1 Introduction
“RegioTriRhena” represents a geographical area around Basel and the upper Rhine knee. It encompasses the northwestern part of Switzerland with its five cantons (Basel-Stadt, Basel-Land, Aargau, Solothurn and Jura), the southern part of Alsace between Colmar and Saint Louis, which includes the Mulhouse metropolitan area, and the part of Baden-Württemberg located south to Freiburg.
Chemical industry started at the beginning of the 19th century in the Alsatian city of Thann as kind of a subcontractor to the important textile industry established since the 18th century in RegioTriRhena, Mulhouse being the leading centre of cotton printing and Basel the leading centre of silk ribbon weaving. During the second half of the 19th century, the economic influence of Mulhouse declined gradually in favour of the neighbouring Basel. Thanks to a close cooperation of industry and university—linking scientific invention and technical innovation—the Swiss city became the hub of new industrial developments, turning from pure chemical and dyestuff production inherited from Mulhouse to the life science–oriented activities that characterize the vigorous Basel industry nowadays. As to Mulhouse, the city and the “École de chimie de Mulhouse” succeeded in keeping at a high level the local tradition of updated technical education and applied research in chemistry.
The following presentation traces in some detail the vivid interplay of scientific invention and technical innovation cultivated to boost the industrial developments in Mulhouse and Basel. Special attention is given to the individual contributors, scientists and entrepreneurs who pioneered over the past centuries the industrial rise of the RegioTriRhena leading from initial textile productions to the present day specialty chemistry and pharmaceutical activities.
2 Development of chemical industries in the “RegioTriRhena” since 1808
During 1808, Charles Philippe Kestner (1776–1846) came from Hanover to the Alsatian town of Thann to launch the first chemical industry in the RegioTriRhena (Fig. 1). His production plant increased gradually in size and is still operating today. It happens to be the very first chemical plant having ever been installed in France. Half a century later, several chemical production plants were launched, predominantly in the canton Basel, also in the Mulhouse metropolitan area and in the southern part of the German state of Baden. In his Ph.D. thesis, Die chemische Industrie im Raume von Basel, which he had submitted to the University of Basel in 1974, Heinz Polivka describes the continuous expansion of chemical industry since the year 1808 in what is named today RegioTriRhena. In 1860, he counted 500 employees in that geographic area, half of them being employed in Thann; a century later he came up with nearly 40,000 employees in the whole RegioTriRhena (Table 1) [1].

The Thann chemical plant in 1823 (engraving).
Employees of the RegioTriRhena chemical industries between 1808 and 1965 [1].
| Year | Total employees | Thann | Mulhouse | Chalampé | Basel area | South Baden |
| 1860 | 530 | 49% | 38% | 13%a | – | |
| 1890 | 1950 | 18% | 13% | 61%b | 8% | |
| 1914 | 6450 | 5% | 4% | 47% | 44% | |
| 1939 | 13,160 | 4% | 1% | 66% | 29% | |
| 1955 | 24,450 | 4% | 4% | 70% | 22% | |
| 1965 | 38,080 | 3% | 2% | 3% | 70% | 22% |
a Including the Lörrach and Schweizerhalle subsidiaries.
b Including the Huningue and Schweizerhalle subsidiaries.
This remarkable industrial expansion lasted until the end of the 1970s. A turning point occurred at that time from which one observes a slow decline of the so-called specialty chemical industries. For example, in 1974, at the height of that turning point, 32,000 employees were working in the two Basel cantons alone; since that time, about one-third of this workforce has been displaced or removed [2].
In 1808, Philippe Charles Kestner (1776–1846) coming from Hanover established a chemical plant in Thann for the production of inorganic chemicals, which are indispensable in the textile industry: sulfuric acid, nitric acid, tartaric acid and alum.
Philippe Charles Kestner was the third of 11 children born to Lotte née Buff. Charlotte, who had been in her youth the heroin of Goethe's novel Die Leiden des jungen Werther, had married Johan Christian Kestner, a privy counsellor to the elector of Hanover.
Philippe Charles having been able to develop a continuous process for the production of sulfuric acid, the banker Mennet helped him to set up his plant, which is the oldest chemical plant in France still in operation. This has to do with the history of chemical industry in France and in the RegioTriRhena area [3,4].
One should take notice that during the last quarter of the 20th century, and even more so during the beginning of the 21st century, pharmaceutical industries had supplanted the purely chemical ones in the RegioTriRhena. As to the production of textile dyestuffs, it has become a minor industrial activity, China and India having taken over that field worldwide. Nevertheless, pigments are still of some importance in the Basel and Thann areas, in terms of both production and applied research.
Whatever the evolution of industrial chemistry and pharmaceutical industry, synthetic organic chemists are always in great demand. As a matter of fact, production of small-sized molecular drugs obviously necessities the active cooperation of synthetic organic chemists. One should keep in mind that the two major pharmaceutical companies Novartis and Roche, their main subcontractor Lonza and their numerous spin-offs like Bachem, Basilea Pharmaceutica or Actelion Pharmaceuticals, and the many chemical and biochemical start-ups of RegioTriRhena require synthetic organic chemists in large numbers.
3 Textile industry initiated the manufacture of dyestuffs in the RegioTriRhena
In 1746, the manufacturing of printed cotton textiles called indiennes had been introduced into the Mulhouse economy. This move represented a major turning point, which had been initiated by three young entrepreneurs coming from leading Mulhouse textile, tanning and business dynasties. These entrepreneurs were Jean-Jacques Schmaltzer (1719–1776), a businessman who had worked in Basel, Samuel Koechlin (1721–1776), the business expert of the new company, and Jean-Henry Dollfus (1724–1802), the artist who took over sketching and engraving. Thanks to the launching of this novel manufacturing process, which had been aided by the Mulhouse banker Jean-Jacques Fehr, the town of Mulhouse became gradually an important hub of the Industrial Revolution in Europe.
In 1753, Johann Friedrich Küpfer, a Swiss cotton yarn producer from Bern, also launched the manufacturing of “indiennes” in Lörrach, a town in Germany, which became the cornerstone of the industrialization of the whole valley located along the Wiese River upstream northeast to Basel.
One should notice that for a long period of time, weaving was performed as one-family business in the RegioTriRhena, and in particular in the old canton of Basel. When Martin Luther launched the Reformation in 1517, religious intolerance resulted, which was followed by civil wars in many European countries. Therefore, religious refugees started to quit their dominantly catholic countries like France, the northern part of Italy and the Spanish Netherlands, and asked to settle in protestant regions of Europe like Basel or Geneva. After the revocation of the Edict of Nantes by Louis XIV in 1685, a second flux of French religious refugees called the Huguenots arrived in the old canton Basel. A large part of these refugees consisted of businessmen and craftsmen who brought along with them a strong know-how, particularly, in banking as well as in the silk business and the weaving of silk ribbons.
More than a century after the arrival of the first religious refugees and thanks to a technical discovery made in 1667, the council of the canton Basel decided in 1670 to permit the instalment of weaving power looms for the production of several silk ribbons at a time. The new and more efficient weaving looms were powered by domestic water mills and could produce up to 16 ribbons at a time. During the proper Industrial Revolution, that is, between the end of the 18th and the middle of the 19th century, steam engines and semiautomatic weaving machines—the latter ones having been invented by Joseph Marie Jacquard (1752–1834) in Lyon—led to the production of silk ribbons of higher quality and to an increase of productivity by the weavers who, by now, worked in large production plants.
Clearly, the Huguenot refugees had turned the canton Basel into an international hub for the production of silk ribbons, which led to considerable enrichment of the city.
Chemical industries were set up in great numbers during the second half of the 19th century for the production of predominantly artificial dyestuffs. The starting materials, indispensable for their industrial production, were extracted by distillation from coal tar products, that is, from the liquid leftovers of gas manufacturing for urban lighting via pyrolysis of coal. Some of these distillates, such as benzene, toluene, xylenes, naphthalene and anthracene permitted multistep syntheses of whole series of the so-called “aniline dyestuffs”, which do not occur in nature and which, for that reason, were called “artificial dyestuffs”.
In the town and the canton of Basel, the pioneers of the dyestuff industry often were French and Alsatian chemists coming from the textile manufacturing centres of Lyon and Mulhouse [5]. One should keep in mind that between the 1871 Frankfurt peace treaty and the 1919 Versailles peace treaty, inhabitants of Alsace were by law German citizens. The Alsatian entrepreneurs launched a series of small companies for the production of aniline dyestuffs, predominantly in the Basel area. Alexandre Clavel from Lyon, who was an expert in the printing of silk fabrics, launched in 1859 the first “start-up” in Basel for the production of fuchsine and some other aniline dyestuffs. After a couple of years, his company was passed on to some new investors to become in 1884 the “Chemische Industrie Basel” (CIBA).
Shortly thereafter, in 1860, Alexander Clavel was imitated by Johann Jakob Müller-Pack, an employee of the Basel Geigy Company, once again to produce some aniline dyestuffs, fuchsine being his first choice. Launched in 1758 by Johann Rudolph Geigy-Gemuseus (1733–1793), Geigy had been a purely commercial company importing and selling overseas products such as spices, medicinal and colonial products, naturally occurring dyestuffs and chemical products. These latter ones were mostly inorganic compounds, which were in great demand for the making of silk ribbons in the RegioTriRhena. Thanks to Müller-Pack, it was decided to turn Geigy into a manufacturing company of aniline dyestuffs.
After this second launching of a dyestuff company in Basel, several other manufacturing start-ups were launched in the RegioTriRhena area, such as Farbstofffabrik Gerber & Uhlmann in 1864 by Alsatian chemists Jean and Armand Gerber-Keller, Durand & Huguenin Company in 1871 by the French associates Louis Durand from Lyon and Edouard Huguenin from Mulhouse and finally the Sandoz company in 1886 by Edouard Constant Sandoz from the Neufchâtel canton. Mulhouse followed in 1892 when the Alsatian Louis Roesler launched his chemical plant, which, in due course, he named “Industrie chimique Mulhouse Dornach”. This company remained the property of the Roesler family for four generations and specialized in starting materials for the production of textile dyestuffs. Over the years, fine chemistry and pharmaceutical compounds became the leading products. In 1985, Industrie chimique Mulhouse Dornach was taken over by the Rhodia Company, which in 2009 decided to shut it down.
At the outset, the dyestuff plants in the “RegioTriRhena” were small-sized companies, which nowadays would be called start-ups. All of them should be considered, at least at their creation, as subcontractors of the textile industry. After several decades, the most prosperous of these chemical companies expanded tremendously by incorporating smaller companies and by diversifying their portfolio into other domains like polymer materials, plant protection products and pharmaceutical compounds [6,7].
One should also notice that the traditional production of silk ribbons diminished during the middle of the 19th century and disappeared entirely from the cantons Basel-Stadt and Basel-Land shortly before World War II (WWII).
Back to Thann, in 1883 Auguste Scheurer-Kestner changed the focus of his company towards organic chemical production by incorporating two small chemical companies located in Mulhouse. The newly created company was called “Fabrique de produits chimiques de Thann et Mulhouse” or “Thann et Mulhouse”, short-named “TM”. Next, an important economic fact appeared at the turn of the century, when “potash” deposits—actually a mixture of sodium and potassium chlorides—were discovered on a large scale in some deep-lying sediments accumulated by a former inland sea, which many eons ago covered the Upper Rhine area. Shortly after World War I (WWI), extraction of potash was performed by the French state-run company “Mines domaniales de potasse d'Alsace” in large quantities until 2004 when the mines were shut down. Furthermore in 1927, a new chemical industry had been launched; it was based on the electrolysis of potassium chloride (KCl) solutions. Named “Potasse et produits chimiques” (PPC), it was a subsidiary both of TM and of Mines domaniales de potasse d'Alsace. A few years earlier, in 1922, another move had already been taken in the local chemical industry, thanks to an agreement signed between TM and the “Société des Terres rares” of Serquigny, in Normandy. It led to the construction of a new plant in Thann for the production of the white pigment titanium dioxide (TiO2) at the TM site. TM was the first industrial plant worldwide to produce TiO2 on a large scale. In 1951, there followed the production of organic bromo derivatives by PPC and in 1957 of TiCl4 by TM. An even larger TiO2 plant was set up by TM in the port of Le Havre, Normandy. In 1971, after several takeovers, both TM and PPC became subsidiaries of Rhône-Poulenc. TM is now part of the Djedda-based Saudi Cristal Company and PPC a subsidiary of the Frankfurt am Main-based International Chemical Investors Group.
In 1957 near the Alsatian village of Chalampé, along the “Grand Canal d'Alsace”, which runs parallel to the Rhine, Rhône-Poulenc launched a plant for the large-scale production of Nylon 66 salt, starting from adipic acid and hexamethylenediamine. Equimolar mixtures of these two components are sold all over Europe to production plants of the Nylon 66 polyamide. Rhône-Poulenc had a good command of the oxidative ring cleavage of cyclohexane into adipic acid. As to hexamethylenediamine, a joint venture with the US Company Du Pont de Nemours allowed its production on a large scale via catalytic hydrocyanation of butadiene. At the outset of the 21st century, the Chalampé production plant, which for a long period of time had been the property of Rhône-Poulenc followed by the Rhodia Company, was taken over by the Belgian Company Solvay (Fig. 2).

The Solvay plant of Nylon 66 at Chalampé.
4 Chemical research at the University of Basel and in chemical and pharmaceutical industries of the RegioTriRhena
Since 1515 and the political alliance of the independent Republic of Mulhouse with the Confederation of the 13 Swiss Cantons of that time, until its “reunion” with France in 1798, economic, military, cultural and religious exchanges operated as a rule between the Swiss Confederation and the “Associated Territory of Mulhouse”, a so-called “Zugewandter Ort”. Therefore, it is not surprising that a few students from Mulhouse got their higher education at the University of Basel, either at the faculty of protestant theology or at the faculty of law. We notice that the Basel and Mulhouse protestant church was called “reformed church”, which is somewhat similar to the Calvinistic faith. It had been elaborated by the Basel reformer Johannes Oecolampade for both towns.
The University of Basel, founded in 1460, a few years after the Council of Basel (1431–1449), has a rather long tradition in alchemy and medicinal chemistry. This tradition dates back to 1527, when Paracelsus (1453–1541) was the chair holder of medicine in Basel, albeit only for a one-year period. To the great surprise of Basel's society and just before his nomination, Paracelsus had been able to heal Johannes Froben (1460–1527), the principal printer and editor of Erasmus of Rotterdam's manuscripts, from a severe ailment. Using healing approaches of his own, Paracelsus treated Froben successfully. Because of his rather new healing methodology, some science historians believe that his medicinal approach is at the origin of chemical pharmacopeia, even the source of modern pharmacopeia and so on.
Since 1527, the very year when Basel adopted the Reformation, until the novel University statutes were edited in 1818, the faculty of philosophy had been subordinated to the faculties of theology, law and medicine. The domain of the “Naturwissenschaften”, to which belong both physics and chemistry, had been taught at the faculty of medicine. As a consequence of it and for three centuries, the University of Basel had asked professors of that faculty to teach chemistry and, at least in part, also alchemy according to the concepts of Paracelsus, whatever that meant….
The University statutes of 1818 were more or less the consequence of Napoleon's reshuffling of the University system in France, which he had brought about in 1806. According to these novel statutes, the Basel faculty of philosophy attained the same level as the three professional faculties. Therefore, the authorities of the canton Basel created a new chair for both chemistry and physics. In 1820, Peter Merian (1795–1883) became his first chair holder for 15 years. He is best known for having created the first chemical laboratory of the University. We notice that the laboratory of the future Mulhouse Chemistry School was launched at about the same time.
In 1835, Christian Friedrich Schönbein (1799–1868) became Merian's successor and proved to be a first-class research chemist. He discovered ozone in 1838 and nitrocellulose in 1844–1845, in the form of guncotton and wet collodion. After his death, Jules Piccard (1840–1933) took over his chair and studied food chemistry and some newly invented aniline dyestuffs. He was the first professor to cooperate with Basel's chemical industry. Jules Piccard was also the founder of a famous dynasty of scientific explorers: his son August (1884–1962), a professor of physics at the Free University of Brussels, who went up vertically to stratospheric heights inside a pressurized tight metallic sphere attached to a hydrogen-filled balloon; his grandson Jacques Piccard (1922–2008) also used a tight and thick-walled metallic sphere built by his father as part of the deep-sea bathyscaphe submarine and went down vertically into the Mariana trench in the deepest part of the Pacific Ocean; and his great-grandson Bertrand Piccard (born 1958), a psychiatrist who in recent years completed both the round-the-world nonstop flight inside a cramped housing attached to a hot air balloon and the multistep round-the-world flight in the electric-driven “solar impulse” plane.
Because chemical industry and physical sciences were developing at a fast rate, more and more students got interested in these fields. Therefore, the authorities of the canton Basel created new teaching and research chairs, which by now specialized in either chemistry or physics.
We shall quote but a few of the many chemical professors of the University of Basel. To begin with, one must cite Johann Friedrich Miescher (1844–1895) who discovered the nucleic acids he called “nuclein” and which today are named DNA. He had isolated them in 1870 and surmised their role in the genetic transfer process. In his honour, the Friedrich Miescher Institute of Biomedical Research (FMI) was created in 1970, that is, 100 years after Miescher's discovery, as a foundation by the CIBA-Geigy Company. In 1996, the FMI was incorporated in the pharmaceutical company Novartis, and in 2012, it became an independent institute whose reputation is worldwide in terms of fundamental biomedical research.
Having obtained his “habilitation” in 1884 with Jules Piccard, the chemist Rudolph Nietzki (1847–1917) succeeded Piccard from 1895 until 1911. He played a decisive role in establishing a new class of dyestuffs, which he obtained chemically from coal tar derivatives, and cooperated with the Basel chemical industry. One of the concepts he developed was the “quinone theory” which bears his name. Nietzki discovered two novel dyestuffs, Bieberich scarlet in 1878 and alizarine yellow in 1887, and is considered to be the founding father of the Basel school of dyestuffs [8].
Let us focus also on Dr. Paul Müller (1899–1965) who had been a student of Hans Rupe (1861–1951) and Friedrich Fichter (1869–1952), both were professors at the University of Basel. He was a biochemist with Geigy and had obtained the Nobel Prize in Physiology or Medicine in 1948 for the discovery of DDT's insecticidal properties: dispersing DDT powder over infested marshlands led to the reduction of the number of insects that propagate malaria fever. Consequently, thousands of lives of human beings could be saved, particularly in Southeast Asia, during and after WWII.
Because of the important industrial production of dyestuffs—the textile industry being still the major economic factor after WWII—the opening in 1948 of the “Institut für Farbstoffchemie” by Professor Robert Wizinger-Aust (1896–1973) was kind of a joint venture between chemical industry and the local University. Wizinger-Aust got interested in substitution reactions and studied, in particular, the relationship between molecular structure and colour. In 1966, Professor Heinz Balli (born 1929) succeeded him as a director of that institute and studied bis-diazonium complexes and diazo transfer reactions. In 1973, Basel's dyestuff chemists organized the International Dyestuff Conference. After the retirement of Heinz Balli and in view of the declining dyestuff industry, the Institut für Farbstoffchemie was closed.
Professor Edgar Heilbronner (1921–2006) must be cited as a leading physical chemist at the University of Basel, because he introduced Hückel's theory of molecular orbitals—the so-called HMO theory—to interpret conjugation effects in benzene derivatives, in general, in annulenes and dyestuffs, in particular, by making use of photoelectron spectroscopy [9].
Besides the domain of dyestuffs, which for a long period of time was the dominant economic domain of Basel's chemical industry, natural products and medicinal chemistry gradually represented an industrial diversification and a new objective in research laboratories at the University of Basel. Professor Tadeus Reichstein (1897–1996), a former research student of Chemistry Nobel laureate Hermann Staudinger (1881–1965) at the Eidgenössische Technische Hochschule (ETH) Zurich took over the Basel Institute of Pharmaceutical Science in 1938 and the Institute of Organic Chemistry 10 years later. Reichstein was awarded the Nobel Prize in Physiology or Medicine in 1950 for the isolation of corticosteroids and, in particular, for the discovery of cortisone and its therapeutic properties against rheumatoid arthritis. Not to be forgotten is the fact that Reichstein had worked out, already in 1933 at the ETH Zurich, the multistep synthesis of ascorbic acid—that is, vitamin C—from glucose. Emil Barrell (1874–1953), the energetic CEO of the pharmaceutical company Hoffmann-La Roche, immediately took over this multistep synthesis of vitamin C and applied it on a large industrial scale. Gradually, his company became a major producer of almost all vitamins worldwide. Quite remarkably, the Reichstein procedure for the industrial production of vitamin C is still in operation today on a large industrial scale with DSM, a Dutch chemical company, which in 2000 took over the department of vitamins and fine chemicals from Roche.
Cyril A. Grob and Christoph Tamm, both of whom had been Ph.D. students of Tadeus Reichstein, became professors of organic chemistry at the University of Basel. Grob (1917–2003) discovered some specific organic chemical fragmentation processes, which proved to be concerted and stereospecific reactions. Nowadays, these dynamic molecular mechanisms called “Grob fragmentation processes” have become part of the modern doctrine of organic chemical reaction mechanisms. As to Christoph Tamm (1923–2017), we owe him the synthesis and biosynthesis of some metabolites, which operate in microorganisms and in plants; we also owe him a better understanding of the DNA replication.
Thanks to Reichstein, Grob and Tamm, the Basel faculty of sciences turned its attention predominantly to the chemistry of natural bioactive products and to medicinal chemistry. This sort of “turning point” had already been taken by Hoffmann-La Roche, a few years only after its foundation in 1896: opium alkaloids and cardiotonic glucosides from foxglove flowers were sold successfully under the trade names Pantopon and Digalen, respectively. As mentioned above, the chemist Emil Barrell, who was the longtime CEO of Hoffmann-La Roche, decided to engage in large-scale production of almost all vitamins. Next, his colleague Dr. Leo Sternbach in Nutley (New Jersey, USA) invented in the early 1960s several benzodiazepine tranquilizers, all of which became profitable blockbusters for his employer Hoffmann-La Roche. Furthermore, in the 1970s, Rocephine®, a powerful third-generation cephalosporin antibiotic that had been invented by the Department of Infectious Diseases was launched by Roche and became a first-class “blockbuster” for several years.
In the 1920s, the Sandoz Company also entered the field of natural bioactive products and of medicinal chemistry. Thanks to the pioneering work of Professor Arthur Stoll (1887–1971), ergot alkaloids were isolated in a pure form and investigated thoroughly. For example, Gynergene, which is but one of these alkaloids, was shown to combat postnatal pain and chronic headache [10]. Furthermore, cardiotonic glucosides from foxglove flowers were also on Stoll's research program.
We should also cite Kurt Wüthrich (born 1938), a professor at the ETH Zurich, who had obtained his Ph.D. at the University of Basel with professor of chemistry Silvio Fallab (1925–1993). He obtained the Nobel Prize in Chemistry in 2002 for deciphering the Fourier transform high-resolution NMR of complex prion proteins whose molecular conformation could turn pathogenic and even lethal, as in the case of the Creutzfeldt–Jakob disease.
For a long period of time, and as in other European Universities, chemical research at the University of Basel was oriented mostly towards fundamental science, whereas the local chemical industry was very much in demand of applied research in the field of novel dyestuffs. The industrial sector of “specialty chemicals” recruited its scientists as “Diplomchemiker” or “Promovierter Chemiker (Ph.D.)” at Universities and “Technische Hochschulen” (TH) in Germany, for example, at the TH Karlsruhe, also at the “Mulhouse Chemistry School”, and quite often at the “Polytechnikum” of Zurich renamed in due course “Eidgenössische Technische Hochschule” [11–14].
5 Sandoz and the immunosuppressive cyclosporine A
At the end of WWI and thanks to the efforts of Professor Arthur Stoll (1887–1971), Sandoz had taken an early turning point leading from a large-scale production of artificial dyestuffs to the study of natural products having medicinal properties like the ergot alkaloids [10].
The long efforts to discover the immunosuppressive effects of cyclosporine A (CsA) were rather complex and required a large array of investigations, some of which proved to be quite frustrating. The serendipitous discovery of CsA represents a major breakthrough in 20th century medicine, particularly in the field of viable organ transplants. It could be achieved, thanks to the interplay and intense cooperative work of a large multidisciplinary team of dedicated scientists of Sandoz-Basel, which were led by Dr. Med H.P. Staehelin. His team included medical doctors, microbiologists, chemists, biologists and pharmacologists. In our opinion, Pasteur's statement “chance only favours the minds which are prepared” applies well to the discovery of Staehelin's team of immunosuppressive CsA, whose brand name became Sandimmune, later to be renamed Neoral.
The discovery of CsA represents a second and remarkable milestone indeed in medicinal chemistry of natural products at Sandoz. Starting in 1957 at the Sandoz Laboratories in Basel, scientists investigated tens of thousands of culture broths of soil microorganisms for cytostatic activity in tissue cultures of chick embryo fibroplasts or in cultures of murine P-815 mastocytoma cells. The active filtrates of the fungus cultures were then extracted and purified by chemists. In the early years of that program, the preparations that were active in mammalian cell systems were also investigated for antimicrobial activity, which became a predominant goal.
In 1969, Staehelin made the key decision to include a test system for immunosuppression in the general screening program. He invented a new procedure, which permitted the use of the same mice for testing both anticancer activity (L-1210) and immunosuppression, that is, inhibition of antibody formation in an “all in one in vivo test system”.
One week, in December 1971, the batch of 20 preparations included “preparation no. 24–556”, a partially purified fungal product, which was later found to consist mainly of CsA. Preparation 24–556 was derived from a fungus, Tolypocladium inflatum (Beauveria nivea), which the microbiology group had found in March 1970 in a soil sample obtained in 1969 from the high altitude Hardangervidda peneplain by a Sandoz microbiologist when on vacation in Norway. This high-altitude plain had caught his eye because it was rich in alpine and subarctic plants. At Sandoz, scientists set out at once to culture this microorganism because of its antifungal effect. Fortunately, and in view of the striking positive results of 24–556, research on its immunosuppressive potential was also performed with success. That potential had been discovered on 31 January 1972 by employees of Dr. Staehelin's group.
CsA was isolated and its three-dimensional (3D) structure elucidated by the Sandoz chemists (Fig. 3). Because most peptides are synthesized by ribosomes, one may be interested to know that CsA is a nonribosomal cyclic peptide, which is built up from 10 amino acids belonging to the l-series and one being a new d-amino acid, a fact that is rarely encountered in nature.
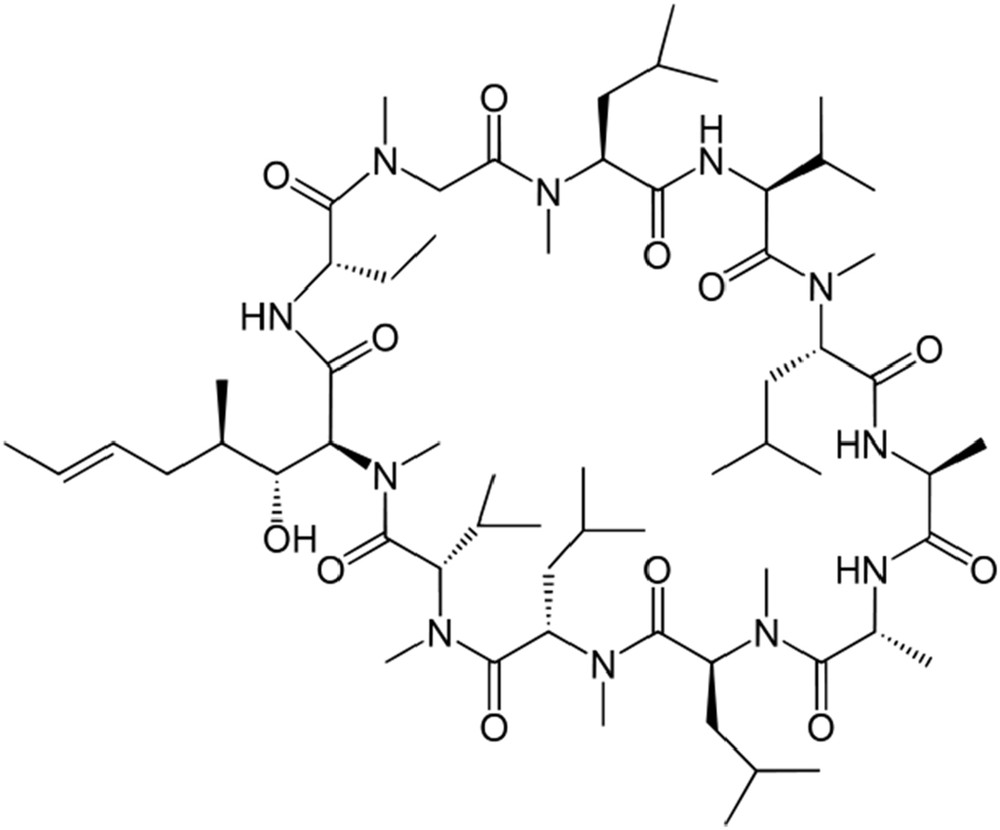
Three-dimensional structure of the naturally occurring product CsA.
In April 1976, Jean-François Borel, also a member of Staehelin's group, gave a conference at the spring meeting of the British Society for Immunology in London. His conference, which was based on the findings of Staehelin's multidisciplinary group, stimulated the interest of many British scientists and clinicians and proved to be of major significance for the clinical development of CsA in Cambridge, London, and Basel. Transplantation experiments were started at once with CsA in animals, then applied to patients with kidney grafts and to those undergoing bone marrow transplantation.
In the long run, immunosuppressive CsA—which became available in the pharmacies under the brand name Sandimmune and somewhat later under the name Neoral—became the first line treatment for preventing rejection of transplanted organs and for certain autoimmune diseases [15–17]. Actually, CsA reduces the activity of the immune system by lowering the activity and the growth of T cells.
Nowadays, this wonder drug is widely used in organ transplantations to prevent rejection. In the long run CsA, which had been isolated from a Norwegian “magic mushroom”, permitted to save the lives of tens of thousands of transplanted patients. To sum up, thanks to Dr. Staehelin's farsightedness and to the many conferences given by Jean-Francois Borel, Sandoz became the world expert in immunology, at least when it comes to immunosuppressing methodologies.
6 Basel Institute of Immunology and the discovery of monoclonal antibodies
Last but not least, one should notice the rather recent and spectacular development of recombinant proteins and even more spectacular the production of monoclonal antibodies. These novel activities of the two major pharmaceutical companies of RegioTriRhena do not require many synthetic chemists to operate, the experts of genetic engineering and biotechnology playing instead the dominant role.
Along with research in the fields of natural products and medicinal chemistry, one should indeed focus the attention on the Basel Institute for Immunology (BII), also called “Institut für Immunologie Basel”, which was one of the major research centres for immunology worldwide. In 1969, the pharmaceutical group Hoffmann-La Roche decided to launch a new type of research project: without imposing any particular conditions upon the scientific members, it was decided to support their research efforts by investing 25 million dollars per year to the BII. The Institute was opened in 1971 under the leadership of immunologist Dr. Niels Kay Jerne from Denmark. Over a period of 30 years, 500 research experts worked at the Institute for various periods of time, 27 of them obtaining major awards (among these, Nobel Prizes of Physiology or Medicine to George Köhler, Niels Kay Jerne and Susumu Tonegawa). In 2000, the Institute was closed and replaced by the Centre for Medicinal Genomics, this time as an internal institution of Roche [18].
According to Wikipedia, “the establishment of the BII coincided with a convergence of a critical mass of young and energetic scientists from around the world in Basel to staff three start-up research ventures—FMI, Biozentrum and BII—to exploit the newly breaking technologies related to molecular biology, gene cloning and development of mouse models. BII was a unique concept in the history of mechanism for funding basic science and the relationship between basic science and industry. Through the influence of Paul Sacher, Swiss orchestra conductor and patron of the arts and sciences, drug company Hoffmann-La Roche committed unrestricted support and freedom of design of the BII to its director Niels Kay Jerne, as well as to his successor Fritz Melchers. Scientists from beginning postdoctoral to senior professor were provided complete freedom of research design without the pressures of individual fund raising, proposal writing, politicking and pressure to fit research to popular demands and funding source. Continuous visits by distinguished visiting scientists from around the world for periods of a day to months enriched the environment. In the 70's it was estimated that 17 different languages were spoken at the institute united by English, the common language of science. Social gatherings between the international staff of the three institutes and heated discussions concerning lifestyles, the arts and in particular science in the pubs of Basel were common in that period [19].”
This private institute came up with some brilliant results during its existence of three decades. The invention of monoclonal antibodies by George Köhler, a member of the BII, and César Milstein of Cambridge University, led to a series of medicinal breakthroughs, which heralded a new pharmaceutical era.
George Köhler had been engaged by the BII in June 1971 […], that is, three years before he got his Ph.D. at the University of Freiburg im Breisgau. In 1975, this brilliant young scientist, who nourished what he called “crazy ideas”, developed as a postdoctoral fellow at age 29 years together with César Milstein, the hybridoma technology for producing monoclonal antibodies. This technology is based on the hybridization of an antibody producing malignant cell with a normal cell of the same type. Ultimately, this technology permitted exploitation of antibodies for diagnostics and therapeutics and represents a remarkable breakthrough and a major scientific discovery of the 20th century [20].
As expected, Nobel Prizes in Physiology or Medicine were bestowed in 1984 to George Köhler of the BII and to César Milstein of the Cambridge Laboratories, as well as to Niels Kay Jerne, the director of BII, for his clonal selection theory and his proposal of the immune network concept. Furthermore, Susumu Tonegawa, also a member of the BII, obtained the Nobel Prize in Physiology or Medicine in 1987, for his discovery of the genetic principle concerning the generation of diverse antibodies.
FMI and BII cooperated with the Biozentrum University and were actively engaged in the teaching of immunology. When Roche closed the BII in the year 2000, it made a bequest of 10 million Swiss francs to the Biozentrum University of Basel for the launching of a chair of immunology. We notice furthermore that Professor Werner Arber, one of the leading scientists of the Biozentrum, had already been awarded the Nobel Prize in Physiology or Medicine in 1978 for his discovery of the restriction enzymes, which permitted the synthesis of recombinant proteins like human insulin and interferon α 2a.
The three Basel institutes cited above had been launched within a few months from each other in 1970–1971, a fact which obviously is not accidental…. Quite remarkably, music conductor and main owner of the Hoffmann-La Roche Company, Paul Sacher, decided to launch and support the BII over a period of several years. In 1978, Dr. Fritz Gerber became the new CEO of Roche. In agreement with Dr. Paul Sacher, he decided to stick to the same policy of research freedom offered to all the scientific members of the BII… for another 22 years. Neither Paul Sacher nor Fritz Gerber was scientist; they had been given top quality recommendations by some excellent advisers. Both accepted to take high risks to gain access to biotechnological discoveries…, which had not even been made.
In addition to the BII the Max-Planck-Institut für Immunbiologie und Epigenetik, which had been founded already in 1961 in Freiburg-im-Breisgau, is another institute of RegioTriRhena that assumes an important role in immunotechnology. In 1984, when George Köhler received the Nobel Prize in Physiology or Medicine, he became the director of this Freiburg MPI, a position he kept until his untimely death in 1995, at the age of 49 years.
The 1970s and 1980s represent a remarkable period of fundamental research in the field of immunology and genetic engineering at all three Basel institutes cited above, which cooperated closely. As a nonforeseen consequence of the “brain-storming atmosphere” in Basel, the local pharmaceutical industry profited in the long run from these high level “scientific think tanks”, particularly in terms of immunotherapy of various forms of cancer. This unprecedented and successful biotechnological high-risk adventure in RegioTriRhena is the consequence of two factors: (a) the fundamental scientific discoveries of the above-cited three institutes, and (b) the takeover of the California-based company Genentech by Hoffmann-La Roche in 1990 as well as the fusion of Roche with the Japanese biotechnology company Chugai in 2002, followed by its takeover in 2014.
7 Applied research at the Mulhouse Chemistry School
In the greater metropolitan area of Mulhouse, textile industry was for a long period of time the dominant economic factor. Consequently, applied research had to be focused on textile chemistry. Clearly, the Mulhouse Chemistry School had to play a leading role in these matters after having been launched in 1822 by the Mulhouse municipality and the owners of the major textile companies [21].
Already in 1811, the city council of Mulhouse had in mind to launch a course in chemistry to train technicians in the field of textile printing industry. Eventually in 1822, a chemistry chair was created by some local industrialists under the driving force of Daniel Koechlin-Schouch (1785–1871). This scientist-industrialist had a good knowledge of the dying processes. He himself had invented a process permitting the direct application of Turkey red on canvas. He had also invented a process by which almost any colour could be printed on top of a red-tinted fabric. He was a remarkable personality, having studied at age 15 years under the guidance of Antoine-François de Fourcroy, a professor of chemistry in Paris and an excellent teacher who was among the very first scientists having embraced the new concepts of Lavoisier.
In 1822, the office of the Mulhouse College Municipal announces the launching of a “new course in chemistry as applied to the arts” (Fig. 4). Thirty years later, in 1855, that course was incorporated in the program of the “École supérieure des sciences appliquées” (ESSA) of which it became in 1866 an “Independent Chemical Section”. After the Franco-Prussian war of 1870, the Mulhouse municipality and the “Société industrielle de Mulhouse” (SIM) reorganized that independent Chemical Section into the “École municipale de chimie industrielle”.

Mulhouse municipal poster dated 16 February 1822.
One century later, in 1930, the Mulhouse Chemical School became a private Foundation of public utility with the new title “École supérieure de chimie de Mulhouse” (ESCM). Last but not least, in 1957, its director Jean Lichtenberger obtained the integration of ESCM as a full-fledged Institute in the University of Strasbourg.
To sum up, since its foundation in 1822 and until the year 1957, the Mulhouse Chemistry School operated as a local institution under the auspices of the municipality and SIM. In 1957, it became an Institute of the University of Strasbourg [21,22].
7.1 The Achille Penot era (1825–1854)
The SIM was founded in 1826. Even before that year, the Mulhouse Chemistry School was launched in 1822 by some dynamic owners of textile industries and the Mulhouse municipality, the objectives being to launch some evening courses in chemistry, a scientific discipline that was clearly needed in the textile printing industry. A chemical laboratory was also installed to permit the various analyses of the natural dyestuff mixtures bought on the world market, and to test dyeing and printing procedures of diverse fabrics. Both the lecture rooms and the laboratory were installed in the Collège de Mulhouse, at 31, Grand Rue. The first courses began on 1 March 1822 and were partly financed by industries whose activities were mostly in the field of dying, printing and finishing of textiles (Fig. 4).
During the first three years, only evening courses were dispensed. As to the laboratory, it operated full time with six appointed experimentalists. The number of students was limited to 44 and the tuition fee rather costly, so that only students send by the leading Koechlin, Schlumberger, Schwartz, Thierry, Zuber, Mieg, Dollfus or Zundel textile dynasties could afford the tuition fees.
In 1825, Achille Penot (1801–1886), a teacher of physical sciences at the Nîmes High School, who over the years had obtained a Ph.D. in physical sciences at the University of Strasbourg, was appointed as headmaster of the courses. He proved to be the right man for that job, which he fulfilled over a period of three decades, teaching courses both in chemistry and physics and supervising experimental work, by now at full time. Penot trained numerous chemistry technicians and attracted students from all over Europe, especially from Germany, Switzerland, Central Europe, tsarist Russia, and even from the Orient. Besides teaching, he also performed a few chemical experiments and proved particularly active at the committee of the SIM where he contributed about 100 articles to the Bulletin de la Société industrielle de Muhouse in various fields, pertaining to social and economic sciences, rather than technical ones.
7.2 The Paul Schutzenberger era (1854–1865)
In 1854, the industrial classes were transferred from the “Collège municipal” to the newly created Professional Training School that had been launched by the municipality. According to the Mulhouse archivist and Professor of History Raymond Oberlé, the launching of such an institution was a remarkable feat, because European nonuniversity towns had rarely such Professional Training Schools of their own, with study programs quite close to those of a University bachelor level. It so happened that almost simultaneously, the French imperial government launched the ESSA, whose study program was similar to that of the Professional Training School. In fact, these ESSAs were officially designed to teach at a level somewhere between a high school program and a University bachelor program, albeit they were more technically oriented. The Mulhouse ESSA started its study program in November 1855 in the new buildings of the rue Huguenin, which had been destined also to house the Professional Training School of the municipality and the Municipal School for Textile Design. Thanks to its reputation, the Chemical Section joined ESSA in the new building.
After the move from the Grand Rue to the rue Huguenin, the study program was extended to 2 years and the resulting Chemistry Section became an independent entity.
At about that time, Paul Schutzenberger (1830–1897) from the Strasbourg University was recruited as an instructor in charge of all chemical courses. His teachings proved to be more scientifically oriented. In addition, he introduced laboratory work in the field of applied chemistry, which was a novelty at that time. Both the municipality and SIM decided to install a second laboratory to permit Schutzenberger to pursue some research topics of his own. This new laboratory also permitted to train students both in qualitative and quantitative chemical analyses. Schutzenberger was an authentic scholar, who had obtained a Ph.D. in chemistry and a second one in medicine at the Strasbourg University. He was able to pursue simultaneously several research topics, mostly in the field of applied science, and must be considered as the pioneer of modern scientific research in Mulhouse.
During the two-year study program, several disciplines were taught. Besides general chemistry as the major topic, principles of physics, analytic chemistry and industrial chemistry were also put on the agenda. These various lectures alternated with practical work in the laboratories, bringing the overall weekly load to 30 h. Sixteen-year-old candidates were permitted to enrol, provided they had acquired the chemistry level of a French “baccalauréat”. After having accomplished 2 years of successful study, the students were handed over a certificate, which one could consider today as an equivalent to the “brevet de technicien supérieur” in chemistry or equivalent to the “diplôme universitaire de technologie”.
Paul Schutzenberger initiated modern scientific research and wrote a detailed treaty about colouring matters [23]. This two-volume book had been written under the auspices of SIM with the financial help of its Chemistry Committee. It soon became the standard reference compendium, particularly for foremen, technicians and engineers who were actively engaged in the production of calico prints and the sophisticated indiennes. Schutzenberger had written this book at a time when the theory of interatomic bonding, valence and molecular structure was in the offing. Only in 1858, did Friedrich Kekulé in Heidelberg and Archibald Scott Couper in Paris propose the tetravalency for carbon atoms. In 1865, this time at the University of Gent, Kekulé proposed the revolutionary hexagonal, planar and symmetrical structure for benzene. This structural proposal was published a short time before Schutzenberger sent his manuscript to the editor. Therefore, it is not surprising that so-called “developed structures of molecules” are totally absent from it, not even hexagonal benzene being represented. In his book, dyestuffs are represented by their gross elemental formulae, which are not always correct. For example, alizarin red was given the elemental composition C10H6O4, then C20H12O6, instead of C14H8O4, but isatin had the correct elemental composition C8H5NO2. As to indigo blue, Schutzenberger proposed the formula C8H5NO, which is exactly one-half of the correct one C16H10N2O2. In our opinion, this result shows the high precision of his analytic experiments, because the centrosymmetric structure of indigo was not known yet. It was demonstrated as late as 1883 by Adolph von Baeyer at the University of Munich.
In 1865, Schutzenberger moved to Paris where in due course he obtained the chair of chemistry at the Collège de France. He became also a member of the “Académie des sciences”. In 1882, the city of Paris founded the “École supérieure de physique et de chimie industrielles” (ESPCI), which was willingly a copy of the Mulhouse Chemistry School, which Schutzenberger knew best. As expected, he became the first director of that engineering school, which nowadays is called ESPCI, ParisTech. Both at the Collège de France and at the ESPCI, Schutzenberger continued his research endeavours he had launched in Mulhouse, particularly in the field of natural dyestuffs. Moreover, he was the first scientist to prepare and identify cellulose triacetate, which became—albeit much later—an important economic factor as a textile fibre, as a lacquer and in the form of various plastic materials. Furthermore, Schutzenberger prepared several metal-carbonyl derivatives and silicon carbide. Even more importantly, he discovered the reducing agent “rongalite”, which is the addition product of formaldehyde and sodium hydrosulfite. Rongalite was taken up quickly by the cotton industry as a bleaching agent for the processing of vat dyes. Last but not least, he wrote a seven-volume Chemistry Treatise [24].
7.3 The Rosenstiehl, Perrey and Goppelsroeder eras (1865–1880)
After Schutzenberger's departure to Paris, the “Chemical Section” became the “École supérieure de chimie” as an annexe to the Mulhouse ESSA. From 1865 until 1868, Daniel-Auguste Rosenstiehl took over the chemistry lectures, followed by Paul Perrey from 1868 till 1871, physics being taught by A. Schneider over a 10-year period, from 1864 till 1874, himself being the successor of former director Achille Penot who had taught the physics course from 1855 until 1864. Rosenstiehl (1839–1916) proved to be a first-class scholar and a superb teacher who attracted excellent students to the École supérieure de chimie. Among his research endeavours, one should cite the experiments he performed with aniline dyes, particularly with aniline black and with various fuchsine derivatives. He was best known for the way he defined the various colours and for his classification of the colour spectrum, which led to the publication of his Treatise of Colours [25].
After the Franco-Prussian war of 1870, Alsace and part of Lorraine were officially detached from France in 1871 to become the “Reichsland Elsass-Lothringen” as part of the Second German Empire. Thanks to the efficient cooperation between the Mulhouse municipality and SIM, the École supérieure de chimie was quickly reorganized into an École municipale de chimie, without any control on behalf of the German authorities. Starting in 1872, the new director of the school was Professor Friedrich Goppelsroeder (1837–1919), a Swiss citizen from the University of Basel. He was able to keep up the reputation of the Mulhouse Chemistry School and give it a new impulse, particularly in favour of scientific research. He introduced electrolysis as a means to perform original syntheses of new dyestuffs for the local textile industry.
It is worth noticing that Professor Christian Friedrich Schönbein (1799–1868), whom we already met at the University of Basel, had quite an experimental flair for novelties. He observed that mixtures of coloured substances migrated in a solution on the filter paper with different capillary velocities. Friedrich Goppelsroeder took over that topic and developed it by making his own observations, even before moving to Mulhouse. Over the years, he was able to build up a new analytic methodology to be called later “paper chromatography”. His first two articles published in 1861 and 1862 about the concept of “capillary analysis” did not draw the attention of the scientific community [26,27]. Some four decades later, in 1904, he published a book about his detailed findings concerning capillary analysis [28]. Professor Daniel Kritchevsky published a full article in 1959 in which he credits Friedrich Goppelsroeder as being the “Pioneer of Paper Chromatography” [29].
7.4 The brilliant Emilio Noelting era (1880–1915)
In 1880, the SIM authorities offered the direction of the Mulhouse Chemistry School to Dr. Emilio Noelting (1851–1922), a 29-year old Danish-German citizen who was born in the Dominican Republic. He became a chemist who had obtained his University diplomas and his Ph.D. at the “Zurich Polytechnikum”, a technical university, which was renamed in 1911 ETH, short form for Eidgenössische Technische Hochschule. The Noelting era proved to be a brilliant one in the life of the Mulhouse Chemistry School during the final two decades of the 19th century and until WWI.
Noelting had shown his scientific capacities during the course of his Ph.D. at the ETH Zurich and, even more so, during the 5 years he spent in chemical industries, first in Lyon and then in Geneva. Thanks to his first-class activities in Mulhouse, both as a pedagogue, as an expert in applied research, and as an administrator over a period of 35 years, the Mulhouse Chemistry School acquired an excellent reputation all over Europe and even worldwide. Noelting added a third year and finally a fourth one to the study program, to permit the preparation of some Ph.D. degrees. Clearly and from that point on, the Mulhouse Chemistry School could be compared with the Department of Chemistry of a German TH. Nevertheless, it was a privately owned institution, which operated independently from any German-run Technical University system during the Reichsland Elsass-Lothringen period (1871–1918).
During his two research periods in industry, first in Lyon and then in Geneva, Noelting improved the production of some dyestuff processes. In cooperation with his colleague Frédéric Reverdin in Geneva, he studied derivatives of naphthalene as starting materials for the production of dyestuffs. The edition of their monography Über die Konstitution des Naphtalins und seiner Abkömmlinge (“About the constitution of naphthalene and its derivatives”) became an editorial success and did influence the production of new naphthalene type dyestuffs [30]. During his years in industry, Noelting invented new polyhalogenated fluoresceins like erythrosine, which became food colourant E-127, or rosebengal and phloxine, these two dyestuffs having been used by biologists as histologic markers.
As soon as he entered the new buildings along the “quai du Fossé” of the Mulhouse Chemistry School, Noelting modified the teaching program to take account of the recent developments of chemical and physical sciences. Consequently, in 1888 he revamped the teaching program and extended it to a three-year period. Furthermore, he addressed his research efforts towards the structure of triphenylmethane dyes, which was not an easy task to cope with. In fact, it was solved at the end of the 19th century by some German chemists. According to Professor Chézeau, Noelting believed the practical job of a textile chemist could not simply rely on recipes, which become quickly obsolete. His know-how had to be based on scientific knowledge and on some scientific research efforts. Noelting's most important contribution was to keep applied research in the teaching program of the Mulhouse Chemistry School, in a way not unlike the one he had experienced himself at the Zurich Polytechnikum [31].
During Noelting's long chairmanship, the Mulhouse Chemistry School chose two well-defined technical and scientific orientations, which became its chemical cornerstones for almost a century:
- • dyestuffs and their precursors,
- • textile ennobling.
On the other hand, precursors of dyestuffs could prove to be of some value in other domains; for example, perfumes could be prepared from aromatic precursors. Noelting was farsighted enough to grasp quickly any possible industrial application of such discoveries. To quote but one domain of application, the era of musk synthesis is worth describing. In 1888, the Mulhouse chemist Albert Bader filed his first patent about the production of trinitro-tert-butyl-toluene, a nitro derivative of a benzene precursor, which proved to be an excellent and strong perfume. Its commercial success was overwhelming; this product quickly replaced natural musk derivatives, which had to be purchased for a high price in Asia [32,33]. Pretty soon, this product was followed by the discovery, again by Bader, of new polynitro aromatic compounds, which proved to be even more intense perfumes. The scale-up of their production was performed by Bader at the TM plant in Thann and by Noelting at the Mulhouse Chemistry School. When that School celebrated its 150th anniversary in 1972, several of these polynitro-aromatic compounds were still on the market under various brand names like musk xylene, musk ambrette and musk ketone.
During the Noelting era, the Mulhouse Chemistry School participated in a few World Fairs where she gathered several distinctions: bronze medal at Chicago in 1893 and gold medal at St. Louis (USA) in 1904. Consequently, the School gained in reputation and the recruitment of new students became quite international. The SIM committee concluded with satisfaction that higher education institutions always take a leading advantage when its student body becomes international and multilingual. During the Noelting era, well-trained textile chemists went back to their home countries to staff the local textile industries, particularly to tsarist Russia, Mexico and Spain, the most thought after specialists being in the field of textile printing. Between 1879 and 1905, the Mulhouse Chemistry School had trained ca. 700 French-born students, 121 Austrians, 115 Swiss, 240 citizens from Russia and Poland, 96 Italians, 37 British citizens, 30 US Americans, 23 citizens from Spain, to quote but the most important student groups.
In 1898, a fourth year was instituted by Noelting to have a study program equivalent to those of the German and Swiss Universities. This fourth year was dedicated to original research as a first Ph.D. year. As a consequence and in recognition of their scientific level, the German authorities delivered to fourth-year students an official diploma entitled Zeugniss wissenschaftlicher Befähigung. And indeed, it permitted students to carry on their research efforts towards a full-fledged Ph.D.
WWI led to a brutal interruption of the Mulhouse Chemistry School's activity. During the first year of the war (1914–1915), it was operating with only 15 students, whereas during the preceding years the average number was 80. On 30 June 1915, the School was closed by the German authorities and Noelting was expelled, the official reason being that he was a “non-German citizen”.
Jean Lichtenberger (1889–1984) left a manuscript in which he described his experience at the “École municipale de chimie de Mulhouse” during the academic year 1913–1914. Here are some excerpts:
“During my sojourn, I became a member of Noelting's private laboratory which ‘was accessible to the best students only’. Like myself, these students appreciated Noelting's scientific competence and experimental know-how, and admired his real scholarly aura. Everybody called him ‘le chef ’ (the boss). All of us sensed his ability to introduce us into real experimental research. Each day Noelting showed up in the lab, inquiring about the progress on the bench and coming up with some new advice. Taking himself to the test tube, he suggested new experiments to be performed, his students listening in silence and with great admiration to such a great scholar [34].”
About 100 years later, in 2016, Professor Yves Lichtenberger of the University Paris-Est Marne-la-Vallée gave his opinion about his paternal grandfather:
“From discussions with my grandfather Jean Lichtenberger, I gathered the opinion that one of his main objectives was to foster strong relationships between ESCM's applied research programs and the textile and chemical industries. This was indeed the trademark of Emilio Noelting which was shared by Martin Battegay, by himself, and by my father Robert Lichtenberger who was a research chemist in industry.”
7.5 The Wild and Battegay eras (1926–1941)
Having reorganized the Mulhouse Chemistry School after WWI, Noelting was succeeded by his long-time colleague Professor Eugène Wild who managed the Mulhouse Chemistry School from 1919 until 1926. Next, Professor Martin Battegay took over until 1939 at Mulhouse and for another 2 years during WWII, successively in Toulouse and Lyon to which the Mulhouse Chemistry School and part of its staff had been transferred. In 1941, the Vichy regime brutally dismissed Battegay from his academic duties by applying the anti-Semitic laws Marshal Pétain's government had instituted. The Mulhouse Chemistry School somehow managed to survive WWII in Lyon, albeit with rather limited means.
When it comes to research matters during the Wild and Battegay eras, one must cite the discovery in 1921 of the indigosol dyestuffs, which Professor Bader had invented. He had been able to prepare these water-soluble sulfuric diesters of leuco-indigo intermediates, which have some practical advantages over the unsubstituted leuco-indigos. Consequently, his invention was applied in textile industry for the dyeing of indiennes and for the cotton-printing processes. The Basel Durand & Huguenin Company used it at once on a large scale [35]. Nowadays, such indigosols are mainly produced in China.
At the end of the Wild era and after a 114-year lifespan of the Mulhouse Chemistry School, let us have a short look at its historical evolution. Right after its beginning in 1822, this learning institution taught practical matters to technicians during evening held lectures until 1831, then full time ones over a one-year period. In 1855, a second school year was added and applied research got under way; in 1888, the Mulhouse Chemistry School became a three-year engineering school specializing in textile dyeing and printing. Finally, in 1898 a fourth year was instituted for those students who wanted to prepare for a Ph.D. Many alumni of the Mulhouse Chemistry School accomplished their careers in textile industries.
In 1934 during the Battegay era, the ESCM introduced a lecture course in the field of elastomers, heralding thereby a new era, which probably represented the very first course about macromolecular chemistry in France. As a matter of fact, macromolecular chemistry, also called polymer chemistry, became an important sector of large-scale chemical companies, a situation that still prevails today. In 1937, Roger N. Wallach from New York, an alumnus of ESCM, offered 320,000 francs to the Mulhouse Chemistry School to set up a research laboratory, specifically for elastomeric polymers. For several years, this laboratory was run by Dr. Léon Denivelle (1905–1992), also a former student of ESCM, who was to become both the chair holder for dyestuff chemistry at the Paris “Conservatoire national des arts et métiers” between 1941 and 1974, and the CEO of the Thann and Mulhouse Company (TM) between 1945 and 1977.
7.6 The Lichtenberger era (1941–1957)
After the 1940 military defeat of France against Nazi Germany and the forced resignation of Battegay, Jean Lichtenberger (1899–1984) took over and was able to manage what was left from ESCM when it was installed at the “École de chimie de Lyon” from 1941 until 1945. Back to Mulhouse after WWII, he could restart ESCM at a normal speed, the School having been kept in good shape, a large number of the students being back to work and the faculty body complete.
Between 1945 and 1957 and thanks to Jean Lichtenberger, study and research programs were resumed at the level that had been brought about by his two predecessors, albeit municipal support and support by SIM had been sharply reduced. Because of the rather limited financial means, Lichtenberger had an important and last objective in mind, namely, let the Strasbourg University incorporate the ESCM Foundation as a new Institute, a task he was able to achieve in 1957. The incorporation of ESCM's Foundation by the Strasbourg University was achieved, thanks to a government decree dated 24 May 1957. Consequently, a much better support could be secured, which permitted the expansion of both the teaching programs and the scientific research topics. Furthermore, the “Centre national de la recherche scientifique” (CNRS) was now in a position to strongly support and orient part of the research efforts of the Mulhouse Chemistry School.
7.7 The Meybeck era and the “Centre de recherches textiles” (1958–1975)
In the early 1960s, when textile industry was still a dominant economic factor in southern Alsace and particularly in the Mulhouse area, Professor Jean Meybeck became the chairman of both the ESCM and Centre de recherches textiles de Mulhouse (CRTM), which is but one of the “Institut textile de France” research centres. To run CRTM, Jean Meybeck was assisted by two colleagues from ESCM. Thanks to that cooperation of CRTM with ESCM, Alsatian textile industries could rely on solid professional and practical advice, as well as on some applied research.
8 The CNRS strongly supports applied research at the Mulhouse Chemistry School
After the incorporation of ESCM in the University of Strasbourg, applied research expanded, several research laboratories being strongly supported by CNRS. We describe herein those laboratories whose research topics are of great interest and whose performances are noticeable.
After 1957 and the incorporation of the Mulhouse Chemistry School in the University of Strasbourg, the Professors named above were followed by several research and teaching Professors as well as by some CNRS Directors. Let us cite these teaching and research experts in a chronological order in the various scientific disciplines they taught at the Mulhouse Chemistry School during the last 60 years:
- • Inorganic chemistry, in particular, zeolite chemistry: Raymond Wey, André Hatterer, Jean-Louis Guth, Henri Kessler, Joel Patarin, Bénédicte Lebeau, Jean-Louis Paillaud, Claire Marichal, Jocelyne Brendle, Jean Daou and Angélique Simon-Masseron.
- • Macromolecular chemistry: Albert Banderet, Gérard Riess, Christine Delaite, Jean-François Stumbé, Maurice Brogly and Sophie Bistrac.
- • Photophysical and photochemical processes: Jean Faure, Jean-Pierre Fouassier, Daniel Lougnot, Christian Decker, Patrick Jacques, Xavier Allonas and Céline Croutxé-Barghorn.
- • Organic and bioorganic chemistry: Jean-Pierre Fleury, Jacques Streith, Michel Rohmer, Jacques Eustache, Claude Le Drian, Céline Tarnus, Serge Neunlist, Nicolas Blanchard and Jean-Philippe Goddard.
8.1 The CNRS Institute of Chemistry for Surfaces and Interfaces (Institut de chimie des surfaces et interfaces)
In the early 1950s, Professor Jean-Baptiste Donnet (1923–2015) initiated at the Mulhouse Chemistry School a physicochemical laboratory associated with CNRS under the heading “Centre de recherche sur la physico-chimie des surfaces solides”. Its research program was predominantly about the interaction of carbon black and silica with elastomeric material and, as a rule, with pronounced cooperation with the chemical industries. The Centre de recherche sur la physico-chimie des surfaces solides had been installed in the centre of Mulhouse and kept growing, so that a transfer from the Mulhouse Chemistry School to a CNRS Institute called “Institut de chimie des surfaces et interfaces” (ICSI) was decided. ICSI was built on the Mulhouse–Illberg campus, but according to its statutes, it is not part of the “Université de Haute-Alsace” (UHA) or of the Mulhouse Chemistry School. Nevertheless, ICSI does keep some loose scientific ties with these academic institutions. After 1985, the director of ICSI became Professor Jacques Schultz for 20 years. In 2005, he was succeeded by Dr. Cathie Vix, a research Director of CNRS.
8.2 Laboratory of silicates with controlled inbuilt porosities
Fig. 5 represents the perspective of a zeolite that had been synthesized in the Mulhouse “Laboratoire de matériaux à porosité contrôlée” (LMPC), a research laboratory launched in the late 1950s by Professor Raymond Wey. Over the years, it progressed under the leadership of Professor Jean-Louis Guth and Dr. Henri Kessler, then of Dr. Bénédicte Lebeau-Talamona, Dr. Joël Patarin, and Dr. Jean-Louis Paillaud, all being research Directors of CNRS. The scientific topics of the LMPC have to do with chemical syntheses of some novel solid-state silicates and alumina silicates showing controlled inbuilt porosities. Their practical applications are in several fields: adsorption, ion exchange, catalysis, enforcement of elastomers and protection of the environment. Over the years, two types of crystalline nanoporous materials were synthesized: 3D zeolite skeleton structures (Fig. 5) and 2D clay silicate structure. Characteristically, zeolites show sharp-defined pore-size distributions, with high volumes and very high specific surfaces. The numerous nanoporous solids that have been synthesized by the LMPC experts led to an impact in the field of nanoscience and nanomaterials [33]. To cite but one example, in 2006–2007 the clay–algae nanocomposite amadeite was invented at the LMPC and then developed on an industrial scale in cooperation with the Olmix group. This nanocomposite is used in animal feed to neutralize mycotoxins.

A germanosilicate zeolite synthesized in Mulhouse.
Technology transfer had been initiated when members of the LMPC launched in 2009 the Zéphir-Alsace start-up, which took advantage of a CNES/UHA/CNRS patent about molecular decontamination during spatial exploration. The patent resulted from a cooperation between the LMPC and the “Centre national d’études spatiales” (CNES) and led to trapping of volatile organic dust, which accumulates in space. In actual fact, a pastille of an absorbing Mulhouse-made zeolite was incorporated into the robot Curiosity launched by NASA in 2011 to explore the surface of the planet Mars.
One should also keep in mind that nowadays nanoporous solids—also called molecular sieves—are used in many applications. They are present inside double-pane windows to trap water molecules, also as washing powder to trap calcium cations in washing waters or in oil refineries to catalytically produce gasoline. In a near future, they may also be used to solve problems like air and water pollution, the recycling of waste, and even for energy storage. In this latter field, which is in the offing, LMPC exerts a leadership in Europe: by applying pressure on water to force it to enter hydrophobic nanoporous solids, one observes shock-absorbing properties of these latter materials, or their spring-like behaviour during the pressure release period. For example, when it comes to spring-like properties, 98% of the stored energy can be released, which represents an unmatched energy efficiency. Since 2009, LMPC extends this research topic to electrolytic solutions and to other nanoporous substances like metal-organic frameworks.
8.3 The laboratory of macromolecular chemistry
The Laboratory of Macromolecular Chemistry should be considered as the continuation of the laboratory for elastomers, which had been launched in 1937 by Professor Léon Denivelle. Having been led successively by Professor Albert Banderet, then for over 35 years by Professor Gérard Riess and presently by Professor Christelle Delaite, this laboratory proved to be quite productive in the field of the synthesis and the characterization of copolymeric materials, colloidal polymeric materials and multimaterial polymers having very defined physical properties. It is well known for the production of grafted copolymers and block copolymers, the applications being in micellar systems, nonaqueous emulsions, polymer alloys and composite materials. Thanks to the concept and experimental expertise of Professor Riess, an emulsion of a liquid in an elastic polymer was used to develop biocidal gloves for surgical purposes, their industrial production having been launched by the French Company Hutchinson Santé [37]. This invention protects medical people, particularly in hospitals, and became a commercial success story. In 1995, Professor Riess has been distinguished for that contribution with the European Invention Prize. Since then, the industrial production of the biocidal gloves has been shifted to Malaysia, the technical transfer having been accomplished by Dr. Pierre Hoerner, a former doctoral co-worker of Professor Riess.
Furthermore, let us cite the discovery by Professor Riess of micellar systems of amphiphilic copolymers, which are used as slow-releasing drug transporters.
Last but not least, we should cite the development of paintings that are applicable underwater during the maintenance of offshore metallic structures. This latter invention by Professor Riess was taken up by the Astral Company, its technological transfer having been accomplished by Dr. Didier Penninck, a former doctoral co-worker of Professor Riess.
8.4 Laboratory of photochemistry and macromolecular engineering
This laboratory had been created in 1969 by Professor Jean Faure and developed since into a large research group, which, at the turn of the century, had an international reputation and is known nowadays under the heading “Laboratoire de photochimieet d'ingénieriemacromoléculaires” (LPIM). Over the years, several research topics have been worked at, like photophysical properties of carbonyl derivatives and mechanisms of photochemical priming during photoinduced polymerization, resulting in applications in polymeric materials and nanoscience. More specifically, the use of polymers to optics requires the characterization of the photosensitive systems by holographic spectroscopy. In the field of information storage, the creation of an optical network whose photoreticulation is variable and controlled is necessary for data saving. Finally, let us cite some industrial applications in the field of fast photoreticulation called instant photoinduced varnishing; also in the field of photografting and the protection of organic materials, thanks to coating by photoinduced cross-linking. Two private start-ups in that last domain, Photon & Polymers and Lovalite, have been launched by the LPIM, respectively, in a suburb of Mulhouse and the town of Troyes.
The main research methodologies are time-resolved laser spectroscopy to study the primary photopolymerization processes, Fourier transform infrared spectroscopy and molecular modelling. This implies photoinduced electron-transfer, triplet–triplet energy transfer, hydrogen abstraction and generation of free radicals or acids. The main interest lies in the reactivity study of photochemical initiator systems and kinetics of photopolymerization, leading to various applications like “laser imaging”, paintings and coatings or optics and microelectronics.
Quite a few of the applied research topics have to do with the car industry, aeronautics, consumer industry and communication technology. A rather large array of physical measurement apparatus permits to address almost any industrial problem and contributes to innovation. Last but not least, the “Agence nationale de la recherche” decided to support the creation of a Chair of Industrial Research within LPIM.
8.5 Laboratory of organic and bioorganic chemistry
Since the 1960s, three professors of the Mulhouse Chemistry School developed new methodologies in organic synthesis. Having invented oxime-tosyl-malodinitrile (OTMD), a new imine type molecule connected to three electron-withdrawing groups, Jean-Pierre Fleury (1927–2015) derived from that “synthon” a whole series of novel heterocyclic molecules. Jacques Streith (born 1933) achieved photoinduced ring expansion of pyridinium N-ylides into isomeric diazepines from which some novel heterocyclic systems could be obtained, for example, a cyclopropapyridine whose 3D structure was ascertained by single crystal X-ray diffraction. As to Claude Le Drian, thanks to the use of homogenous polymer-supported palladium catalysts, he was able to create site-specific CC bonds between predetermined pairs of aromatic ring systems.
The turning point towards natural product chemistry and medicinal chemistry—which had already been taken in Basel during the first decades of the 20th century, both in the pharmaceutical industry and at the University (see above)—was initiated in Mulhouse in the early 1970s by the above cited three professors and some CNRS research associates.
In the laboratory of Professor Streith, CNRS associates Dr. Albert Defoin and Dr. Théophile Tschamber used optically pure educts ex-chiral pool to synthesize potential glycosidase inhibitors. Dr. Defoin used acylnitroso dienophiles obtained from optically pure amino acids to synthesize chiral azacarbohydrates, Diels–Alder cycloaddition reactions being the key steps. As to Dr. Tschamber, he made good use of ascorbic and iso-ascorbic acids to attain chiral imidazolocarbohydrates. The glycosidase inhibition parameters of their target molecules were determined under the guidance of Professor Céline Tarnus, an enzymologist who had left the pharmaceutical company Sanofi-Aventis in Strasbourg.
During his 15-year-long teaching and research period in Mulhouse (1979–1994), Professor Michel Rohmer studied the biosynthesis of some naturally occurring triterpenoids named biohopanoids and discovered a new metabolic pathway, which had not been described yet by the scientific community. This pathway leads to isopentenyl diphosphate and dimethylallyl diphosphate, both being the well-known and universal precursors of all isoprenoids. The Rohmer pathway, called methylerythritol phosphate pathway (MEP), is quite different from the one—called the mevalonate pathway—which had been discovered and rationalized half a century earlier for the biosynthesis of cholesterol. Furthermore, Rohmer discovered that the MEP pathway is often encountered in bacterial biosynthesis and that this pathway is quite ubiquitous in chloroplasts of phototrophic organisms. In modern biochemistry textbooks, the MEP pathway is often called the “Rohmer Biogenetic Pathway”. For his biosynthetic discoveries in Mulhouse, Michel Rohmer obtained the gold medal of the Alfred & Valentine Wallach Foundation. In 1994, he left Mulhouse to join the StrasbourgUniversity where he carried on his research to unravel the details of the MEP pathway [38]. As expected, he was elected member of the German National Academy of Sciences Leopoldina in 2000 and member of the French Academy of Sciences in 2003.
Professor Jacques Eustache, a medicinal chemist who had left the pharmaceutical research laboratory of Sandoz-Vienna, joined the Mulhouse Chemistry School in 1995. He used metathesis extensively as a means for stereospecific syntheses of natural products and some derivatives thereof, by making good use of homogenous molybdenum and ruthenium catalysts.
More recently in the laboratory of Professor Céline Tarnus, Dr. Sébastien Albrecht and his collaborators achieved the synthesis of a specific amino-benzosuberone, which proved to be an inhibitor of aminopeptidase M1 of the malaria parasite, the new inhibitor being presently in preclinical test studies. For this discovery, Dr. Albrecht obtained the “grand prix scientifique” of the Alfred & Valentine Wallach Foundation in 2016 in Mulhouse. This prize was attributed by the “Académie des sciences, lettres et arts d'Alsace”.
Last but not least, two CNRS scientists, Dr. Nicolas Blanchard and Dr. Philippe Bisseret, made good use of transition metal catalysts to synthesize some toxins, which occur in human mycobacterial infections, like the Burundi ulcer.
9 The Mulhouse institute of material sciences (“Institut de science des matériaux de Mulhouse”)
In 2005, the CNRS decided to put together all the institutions he already was more or less in charge of, that is, its own ICSI, the LMPC and the “Laboratoire de physique et de spectroscopie électronique”. These three laboratories were combined into a single entity called “Institut de science des matériaux de Mulhouse”, which is no longer part of either the Mulhouse Chemistry School (ENSCMu) or the “Université de Haute-Alsace” (UHA). This new research establishment, whose first director is Dr. Cathie Vix, is by far the largest research institution in Mulhouse. It is composed of chemists, physical chemists, physicists and crystallographers. Albeit being an independent CNRS institution, it keeps some lose scientific connections with ENSCMu and UHA. Its main task is about multidisciplinary chemical and physical research programs dealing with surfaces, interfaces and porous materials.
10 Since 1854 the Mulhouse Chemistry School was a precursor of applied research in the RegioTriRhena
Since 1854 and the arrival of Paul Schutzenberger, the Mulhouse Chemistry School (Fig. 6) was dedicated to applied research. This historical drive came from the needs of the textile industry and was amplified through the active support of both the municipality and the SIM. Hiring some outstanding scientists as directors of the Mulhouse Chemistry School established a model for teaching and conducting applied research.

The Mulhouse Chemistry School: the “École nationale supérieure de chimie de Mulhouse”.
Although research topics were mostly related to textile dyestuffs and textile finishing during the 19th century, the scope broadened during the 20th century when some new research fields like macromolecular and inorganic chemistry as well as physical chemistry of surfaces and interfaces entered the scene. In the early 1970s, these were joined by disciplines like chemistry of natural products and medicinal chemistry. But most important were always both the direct link to industry and the transmission to students of a passion for applied scientific research. This later point is best documented by looking at the careers of some brilliant alumni of the Mulhouse Chemistry School who are working mostly in the industry.
As already discussed in the beginning of this article, the very first chemistry lectures as applied to textile industry opened in 1822. Simultaneously, a laboratory had been instituted to test the quality of natural colours and chemical products, which are of great use in the textile industry, and also to check the ennobling processes.
Thirty years later, the one-year teaching program was replaced by a two-year program, a new laboratory was opened and applied research introduced in 1854 by Professor Paul Schutzenberger. From that time on, the Mulhouse Chemistry School became the only research-oriented chemistry school in France. It had been initiated by the local municipality and was run by the SIM, a rather efficient “think tank” that had been launched by leading entrepreneurs of the textile and mechanical industries, like the members of the Dollfus and Koechlin dynasties, respectively, as well as by members of the local business community. During the first 100 years of its existence, the Mulhouse Chemistry School developed gradually into an establishment for the training of chemists under the guidance of teachers like Paul Schutzenberger, Daniel-August Rosenstiehl and Emilio Noelting, who were notorious in their domains of applied research. Furthermore, they developed a scientific and professional education, thanks to the continuous support of the local municipality and in cooperation with the newly launched ESSA, which had been established in Mulhouse by the French government in 1855.
10.1 Contributions from alumni of the Mulhouse Chemistry School working in research departments of the chemical industry
Nowadays, this well-established model is more alive than ever. For many decades, the teaching programs of the Mulhouse Chemistry School were closely intertwined with the development of textile industry until about WWI. During the 20th century, the local scientific discoveries became more varied and proved to be mostly in the field of applied rather than of fundamental research.
The transmission to students of the passion for research is best documented by looking at scientific contributions from alumni of the Mulhouse Chemistry School who act in research departments of the chemical industry [39]. Let us mention some of these contributions, in chronological order:
- • Artificial musk perfumes, which were synthesized via nitration of benzene derivatives by Albert Baur.
- • A large-scale production of uranium hexafluoride for the separation of uranium isotopes by Robert Lichtenberger.
- • A novel Merox process by André Gislon and Joseph Quiquerez, which permits the oxidation—thanks to some chelate catalysts—of corrosive and malodorous mercaptans into stable and odourless disulfides, this process being used in many petroleum refineries worldwide.
- • New industrial syntheses of acrylonitrile and hydrazine by Francis Weiss.
- • Production of synthetic ruby stones—an adaptation of the Verneuil process—by Vahan Djevahirdjian and his daughter Katia Djevahirdjian, the ruby jewels being used as “stones” in mechanical watches; synthetic ruby stones were also used to precisely measure the earth-to-moon distance by laser light.
- • For over a century, the development and massive production of new synthetic dyestuffs had been dominant in RegioTriRhena's chemical industries, mostly by CIBA—particularly during WWI when the overall sales had been multiplied by seven—and by CIBA-Geigy after the 1970 merger. This outstanding long-lasting dyestuff-producing era came gradually to an end in the late 1990.
Nevertheless and not merely as a reminder, we would like to draw the attention to the scientific contributions of Dr. Pierre Galafassi and Dr. Jean-Pierre Luttringer during the final stages of that long and brilliant dyestuff producing era.
Both chemists were alumni of the Mulhouse Chemistry School and had obtained their Ph.D. at a time when the invention, development and large-scale production of textile dyestuffs was still a major industrial outcome in RegioTriRhena. They spent their professional career with Ciba-Geigy, which for a long period of time was the world leader for the invention of novel dyestuffs, until divestment of this segment at the end of the 20th century.
Both Dr. Galafassi and Dr. Luttringer contributed to research, application and marketing to develop and launch several ranges of innovative dyestuffs, improving steadily their technical and environmental performance. For example, the new Cibacron bi- and tri-reactive dyes for cotton featured much higher fixation rates than conventional reactive dyes do; thus, they dramatically reduced the load in the effluents and significantly increased the fastness in use of the dyed fabrics. They also markedly improved the dyeing reproducibility, which is an important economic issue. For polyester fibres both scientists developed new generations of disperse dyes: for “high-tech” apparels like sportswear made out of microfibers, a new class of Terasil disperse dyes, based on an innovative chemistry, allowed one to solve the “bleeding” problems caused by traditional dyes during household washing. For the car industry, Teratop disperse dyes were specially developed for automotive textiles, these dyes contributing to improve significantly the durability of automotive interiors, due to their very high lightfastness under severe conditions of temperature and humidity.
10.2 What about the Mulhouse Chemistry School and its academics?
Although the unique history of the Mulhouse Chemistry School explains the emphasis put on industrially relevant research, one should not overlook the relatively large number of alumni who became successful academics and researchers in very diverse areas of chemistry and related fields. A survey of their contributions is beyond the scope of this historical chronicle, but one example will be mentioned to illustrate this point.
Born in Mulhouse in 1947, Pierre Braunstein graduated in 1969 from the Mulhouse Chemistry School. He became Research Director within the CNRS at the University of Strasbourg and made major contributions in the coordination and organometallic chemistry of the transition and main group elements, where he has authored or coauthored close to 600 scientific publications and review articles. His achievements have covered very diverse and important areas, ranging from carbonyl cluster chemistry, bimetallic silicon chemistry, functional phosphorus- and nitrogen-containing ligands, carbenes and metal complexes for applications in catalysis and nanosciences, to strongly dipolar, potentially antiaromatic organic quinonoids with delocalized π systems and their use for electronic communication in mixed valence complexes and the formation of organized monolayers relevant to spintronic applications. Quite remarkable coincidences, he directed a laboratory on Coordination Chemistry, the very field founded by the Swiss citizen—but a Mulhouse-born Frenchman—Alfred Werner, a domain for which Werner received the Nobel Prize of Chemistry in 1913, worked on metal carbonyls, the first such complex (cis-PtCl2(CO)2) having been reported by Paul Schutzenberger in 1868–1870, and also produced novel organic quinonoid dyes, although for different applications than in the Mulhouse tradition.
In 2005, Pierre Braunstein became the first alumnus of the Mulhouse Chemistry School to be elected a member of the French Academy of Sciences and the German National Academy of Sciences Leopoldina.
11 The turning point towards natural products and medicinal chemistry at the Mulhouse Chemistry School
The turning point towards natural products and towards chemical synthesis of therapeutic products was taken at the Mulhouse Chemistry School in the early 1970s. Ten years later, this move gained momentum and scope. The importance of the pharmaceutical industry increased tremendously in the greater Basel area and became the principal economic factor in the whole RegioTriRhena. As a consequence, the interest rose to orient organic chemists of the Mulhouse Chemistry School—at least those who had obtained a Ph.D. in organic chemistry—towards careers in the expanding pharmaceutical industry, particularly in medicinal chemistry and even in the biotechnological sciences. Since 1981, the universities of Basel, Freiburg-im-Breisgau and Mulhouse organize the so-called Regio-Symposia in organic and bioorganic chemistry within the precincts of RegioTriRhena, the objective being precisely to accompany Ph.D. students who are working along the turning point towards natural products and chemical syntheses of therapeutic products. These 3-day long meetings take place yearly in September in remote areas located either in the Vosges-, Jura- or Black Forest mountains. They promote organic and bioorganic chemical research for graduate and doctoral students who get to know each other and who present their research results orally and during poster sessions, either in German or in French, but mostly in English. In addition, eight plenary lectures are delivered by invited scientists from academia and industry. Furthermore, a few research scientists participate who are send by chemical industries of RegioTriRhen (e.g., by BASF) and by pharmaceutical industries (e.g., by Roche and DSM [40,41]).
Increasing number of Ph.D. students of the Mulhouse Chemistry School pursued their careers in pharmaceutical research departments, indeed, some of them obtaining remarkably good results as exemplified below by several outstanding alumni.
After having obtained their Ph.D. in the 1970s at the Mulhouse Chemistry School, Dr Marc Lang and Dr. Christian Hubschwerlen joined the Woodward Research Institute, a foundation dedicated to research, which had been created by Ciba in Basel in 1963—and continued by Ciba-Geigy after the 1970 merger—under the direction of Harvard Professor and chemistry Nobel Prize winner Robert Burns Woodward. The mission of that Institute, which was in operation until Woodward's death in 1979, was to provide cutting edge research, essentially in the field of new antibiotics, and to guarantee the formation of highly qualified research fellows for the pharmaceutical industry. After their two-year postdoctoral periods at the Woodward Research Institute, both scientists joined pharmaceutical research departments in Basel, Dr. Lang with Ciba-Geigy and with Novartis after 1996 and Dr. Hubschwerlen with Hoffmann-La Roche, followed more recently with Actelion Pharmaceuticals.
Dr. Marc Lang spent his full career in the pharmaceutical research department of Ciba-Geigy, which in 1996 became part of the new pharmaceutical company Novartis. He contributed directly to the discovery and development of three marketed molecules: Femara, an aromatase inhibitor for treatment of certain breast cancers; Reyataz, an HIV-protease inhibitor for treatment of AIDS; and Tasigna, a bcr-abl kinase inhibitor for certain leukaemic diseases. As an executive Director in charge of the medicinal chemistry unit in Basel, he was member of the Oncology Decision Board at the Novartis Institute for Biomedical Research.
In the field of antibiotics, Dr. Christian Hubschwerlen at Hoffmann-La Roche in Basel led the effort leading to the discovery of the fifth cephalosporin generation ceftobiprole medocaril and the monobactam carumonam. The latter required the development of an industrial process for the production of (l)-glyceraldehyde acetonide, starting from vitamin C, and a novel access to chiral monocyclic β-lactams. Later on, with Actelion Pharmaceuticals, he also discovered the antibiotic Cadazolid, designed specifically to treat Clostridium difficile-associated diarrhoea resulting from the overgrowth in the colon of toxigenic strains of C. difficile, generally during or after therapy with broad-spectrum antibiotics. This molecule has recently finished its clinical phase-III tests.
High-risk start-ups and spin-offs in the pharmaceutical industry and Dr. Hubschwerlen's career in the field of novel antibiotics, by Dr. Jean-Paul Clozel, founder and former CEO of Actelion Pharmaceuticals.
During his career at Hoffmann-La Roche in Basel, Dr. Christian Hubschwerlen had been closely associated with the discovery of two new antibiotics and coheaded the research efforts in the Infectious Diseases Department. My career path as a medicinal researcher at Roche was in many respects similar to his career at that time, because I had to lead the research group in the Cardiovascular Diseases Department. We were colleagues for many years, until the day when, together with three other Roche scientists, I founded Actelion Pharmaceuticals as a Roche spin-off in Allschwil, whereas Christian together with a biologist set up the Swiss branch of the Munich start-up Morphochem. After 4 years, the management of Morphochem decided to close the Basel site for financial reasons, although it had identified a clinical candidate. Christian found himself on the market place with his team of 18 skilled people. Knowing the scientific and human qualities of his multidisciplinary team, I immediately proposed to integrate the entire team within Actelion by opening the new section “infectious diseases”, which initially was not part of our business plan. The integration of a team of skilled experts who were used to work together and initiate and carry out projects was, in my view, a clear competitive advantage. We have not regretted this decision, because the team has already created a new antibiotic and so on.
Worthwhile to be mentioned is the performance of Dr. Gérard Wolff, who after his Ph.D. at the Mulhouse Chemistry School was active with Rhône-Poulenc. In due course, he became director of the industrial Adisseo Research Department, which specialized in the production of animal feed additives. Dr. Wolff conducted the development of processes and production capacities of the amino acid l-methionine in France and Spain. In addition, he developed an industrial process for Smartamine and Metasmart, both being derivatives of methionine used in the feed industry, which is essential for milk production and milk protein synthesis. The building up of new research centres—in Saint-Fons dedicated to chemistry and in Rangueil to biochemistry—was essential for the optimization of industrial production processes of enzymes like Rovabio, Rovabio Advance and of some antioxidants like Selisseo.
A few years later in 1997, after having obtained his “ingénieur” diploma and Ph.D. at the Mulhouse Chemistry School, Dr. Jean-Luc Schuppiser joined the Rhône-Poulenc Research Centre in Aubervilliers, which was specialized in the synthesis of functional polymers. In 1983, he had to build up and manage R&D in the domain of specialty chemicals, that is, industrial additives, as well as oil additives, additives for pharmaceutical, food and cosmetic products. In 1990, he was sent to the USA where he had to regroup and rationalize research activities of several chemical plants, which had been acquired in the USA during the five preceding years. In 1992, all of them had been regrouped on one site, at Cranberry (New Jersey). That objective having been fulfilled, Dr. Schuppiser came back to France and joined the Rhône-Poulenc headquarters in Paris, where he had to manage all the research activities dealing with specialty chemicals.
In 1996, shortly before Rhône-Poulenc was split in two parts (Rhodia and Aventis), he was given the opportunity to join Essilor International—a worldwide leader in the field of spectacle lenses—where he took over the entire R&D of the group. As a member of the executive committee, he had to report directly to the CEO. That executive position permitted him to deal both with material technologies and material sciences and with human health problems. He exerted that responsibility for 16 years, during a period of almost explosive expansion of the Essilor International group. In 2013 when Dr. Schuppiser retired, Essilors's R&D comprised about 500 people working in three company-owned research centres—at Saint-Maur-des-Fossés (France), St. Petersburg (USA) and Singapore—two research centres in partnership with Nikon (in Tokyo) and the University of Shanghai, and with two major research projects in France with CNRS at Toulouse (Laboratoire d'analyse et d'architecture des systèmes) and with the “Commissariat à l'énergie atomique” at Grenoble (“Laboratoire d'électronique et de technologie de l'information”).
Finally, we must cite Dr. Bernard Marchand, who got his diploma at the Mulhouse Chemistry School and his Ph.D. at the Louis Pasteur University of Strasbourg. Next, he became a postdoctoral fellow at the University of California at Irvine, specializing in natural product chemistry. In 1983, he joined the Servier Pharmaceutical Company as Head of the Chemistry Department and soon was promoted Director of Kinetics (France and the UK) in 1988; in 1994, he was appointed as General Manager of Biopharmacy and moved on in 2007 as Head of the Discovery Research. Since 2013, he is Vice president R&D and Director of R&D Operations. During his career, he actively participated in the development of the Coversyl, Procoralan, Valdoxan, and Protelos drugs.
Several younger organic Ph.D. chemists of the Mulhouse Chemistry School emerged in the footsteps of their successful precursors we just cited. They completed their Ph.D. research expertise by spending postdoctoral periods in outstanding places like Stanford at Palo Alto or ETH at Zurich. Their excellent scientific education gave them access to the medicinal chemistry research departments of the following pharmaceutical companies: Actelion Pharmaceuticals, Basilea Pharmaceutica, Eli Lilly (in Indianapolis and in the London area), Galderma, Novo Nordisk, Novartis, Orion Pharma, Roche, Sanofi and Servier (see Fig. 7).

Business centre of the Actelion pharmaceutical company.
Without pursuing any Ph.D. diploma, many alumni of the Mulhouse Chemistry School also entered pharmaceutical companies—in particular in the RegioTriRhena area—where they occupy technical positions in research, development, production, and administration.
12 Conclusion
The industrial development in the RegioTriRhena essentially dates back to the 18th century and was thereafter carried forward by dynamic entrepreneurs, who acted in accordance with a liberal value system and were influenced by Protestant ethics. Furthermore, industrial progress requires scientific inventions and technical innovations and is implemented by properly educated agents.
Such was the case for 18th century Mulhouse, when young entrepreneurs launched the manufacturing of printed cotton, initiating thereby the process of local industrialization. Equally favourable was the first half of the 19th century, when Mulhouse enjoyed the rich economic expansion that earned the city the name of French Manchester. In those times, the growing demands of the markets boosted modernization and diversification of the traditional textile and dyestuff industries. The Mulhouse Chemistry School was launched at that time to promote an advanced technical education and, in due course, to promote also applied research, both under the supervision of the SIM in a dynamic spirit that is still alive today.
With the annexation of the whole of Alsace and part of Lorraine into the Imperial “Reichsland” (1871), the optimal working conditions of the local manufacturing industries were notably disturbed by the modification of sociopolitical and custom regulations, and by the emigration of many influent entrepreneurs and managers. Thereby Mulhouse lost its significance as the prime industrial place in the RegioTriRhena. The leadership was taken over by the nearby city of Basel, with the advantage of being preserved from the harmful consequences of the two coming French–German conflicts.
On the rise to become the flourishing internationally oriented economic and academic capital city known today, Basel could offer the best opportunities for a timely expansion of the local industry. Founded during the 19th century decades, the pioneering enterprises in Basel (Geigy, Ciba, and Sandoz) deserted soon the traditional field of dyestuff production inherited from Mulhouse, in favour of more promising chemical and life science–oriented activities. As to Roche, it was launched in 1896 by Fritz Hoffmann and his family as a purely pharmaceutical company. Since then, these four local firms efficiently drove their development. In Basel, the link of technical innovation with scientific invention was commonly cultivated by these four industrial enterprises through a close partnership with high quality research institutes, be they privately owned or part of the University. Most of the innovating products and processes—that marked the progressive rise of the local pharmaceutical firms to their current rank among the largest in the world—originated from the results of autonomous scientific research.
As for Mulhouse, the industrial and academic city kept alive its original spirit, linking advanced technical teaching and applied research to the contemporary industrial needs in the “RegioTriRhena. From the beginning, this contributed to the worldwide reputation of the Mulhouse Chemistry School. Teaching programs were conducted by outstanding scientists who also conveyed their passion for scientific research. Some precursors like Paul Schutzenberger (who joined in 1854), the pioneer who introduced applied chemical research, Daniel Rosensthiel (who joined in 1865) and Emilio Noelting (who joined in 1880) [18,19,36] were the leading figures during the 19th century. They were successfully followed by many other first-class professors in the 20th and 21st century. In recognition of these pioneers and the successful role model of the Mulhouse Chemistry School, the efficient support of the Strasbourg University and CNRS led to a substantial increase in the number of professors and lecturers (“maîtres de conferences”), as well as of CNRS research directors and CNRS research associates (“chargés de recherche”), thus permitting ENSCMu to pursue a large spectrum of adequately funded research programs. The strongly enlarged teaching and research staff guarantied an excellent formation of the future chemists and therefore strengthened the contribution of the Mulhouse Chemistry School in the RegioTriRhena area and beyond.
Acknowledgments
The author thanks all those who were willing to evaluate, correct and validate all or part of this text or who have send bibliographic documents: Dr. Carole Billod, director of the Novartis Archives, Basel; Dr. Jean-Paul Clozel, founder and former CEO of Actelion Pharmaceuticals, Allschwil; Dr. Christian Hubschwerlen, former director of the Department of Infectious Diseases at Hoffmann-La Roche, Basel; Dr. Marc Lang, former research director of oncology at the Novartis Institute for Biomedical Research, Basel; Dr. Béatrice Molac, former group head, Patent & Trademark Department at Novartis and president of the Mulhouse Chemistry Alumni Association; Dr. Beat Münch, former “Adjunct” of several Basel University Rectors; Dr. Théophile Tschamber, former CNRS research associate at the Mulhouse Chemistry School and Dr. René Voltz, former professor of physics and expert historian of the “Naturwissenschaften” and “Geiteswissenschaften”, and taught at the various Strasbourg Universities since 1538 during reformation times, in Latin, German or French.


