 12 Hz, 1H, CH2), 3.83 (s, 3H, OCH3), 3.27 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 167.7 (C10′), 166.6 (C2), 163.4 (C4), 162.3 (C8′), 161.2 (C14′), 156.3 (C2′), 142.1, 137.4, 133.8, 132, 130.9, 129.4, 128.7, 128.4, 127.7, 127.3, 127, 126.5, 125.6, 122.4, 122.3, 121.3, 114.5, 112.6, 101.7 (C9′), 61.8 (C7′), 55.5 (C15′), 51.3 (C1″), 43.3 (C3); Anal. Calcd for C33H27N3O4 (529.20): C, 74.84; H, 5.14; N, 7.93; found: C, 75.01; H, 5.18; N, 7.83.
4.6.14 1-Benzyl-4-(2-((3-(4-chlorophenyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7n)
(178 mg, 0.33 mmol, 67%); isolated as a yellow solid; mp 133–135 °C; 1H NMR (300 MHz, CDCl3) δ = 7.59 (d, J = 9 Hz, 2H, ArH), 7.42–7.45(dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.23–7.34 (m, 5H, ArH), 7.10–7.18 (m, 5H, ArH), 6.94–7.02 (m, 4H, ArH), 6.52 (s, 1H, CH-isoxazole), 5.19–5.24 (m, 2H, CH2), 5.31 (d, J = 15 Hz, 1H, CH2), 4.92 (d, J = 15 Hz, 1H, CH2), 4.14 (d, J = 12 Hz, 1H, CH2), 3.18 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 168.4 (C10′), 166.6 (C2), 163.3 (C4), 161.7 (C8′), 156.2 (C2′), 142, 137.3, 136.3, 133.8, 132, 131, 129.4, 129.3, 128.7, 128.3, 128.2, 127.7, 127.3, 127.3, 126.9, 126.6, 12 5.6, 122.4, 122.4, 112.6, 101.8 (C9′), 61.7 (C7′), 51.3 (C1″), 43.3 (C3); Anal. Calcd for C32H24ClN3O3 (533.15): C, 71.97; H, 4.53; N, 7.87 found: C, 71.76; H, 4.51; N, 7.79.
4.6.15 1-Benzyl-4-(2-((3-(4-nitrophenyl)isoxazol-5-yl)methoxy)phenyl)-1,5-benzodiazepin-2-one (7o)
(176 mg, 0.33 mmol, 65%); isolated as a yellow solid; mp 144–146 °C; 1H NMR (300 MHz, CDCl3) δ = 8.20 (d, J = 9 Hz, 2H, ArH), 7.85 (d, J = 9 Hz, 2H, ArH), 7.62–7.65 (dd, J = 9 Hz, J = 3 Hz, 1H, ArH), 7.83 (d, J = 9 Hz, 2H, ArH), 7.47 (d, J = 9 Hz, 1H, ArH), 6.95–7.43 (m, 12H, ArH), 6.65 (s, 1H, CH-isoxazole), 5.20–5.25 (m, 3H, CH2), 4.93 (d, J = 15 Hz, 1H, CH2), 4.14 (d, J = 12 Hz, 1H, CH2), 3.19 (d, J = 12 Hz, 1H, CH2); 13C NMR (75 MHz, CDCl3) δ = 169.2 (C10′), 166.7 (C2), 163.2 (C4), 160.9 (C8′), 156 (C2′), 148.9, 142, 137.3, 134.9, 133.8, 132, 131.1, 129.4, 128.7, 127.9, 127.7, 127.4, 126.9, 126.6, 125.7, 124.3, 122.5, 122.5, 112.6, 102.1 (C9′), 61.6 (C7′), 51.2 (C1″), 43.3 (C3); Anal. Calcd for C32H24N4O5 (544.17): C, 70.58; H, 4.44; N, 10.29 found: C, 70.48; H, 4.47; N, 10.24.
Acknowledgments
This work was partially supported by Rouen University, INSA Rouen, Rouen University, the Centre national de la recherche scientifique (CNRS), the Region Haute-Normandie (CRUNCh network), and the Labex SynOrg (ANR-11-LABX-0029). The authors are grateful to Mrs Amna Benzarti and Miss Nadia Msaddek, NMR service at the Faculty of Monastir, University of Monastir for the NMR analysis and to the Ministry of Higher Education and Scientific Research of Tunisia for financial support (LR11ES39). The authors also thank Albert Marcual (CNRS) for HRMS analyses, Émilie Petit (CNRS) for elemental analysis, and Dr Morgane Sanselme (University of Rouen) for X-ray diffraction analyses.


