1 Introduction
Since Goldschmidt [1] presented his pioneering vision to study element distribution in various natural materials, geochemists have produced a large body of data describing the abundance of elements and isotopes in natural objects. Such data have lead to Earth Scientists to identify the geologic processes operating on the planet today. An Earth scientist was asked to give a summary about F in the Earth at Colloque Français de Chimie du Fluor 2017, Murol (CFCF 2017). Luckily, we have just completed a review article discussing the significance of F and other halogens in the nature [2]. This article aims to introduce the discussion given in that article to the community of CFCF 2017 and beyond geochemical perspectives about F found in water and rocks.
Fluorine plays an important role in geochemical and biogeochemical systems despite its relatively low overall abundance on the Earth and in the Cosmos. For example, F can exert a significant control on the behavior and composition of magmas [3], the composition of hydrothermal fluids, and the transportation of ore metals in the crust [4]. Fluorapatite is a well-known mineral constituting teeth of many animals. Furthermore, it has been reported that a geologic input of F in nature affects the environment and human health [5]. Knowledge of the key F reservoirs can be used to address many ongoing areas of research for geochemists. For example, these include tracing the evolution and source of hydrothermal fluids and how they play a role in ore deposit formation, metamorphism and subsolidus alteration, tracking mantle to crust mass transfer associated with subduction, and the modeling marine-land biogeochemical cycling. In this review, we present descriptions of the distribution of F in various terrestrial and extraterrestrial rocks, fluids, and reservoirs. Some representative data sets from these media are presented, and a short discussion is also presented on the Earth's F mass balance.
2 Origin of fluorine in the solar system
Fluorine was produced via nucleosynthetic processes in stars, which predate the solar system [6]. Specifically, fluorine, with a single mass
The abundance of F in the solar system has been assessed by several methods, including spectrometry of the Sun's photosphere and corona, and measurements of particles derived from the Sun (such as solar wind) and meteorites that are rich in volatile element (such as CI chondrite). However, data of high-energy particles from the Sun (known as solar energetic particles) provide a strong constraint on its composition, they are also biased to lower abundances for the atoms with high first ionization potential [9] (note first ionization potential of F is 17.4228 eV; CRC Handbook). Absorption signals from sunspots have also been used for photosphere abundances of F, but the data have significant uncertainty [10]. Recommended solar abundance values for F are therefore tied to assessments of CI chondrite composition. This is because the abundance of volatile elements (including F) decreases from CI to CV (Fig. 1), indicating CI as the best representation of an early condensed object. Despite the challenges faced by different methods, the abundance data agree within the data uncertainties, which are 10% (Table 1).
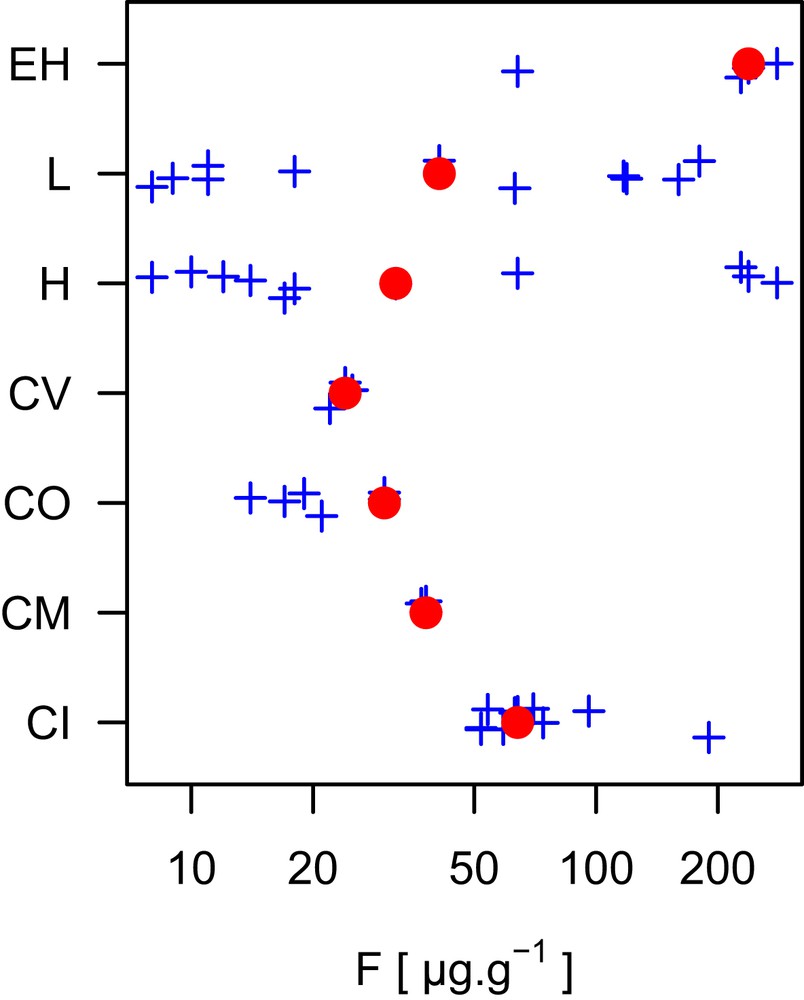
Abundance of fluorine found in different classes of meteorites. The meteorites classifications are indicated as CI, CM, CO, CV, H, L, and EH. They are all chondritic meteorites of which CI is rich in volatile element and considered as the closest representation of the solar system. Note the concentration axis (horizontal) is on the log scale. The filled red circles are published compilation values [58]. Blue crosses are individual values.
Solar system abundance of fluorine (Si = 106).
| Solar photosphere | CI [9,43] | CI [44] | CI [8] |
| 812 (617) | 843 (126) | 806 (121) | 804 (121) |
There are several assessments of F abundances in CI chondrites, and reported values differ by some degrees (Table 2). These variations reflect an increased number and improved quality of measurements in recent years. In addition to these changes, the variation in the solar F abundance is also due to the choices of the average Si content for CI chondrites and the statistical weight of Longueuil meteorite (France), which is the largest CI chondrite meteorite with an observed fall. In consequence, there are more studies of Orgueil than other CI chondrites. Nevertheless, the variations in the reported average F abundance of CI chondrites are comparable to other relatively well-characterized elements. We conclude that F abundance in the solar system is 59 ± 5 μg g−1 as a weighted average of the values in Table 2.
Abundance of fluorine in CI chondrite (ppm = μg g−1).
| Orgueil [43] | CI [9] | CI [44] | CI [8] |
| 58.2 (8.7) | 60.7 (9.1) | 58.2 (8.7) | 58.2 (8.7) |
Although there is a systematic decrease in abundance from Cl, to Br, to I (halogens) in the solar system, F is notably less abundant than the heavier element Cl. In addition, compared to neighboring elements, namely C, N, O, and Ne, F abundance is lower by 3–four4 orders of magnitude (Fig. 2). These illustrate that F is less likely produced during nucleosynthesis reactions, and/or F is more likely consumed during the formation of heavier elements. Overall, F is the 24th most abundant element in the solar system, after Zn, ahead of Cu [9].
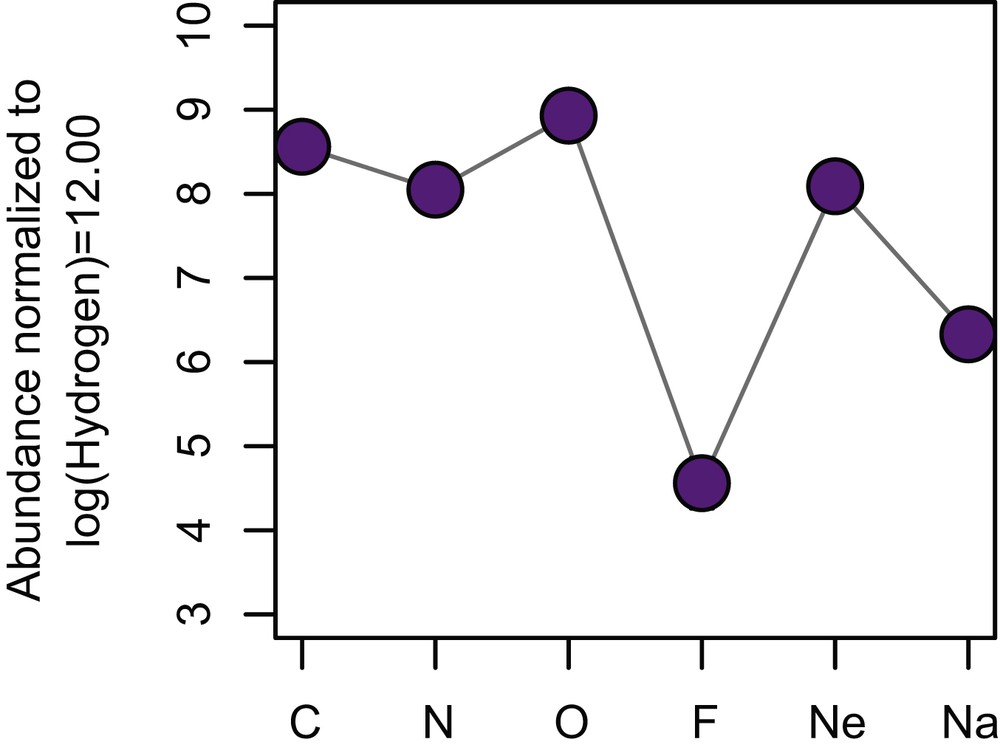
Fluorine abundance in the solar system compared to its neighboring elements. The abundance is expressed in decadic log scale, normalized to a fix quantity of H atoms. Note F is significantly lower than other elements with similar atomic numbers.
3 Fluorine abundance in the Earth
3.1 The hydrosphere
3.1.1 Surficial and ground waters
Seawater, representing only 0.02% of Earth's mass, is a major reservoir of Cl (∼24% by mass of the Earth's budget) but is a negligible host for F, containing less than 1% of the bulk Earth budget. When considering only the hydrosphere, seawater overwhelmingly dominates the mass balance. The preferred mean seawater value of F concentration is 1.3 μg g−1 [11]. In rainwater, rivers, and other surface runoffs, where the F ion abundances are usually not measured, it is considered to be lower than that of seawater, as the mean halide ion abundances are very low and systematically lower than seawater. Fluorine in the surface runoffs is controlled dominantly by its content in the parental material, such as rock types in contact with water [12]. Volcanic plume emissions are reported to significantly increase the F− contents of local surface waters [13].
Formation waters, underground waters often with high salinity trapped deeply in sedimentary basins, can be rich in dissolved chemical components. As it is the case for the surface water, F abundance in formation waters does not correlate with other halogen ions, and rather the abundance seems to reflect its content in the local material, varying from 0.5 to 50 μg g−1 [2b]. Fluorine abundance of underground waters derived from crystalline basements (such as granite) shows the similar range of variations (Fig. 3). In summary, F− is not an abundant anion in the Earth's hydrosphere; however, with an increase of dissolved halide in fluid, F− potentially increases up to the level of 50 μg g−1.
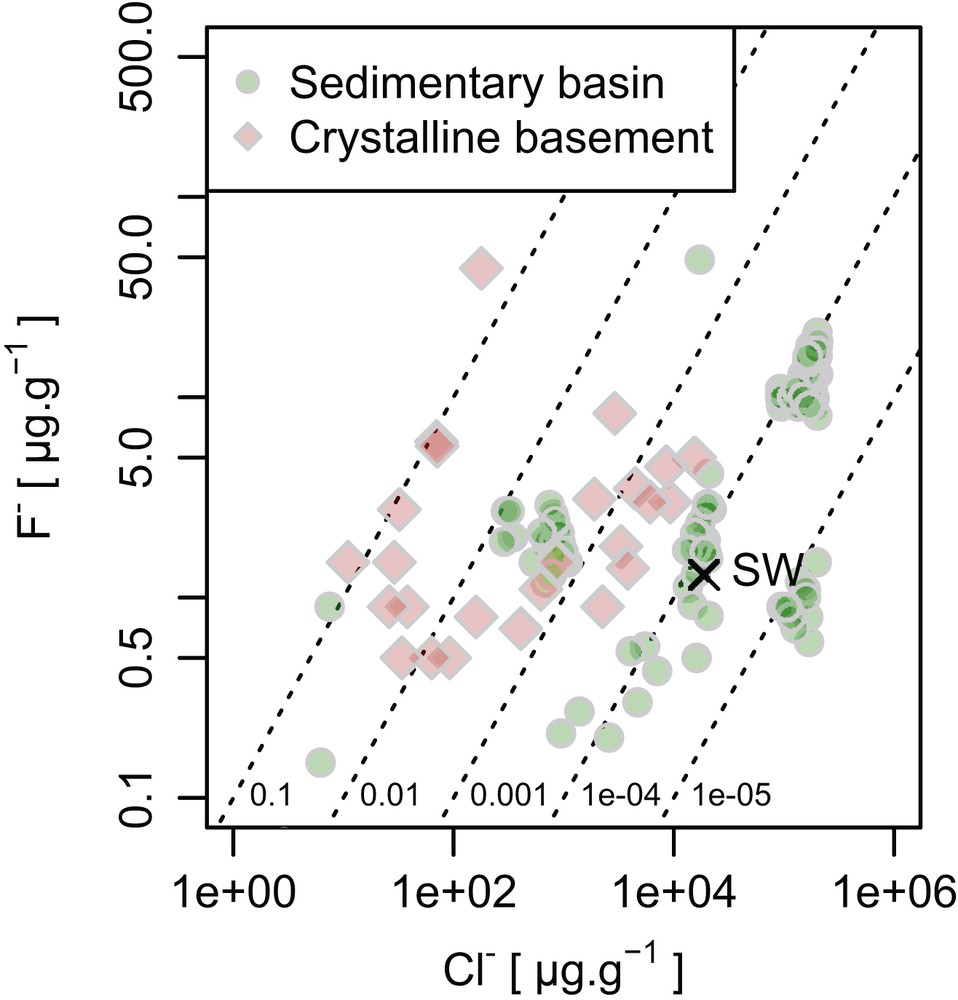
Plots summarizing abundance and ratios of F ions in some terrestrial fluid types (formation waters from sedimentary basins; shallow, brackish, saline, and briny waters from crystalline shield environments and granites, and surficial waters/sediments including seawater [SW], rivers, and fluvial particulates). Diagonal lines represent constant F−/Cl− ratios.
3.2 The crust
3.2.1 Continental crust
Fluorine in the continental crust (CC) is distributed differently in various rock types; however, an average CC value is modeled to be 553 μg g−1 [14]. A CC is subdivided into two parts: the upper CC mostly constituted of sedimentary and low-grade metamorphic rocks, and the lower CC mostly constituted of high-temperature metamorphic rocks. The upper CC is enriched in F by 1.4 times relative to the lower CC, with the values of 611 and 429 μg g−1, respectively [15]. With the exception of some phosphate shales (with very high F contents) and evaporites [16], there is no systematic variation in the F abundance related to rock types. In fact, the abundance can vary by several orders of magnitude in sedimentary rocks of both clastic and chemical types (Table 3; Fig. 4). Difficulty arises in accurately evaluating the relative contributions of rock types in the CC, because F abundances are not tied exclusively to the rock types or their mineral constituents. Further modification of the crust during hydrothermal alteration, mineralization, and weathering, and the overall high mobility of fluorine have led to an order of magnitude variations in the abundance, locally. Despite all this complications, F abundances of the crustal rocks are predominantly on the order of 100 s μg g−1.
Fluorine abundances in some continental crustal rock types and sedimentary particulates (means and ranges).
| Example | F (μg g−1) | Source |
| Metamorphic | ||
| Amphibolites | 659 | [15a] |
| Felsic granulites | 413 | [15a,45] |
| Intermediate granulites | 582 | [15a] |
| Mafic granulites | 845 | [15a] |
| Sedimentary | ||
| Carbonates | 459 [454–465] | [15a,46,47] |
| Sandstones | 485.5 [482–489] | [15a] |
| Phosphorites | 10,115.5 [8796–11,435] | [16b] |
| Phosphatic shale | 16,301.5 [2.99–32,600] | [16a] |
| Marine shale | 740 | [47] |
| Oceanic sediment (noncarbonate) | 826 [393–1259] | [24] |
| Marine pelagic clay | 1300 | [11,47] |
| Manganese nodules | 200 | [48] |
| Pelites | 860 [780–940] | [15a] |
| Mantle ultramafic | ||
| Spinel lherzolites | 5.5 [5–6] | [49] |
| Spinel peridotites | 88 | [50] |
| Igneous, mafic intrusive | ||
| Mafic intrusions | 660 | [15a] |
| Norite | 13 | [51] |
| Gabbro | 13 | [51] |
| Igneous, mafic extrusive | 681 | [15a] |
| MORB | 267 [100–600] | [52,53] |
| OIB | 35 | [52,53] |
| Igneous, intermediate | ||
| Andesite | 500 | [24,52] |
| Diorite | 631 | [15a] |
| TTG | 539 [514–564] | [15a] |
| Igneous, felsic | ||
| Felsic volcanics | 846 | [15a] |
| Granites | 613 [533–692] | [15a] |
| Felsic volcanics | 441 | [15a] |
| Silica-oversaturated felsic rocks | 5567 [200–15,000] | [52] |
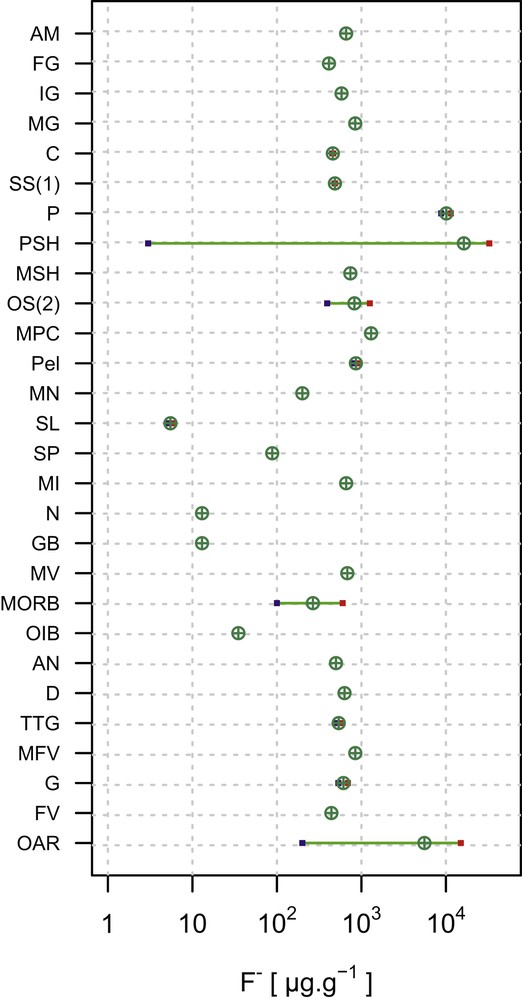
Abundances of F in some continental and oceanic crustal rock types showing mean and ranges. A graphical representation of the data summarized in Table 3 (references for data listed at the bottom of the table). Abbreviations (for rock types): AN, andesites; AM, amphibolites; C, carbonates; D, dolomites; FV, felsic volcanics; G, granites; GB, gabbro; IG, intermediate granulites; MFV, metafelsic volcanics; MG, mafic granulites; MI, mafic intrusions; MN, manganese nodules; MORB, mid-ocean ridge basalt; MPC, marine pelagic clay; MSH, marine shales; MV, mafic volcanics; N, norite; OAR, oversaturated acid rocks; OIB, ocean island basalt; OS(2), oceanic sediment (noncarbonate); P, phosphorites; Pel, pelites; PSH, phosphatic shale; SL, slate; SP, spinel peridotites; SS(1), sandstones; and TTG, tonalites–trondhjemites–granodiorites. Masquer
Abundances of F in some continental and oceanic crustal rock types showing mean and ranges. A graphical representation of the data summarized in Table 3 (references for data listed at the bottom of the table). Abbreviations (for rock types): ... Lire la suite
Fluorine content of different igneous (i.e., magma-derived) rock types in the CC (Table 3; Fig. 4) is somewhat variable. This reflects the range in corresponding abundances in silicate glasses and F-bearing silicate and phosphate minerals (predominantly) derived from magmas. This variation may also reflect the influence of the original F budget of igneous liquids. In addition, fluorine abundance in magma can be diminished by degassing [17] or increased by crystallization concentrating it in residual liquid [18]. Fluorine contents typically are in the range of hundreds to thousands of micrograms per gram, with highly fractionated peraluminous and peralkaline granitoids and some basaltic rocks showing the highest values. During the weathering of volcanic products, glass devitrification results in mobilization of F [19]. Data for F in metamorphic rocks are rarely reported, except for granulites, amphibolites, gneisses, and metapelitic rocks (Table 3). The relationship between metamorphic grade and F abundance is unclear.
3.2.2 Oceanic crust
The oceanic crust, which is the crust beneath ocean, comprises igneous rocks overlain by variable amounts of sediment. The Penrose ophiolite model [20] describes that an oceanic crust consists of a stratigraphic sequence of sediments, extrusive basaltic lavas, sheeted dikes, and plutonic gabbros, underlain by peridotite (i.e., mantle rock), in which igneous rocks originate from mantle-derived basaltic magmas. It should be noted that some oceanic crusts show variations from this standard model, having poor lava coverage as well as a lack of sheeted dikes, and instead, are dominantly composed of serpentinized peridotites (a hydrothermally modified mantle rocks) with some sediment cover [21]. Because variable magmatic activity occurs along mid-ocean ridges, the representative concentrations were assessed for each lithology in an attempt to provide an estimate of F abundance in the oceanic crust. These included fresh magmatic crust, hydrothermally altered magmatic crust, sediments, and serpentinite (Table 4). A combination of these lithologies in varying proportions, depending on the magmatic activity at mid-ocean ridges, should represent the F content of an oceanic crust.
Compositions of oceanic crust lithologies (ppm = μg g−1).
| Primitive MORB | AOC | Sediments | Serpentinite |
| 130 (40) | 400 | 393–1259 | 51–320 |
Table 4 reports the average F content of primitive basalts as a representation of the fresh igneous portion of the oceanic crust. The bulk F content of altered oceanic crust is difficult to assess, because it has been frequently demonstrated that F increases to various degrees with alteration of basaltic igneous rocks [22]. Furthermore, the degree of alteration of oceanic crust is highly variable [23]. A single value representing the composition of the altered ocean crust is probably only accurate to an order of magnitude at best. Several bulk measurements of marine sediments are reported as ranges in the F content [24]. Altered mantle (i.e., serpentinite) also experiences a significant increase in the F content through serpentinization (mineralization of serpentine), as the F content in serpentine (reaction product) is significantly higher than olivine and pyroxenes (reactant) by a factor of 20–1000 [22c]. Accounting for these, there are sufficient data to model the bulk F abundance of the altered ocean crust as a function of the degree of alteration and the proportions of sedimentary, mafic, and ultramafic lithologies. A recent estimate is 400 μg g−1 [25]. As it was the case for the CC, F abundance of the oceanic crust is on the order of 100 s μg g−1.
3.3 The mantle and core
3.3.1 The mantle
The mantle is the most dominant medium in the Earth representing 67% of its mass. Furthermore, majority of magma is derived from the mantle. Therefore, the Earth's mantle is the most important reservoir providing the ingredients constituting the surface today. The mantle abundance of elements present in trace quantity, including F, is commonly assessed through compositional correlations and systematic chemical variations among primitive basalt, peridotite xenoliths, and chondritic meteorites [14,26]. The modeled F content of the bulk silicate earth (BSE, Table 5), which is a portion of the Earth excluding the core, hydrosphere, and atmosphere ranges from 18 to 25 μg g−1, agreeing within uncertainty [27]. Mid-ocean ridge basalts are derived from a depleted mantle (DM) relative to the BSE due to the formation of CC. There are two studies that assessed the composition of DM [27d,28], while the methods and data slightly differ. Salters and Stracke [27d] report a F abundance (11 ± 5 μg g−1), which is derived from an estimate of another better constrained element P, and an F/P = 0.27 ± 0.11, a ratio found to be nearly constant among mantle-derived magmas. The application of the F/P ratio to the P abundance by Workman and Hart [28] gives F = 22 ± 9 μg g−1, a value higher by factor of 2. However, Workman et al. [29] advocate use of another ratio, F/Nd = 20.1 ± 5.8, finding a F abundance of 12 ± 3 μg g−1. Recently, the variability of F content in DM is assessed through new studies. On the basis of the new Nd isotope study, F abundance in DM is possibly slightly lower, ranging from 6 to 12 μg g−1 [30]. Also, Shimizu et al. [31] report enriched and varieties of DM compositions from an extensive submarine glass data set, F = 31 and 8 μg g−1, respectively. Possibly, F abundance in mantle may vary over a factor of 5. As an approximation by taking median values, the F abundances in BSE and DM are 20 and 10 μg g−1, respectively.
Fluorine abundance in mantle (ppm = μg g−1).
| Xenolith [32] | BSE [14] | BSE [27b] | BSE [27c] | DM [27d] |
| 10.5 (3.5) | 25a | 25 (10) | 18 (8) | 11 (5) |
a Standard deviation is not reported.
Data for the bulk F content in mantle xenoliths, rocks of mantle brought to the surface, are quite limited. For the few data available, variability appears to be significant, ranging from 7 [32] to 400 μg g−1 [33]. In addition to the paucity of data, the composition of the subcontinental xenoliths has commonly been modified by metasomatic fluid injections. Therefore, an independent assessment of F abundances in the mantle, using only peridotite xenoliths, is not possible. A significant increase in the size of the whole rock xenolith database in the future could lead to independent constraints on the F abundance in the mantle.
Rare types of mantle-derived magmas, carbonatites from Ol Doinyo Lengai, Tanzania, contain between 1.2 and 3.5 wt% of F [34], which takes the form of an immiscible halide melt [35]. Halide melt inclusions are also found in magmatic minerals from an intrusive carbonatite from Saint-Honoré, Quebec, Canada [36]. Similarly, F-rich micas and amphiboles are reported from various mantle nodules. For example, up to 8 wt % F is reported for phlogopite found in peridotite xenoliths from ultrapotassic lavas [37]. Fluorine contents of 0.05–0.4 wt % have been reported in amphibole and mica in xenoliths from Kerguelen Island [38]. Furthermore, fluoride melt has been reported in peridotite xenoliths [39]. From these examples, it is clear that significant enrichment of F occurs in the mantle, suggesting significant mobility of F. However, high F content magmas are volumetrically insignificant and their contribution to the Earth's F budget is negligible.
3.3.2 The core
There is little direct information regarding the presence of F in the Earth's core. Even if the F content of the core is lower than that of mantle by 1/30th, given that the weight fraction of the core is 32% of the Earth, the core could contribute 1% of F budget of the Earth. Further research efforts will hopefully provide better constraints on this uncertainty. Poor F incorporation in the phases that may constitute the core (metals, metal alloys, and sulfides) can be inferred from experimental results with other halogens [40]. The interaction of F with Fe metal under atmospheric conditions commonly results in corrosion reactions, which form new fluorides. In general, F is not expected to form extensive solid solutions with metal alloys or Fe sulfides. A consequence of such observations leads to a common assumption that the most of the Earth's F budget is found outside of the core. In addition, we are not aware of any significant detection of F in iron meteorites, which are considered as fragments of planetary cores.
4 Earth's fluorine distribution
Given the estimates of average F concentration in different domains of the Earth, it is possible to assess their mass distributions in the Earth. Our estimates are summarized in Table 6. This exercise illustrates some intriguing character of F in the Earth. For example, essentially all F are found in the mantle and the crust. The budgetary contribution of F in the atmosphere, hydrosphere, and the core is negligible. This is due to the nature of element in which F, an element with its highest electronegativity, has a strong tendency to form ionic bonds. For example, naturally occurring metal fluorides are nonvolatile and poorly soluble compounds, and F does not alloy with metal to be incorporated into the core. Silicate mineral–dominated mantle and crust are constituted of oxygen-cation compounds, minor quantity of F is easily incorporated into such minerals.
Distribution of fluorine elements in the Earth.
| Fluorine reservoir | Mass (Yg) | F (Zg) |
| Atmosphere | 5.2 × 10−3 | |
| Ocean [11] | 1.35 | 1.8 × 10−3 |
| Crust | ||
| Oceanic crusta | 4.80 | 1.2 |
| Continental crust [15b] | 21.7 | 12 |
| Evaporiteb | 1.0 × 10−4 | |
| Saline formation watera | 0.15 | 7.5 × 10−4 |
| Mantlea | 4019 | 80 |
| Corea | 1932 | 0 |
| Total sum | 94 | |
| Bulk earth estimate [56] | 5974 | 90 |
Concentrations of F in the atmosphere, ocean, CC, and bulk earth are taken directly from the references cited above (cf. Table 6 footnotes). For other reservoirs, we have chosen values from a variety of existing estimates. Specifically, the value used in Sharp and Draper [41] is adapted for saline formation waters. Then, we calculated the total mass of the formation water by assuming the Cl concentration as 10 wt %. The abundance of F in the formation water was derived from Fig. 5. Fluorine in formation water is considered as a part of CC. We caution that the values shown here are an approximation because (1) the estimates of average concentrations are accurate within a factor of 2, and (2) there is no valid constraint of the total terrestrial F abundance to verify the mass balance of the calculation. Although based on the aforementioned discussion, we have selected our preferred values judiciously, the total F mass in the Earth is 94 Zg comparable to earlier estimate (90 Zg, Table 6).

Pie charts of the fluorine budget in the Earth, which represent a graphic illustration of the data in Table 6. Note the core and ocean are negligible reservoirs for this representation.
Fluorine studies in Earth Science are far from complete. As concluding remarks, we summarize four challenges discussed above. (1) Behavior of F in hydrosphere: data on F abundance in various surface waters, especially other than ocean, are required for understanding the F transfer between hydrosphere and crustal rocks during erosion and weathering. In addition, the role of biology in surface F cycle needs to be assessed quantitatively. (2) F abundance in metamorphic rocks: amount of F present in various types of metamorphic rocks is not studied extensively so far. Because of this, geochemical transport of F during the metamorphism is yet to be understood, except for certain cases related to a prograde metamorphism during the subduction of an oceanic lithosphere [22c]. (3) F abundance in mantle peridotites: data on F abundances in peridotites are also scarce. This prevents an assessment of F abundance in the mantle from the peridotite data. Fluorine is also expected to provide an additional geochemical vector characterizing the metasomatic process in the mantle beneath continents, for example, providing insights into the origin of kimberlite (a diamond-bearing magma). (4) F incorporation in metallic core: although F is not expected to incorporate into the metallic more in a large quantity, even a small quantity can influence the terrestrial budget significantly. Furthermore, at the extreme high pressure and temperature, new F-bearing minerals can be formed. Investigation of chemical reaction at the extreme conditions therefore potentially brings in new discoveries regarding F behavior deep inside the Earth.
Acknowledgments
This manuscript was a result of the organizers' effort in bringing in broader community to the “Colloque Français de Chimie du Fluor”. The authors would like to thank the committee led by Professor M. Dubois for this initiative to organize a cross-disciplinary workshop under the banner of fluorine chemistry. This research was financed by the French Government Laboratory of Excellence initiative no. ANR-10-LABX-0006, the Région Auvergne, and the European Regional Development Fund. This is Laboratory of Excellence ClerVolc (contribution number 287).



Vous devez vous connecter pour continuer.
S'authentifier