1 Introduction
Wood is considered as one of the main renewable materials used for a huge number of end products such as wooden frames, pulp and paper, wooden panels furniture, and so forth. Besides the ecological and economic aspects, which appear as an evidence, this raw material is light, stiff, and resistant and is available in large quantities and at low cost. All these advantages make wood a key material. However, its hygroscopic character results in high sensitivity to ambient humidity as well as changes in temperature. Swelling and shrinkage caused by water absorption and desorption result in cracks and volume deformation in the wood [1–3]. With the aim to decrease the wood's hydrophilic character, several studies and various treatments have been developed, for example, thermal treatment [4,5], electric discharge [6,7], or chemical treatment [8–14]. Nevertheless, most of them require toxic products and high energy costs because of their low yield or energy efficient aspects; the application at the industrial scale is then difficult. Wood pieces are not the only materials that can be used. Natural fibers and wood flour are increasingly used in bio-based composites. In the context of reduction of the amount of fossil carbon produced every day, the aim consists in the replacement of usual fillers, aramid fibers, carbonaceous materials, either fibrous or nanometric, or glass to reinforce the polymer matrix in composites. The main difficulty encountered when processing wood polymer composites is the lack of compatibility between the wood flour and the polymer matrix [15–17]. The intrinsic incompatibility between the hydrophobic polymer, which contains nonpolar groups, and the filler, which is hydrophilic with strongly polarized groups [18], does not favor good wettability of the filler in the matrix, that is, good adhesion, and, as a consequence, a homogeneous dispersion of the wood flour into the commonly used hydrophobic polymer [16]. This results in pores in the composite at the polymer/wood interface that alters the mechanical properties because of two main factors. On one hand, the mechanic strain is not efficiently transferred from the matrix to the fillers and, on the other hand, this porosity allows water absorption to occur; this latter effect is favored by the intrinsic hydrophilicity of the wood flour filler. Water is absorbed into the filler once incorporated into the polymer, leading to dimensional instability and thus to a degradation of the composite mechanical properties [19,20].
In this study, we propose to apply direct fluorination to both wood flour and massive wood pieces to broaden the application range of wood pieces, especially for outdoor uses and to enhance the properties of wood polyester composites. Considering wood as a complex biocomposite, involving hemicellulose and lignin polymer matrix and cellulose as the “filler”, a chemical treatment efficient to polymers may be considered: direct fluorination [21]; it is commonly used to treat the surface of polymers to improve their barrier properties against oil, increase their resistance to chemical solvents' attack, and make them more hydrophobic [22]; the best industrial process example is the surface treatment of petrol tanks to achieve barrier properties. Direct fluorination is also performed for the synthesis of fluorinated carbons [23]. To lower their hydrophilicity and enhance their adhesion to various polymers, fluorination has also been used to treat synthetic reinforcements, such as aramid fibers [24]. As a good indication that fluorination may be efficient for wood, some studies have been devoted to the surface treatment of paper; fluorination results in a significant decrease in the product's surface energy, that is, a gain in hydrophobicity [25].
2 Experimental section
2.1 Materials
Silver fir wood samples were selected from a French sawmill located in Auvergne. The logs processed by this sawmill come from plantations harvested in the sawmill proximity. The tested samples were selected randomly and their size reduced to 10 × 1 × 0.8 cm to fit our experimental devices.
The wood flour under study was a mix of spruce and silver fir species obtained from sawmill coproducts in Auvergne, France. Its density was measured by the solvent method using xylene and toluene and was found equal to 1.41 ± 0.17. The flour was sifted so that its grading was smaller than 250 μm.
The polymer used to process composites was unsaturated polyester Norsodyne G703, from Cray Valley, whose density was 1.17. Wood polyester composites with a reinforcement weight fraction of 45% (corresponding to a volume fraction of 40%) were processed by hot compression molding using an SATIM hot press. The wood/polyester mixture was poured into an aluminum mold of 100 mm × 100 mm × 2 mm size covered with 0.12 mm thick polytetrafluoroethylene sheets on each internal face (aimed at easing the final demolding). Then, the closed mold was placed in the press and kept under pressure of 60 kN and temperature of 80 °C for 2 h, so as to ensure the resin cross-linking. The cooling to ambient temperature was performed using pressurized air. Treated and untreated wood flour composites were processed in exactly the same way, without any precuring of the materials.
2.2 Fluorination
Fluorination by pure molecular fluorine was conducted in dynamic mode (continuous flux of reactive gas in an open reactor) or static mode (a defined amount of F2 molecules is injected into a close reactor). Gaseous fluorine was purchased from Solvay Fluor (purity 98–99% v/v with HF max. 0.5% v/v and other gases, primarily O2/N2 at approximately 0.5% v/v). Before each fluorination whatever the mode, samples were outgassed overnight under primary vacuum (10−3mbar) at 80 °C to eliminate physisorbed molecules on the particle surface or porosity. The reactor was flushed for 1 h with nitrogen gas to remove all traces of air and moisture. Whatever the means of fluorination, a passivated nickel reactor was used (covered with NiF2).
About 5 g of wood flour was placed in a passivated nickel reactor. The thickness of the powder deposition was less than 2 mm to favor the diffusion of fluorine gas in the whole volume and avoid inhomogeneous treatment. The gas inlet was located to the left of the sample. This configuration may result in higher fluorination rate close to the inlet. The reaction oven was then divided into three parts: it was set at 42 °C on the left, 55 °C in the middle, and 70 °C on the right. Such a temperature gradient allows both the control of the fluorination process and a homogenous treatment. A static mode was used: a partial vacuum was applied to the closed reactor (−20 mbar) and F2 gas was injected in addition to N2 gas to reach 1 atm. The total pressure inside the closed reactor was maintained constant for 3 h, and five additions of fluorine were performed to compensate for the consumption of molecular fluorine because of the reaction. Finally, after the removal of all the F2 molecules over a period of 2 h thanks to a flow of N2 gas at 150 °C and then cooling to ambient temperature for 11 h, the flour was heated again to 150 °C with a flow of N2 gas for 1 h to eliminate traces of adsorbed F2, HF, and CF4 molecules.
Silver fir samples were treated under dynamic fluorination in a continuous process, without a vacuum step in a reactor. This latter step is performed in a preliminary step outside the reactor, the transfer into the reactor being fast —about 1 min). This process is more appropriate for continuous treatment of large pieces. Samples were exposed to the reactive F2/N2 (1/2 volume ratio) gaseous flow at room temperature. The fluorination durations tested were 1, 5, 10, and 20 min. After the reaction, the fluorine flow was stopped and the reactor was flushed with N2 for 1 h.
2.3 Physical–chemical characterization
The morphology of the samples was investigated by scanning electron microscopy (SEM) to evaluate the potential effect of fluorination on their surface. The energy of the electron beam was fixed at 3.00 keV and the working distance was over 4–6 mm range.
19F NMR experiments were carried out using a Bruker Avance spectrometer with a working frequency of 282.2 MHz. A magic-angle spinning (MAS) probe operating with 2.5 mm rotors was used allowing a 30 kHz spinning rate. For 19F MAS spectra, a simple sequence was used with a single π/2 pulse duration of 4.0 μs. 19F chemical shifts were externally referenced to CF3COOH and then referenced to CFCl3 ().
FTIR spectrometer NICOLET 5700 (Thermo Electron) was used to record IR spectra using ATR mode. One hundred scans with 4 cm−1 resolution were collected to acquire each spectrum between 4000 and 400 cm−1.
To evaluate the hydrophilicity/hydrophobicity character, static and dynamic water contact angle measurements were recorded using an Attension Theta Lite Optical Tensiometer with an imaging source camera. A water microdrop of 5 μL was deposited on the surface of either the silver fir pieces or the pellets made from wood flour. Once the drop was stabilized, the picture was taken and analyzed using the Attension software that evaluates directly the contact angle. Contact angles were calculated as the average of five measurements taken at different locations on the sample surface. For samples with a strong hydrophilic character, as water was absorbed into the pellet, the droplet was pinned and the contact angle decreased with time. The time dependence of the values was recorded until complete absorption. Pellets were prepared using a pressure of 2 tons on the compacted powder. The Lifshitz–van der Waals method was used to estimate total energy and polar and dispersive components. Three solvents, among them two are polar, were used: water, formamide, and dimethyl sulfoxide.
3 Results and discussion
3.1 Fluorination mechanism
The superficial structure of silver fir has been checked by SEM (Fig. 1). The fluorination does not lead to major changes for short fluorination durations. However, a 20 min treatment implies the initiation of surface deterioration that is revealed by some strongly deteriorated tracheid. The SEM images of untreated wood particles and fluorinated wood particles are also represented in Fig. 1. The tracheid physical structure appears preserved after direct fluorination; even the wood pits were maintained intact.

Scanning electron micrographs of pristine (a) and silver fir fluorinated for 5 min (b), 10 min (c), and 20 min (d). Raw (e) and fluorinated (f) wood flour images are also shown.
Fluorination results in wood browning; the higher the fluorination duration, the higher the chemical conversion on the surface and the samples appear to brown significantly (Table 1).
Color change in silver fir during fluorination.
| Fluorination duration | Raw | 1 min | 20 min |
To estimate the interaction between samples and water molecules, the contact angle between a water drop and the wood surface has been measured. Table 2 summarizes the contact angles and the adsorption time of a water drop onto a wood sample before and after fluorination. The hydrophilic character of the untreated silver fir is revealed by the low water contact angle (65°). Moreover, its strong affinity with the water is confirmed by the fast water drop adsorption (11 min for a complete disappearance of the drop).
Contact angle and adsorption time of a water drop on the silver fir surface and on pellets made from wood flour.
| Sample | Untreated | Fluorinated 5 min | |
| Silver fir | 2 s after the water drop deposit | ||
| 15 min after the water drop deposit | |||
| Douglas | 2 s after the water drop deposit | ||
| 20 min after the water drop deposit |
These two values significantly increase after a few minutes of fluorination (below 20 min) and the silver fir acquires a hydrophobic character (110–115°). The hydrophobic character is manifested for the case of wood flour pellets by a significant gap between the contact angle before and after treatment, that is, between 80° and 95°, respectively. The absorption time into the pellet is 28 s; thus, significantly shorter than silver fir because of diffusion of water molecule into the interparticular porosity.
Both silver fir and wood flour acquire a hydrophobic character after fluorination. Because the morphology of the surface is not significantly changed (see SEM images), this conversion may be due to the change in the surface chemistry. IR and NMR spectroscopies were performed to investigate it. The observation is similar for wood flour and silver fir. Whatever the species and its nature, massive or powder, wood fluorination results in the appearance of carbon–fluorine bonds, proved by the vibration bands at 1080, 1160, 1200, and 1280 cm−1 (dashed lines in Fig. 2) on FTIR spectra. It is to be noted that the intensities and positions of CFx vibration bands (x = 1, 2, or 3) were obtained by a careful fit of the spectra and comparison with the spectrum fit of the pristine samples. This phenomenon is combined with a significant decrease in the hydroxyl group contribution (around 3320 cm−1). Therefore, the wood fluorination consists in the substitution of OH groups by fluorine atoms. FTIR spectra highlight that this substitution increases in line with the fluorination duration for both wood species. Furthermore, hydroxyl groups favor water absorption in wood because of their strong interaction with water molecules by hydrogen bonding, whereas fluorinated surfaces are known for their hydrophobic and even superhydrophobic character [26–29]. The substitution mentioned above explains lower interaction between wood and water after fluorination.

ATR IR (a) and 19F MAS NMR (b) spectra of raw and fluorinated silver fir.
Lignin results from the polymerization of three phenolic alcohols: p-coumaryl alcohol, conferyl alcohol, and sinapyl alcohol. Because of the diversity of chemical functions in lignin, which appears as the component of highest reactivity with F2 gas, the question of mechanism is of high complexity. CHF, CF2, and in less amount CF3 groups are evidenced by solid-state NMR [30] and FTIR. CF3 groups result from the disruption of chains. Aromaticity of the phenolic group is probably lost to form CHF and CF2 groups. HF is evolved during the conversion by F2 molecules of CH bonds into CF ones. Moreover, COH relative content decreases after fluorination and a conversion reaction of COH into CF may be proposed.
The total surface energy was estimated using the Lifshitz–van der Waals method [31]. These data, as well as the dispersive and polar components, are reported in Fig. 3. Fluorination for very short duration, even 1 min, results in the decrease in both the polar and dispersive components. The conversion of hydroxyl groups COH into CF explains the decrease in the polar component for short duration. The longer the treatment duration, the higher the polar component, whereas the dispersive component is still nearly constant. The total energy then increases but still lower than the raw silver fir.
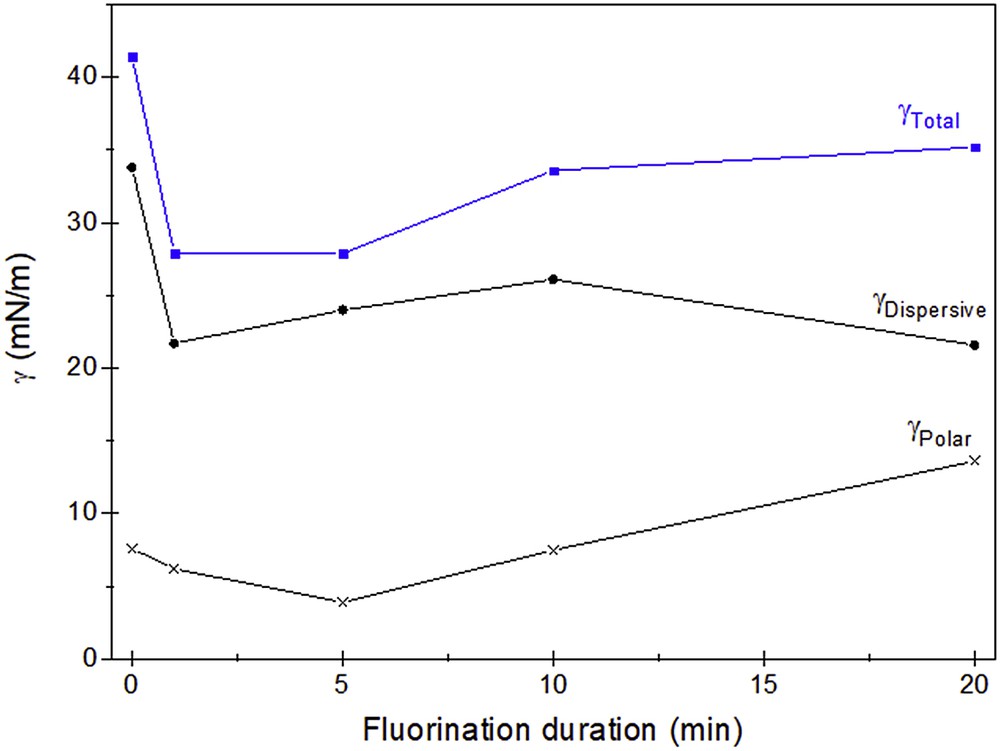
Change in the surface energy (■) of silver fir as a function of the fluorination duration; the dispersive (●) and polar (×) components are also reported.
Chemical change, that is, conversion of COH groups into CF containing groups, explains the hydrophobicity. To further understand the effect of fluorination, that is, hydrophobicity without major structural change, the microstructure of wood must be considered. The basic unit is the plant cell with size in diameter range from 10 to 100 μm. An extracellular matrix, called the cell wall, acts as a supportive framework and surrounds the plant cell. It is made of a network of cellulose microfibrils embedded in a matrix of lignin and hemicellulose, which are polysaccharides. The tubular cell wall has a layered structure where further cells are aligned parallel to the cell axis. Fig. 4 summarizes the various walls and layers and their notation, that is, M, P, S1, S2, and S3 for middle lamella, primary cell, inner, middle, and inner layer, respectively, from the outer part of the cell toward the inner core. It is of primary importance to note that the relative contents of cellulose, hemicellulose, and lignin depend on the considered component (middle lamella, primary wall, or secondary wall).
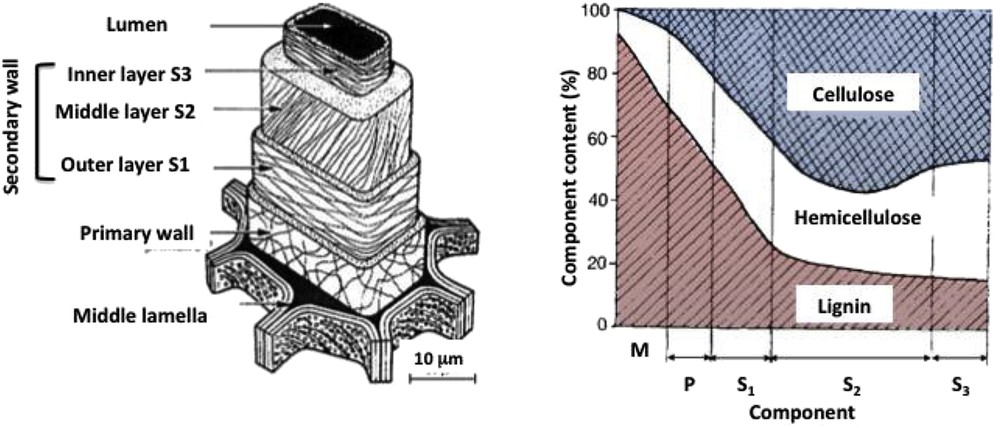
Components' relative content and microstructure of wood [32,33].
A recent study about fluorination of natural fibers [27] emphasizes that the reactivity is proportional to the content of lignin and cellulose. Indeed, the presence of lignin seems favorable and even necessary to achieve the treatment, whereas cellulose tends to inhibit the reaction; high lignin content tends to accelerate the treatment, whereas high cellulose content seems to inhibit the reaction. Regions where the lignin content is the highest, that is, middle lamella and primary cell, will consume fluorine gas first and fix fluorine atoms. This may localize the covalent grafting of fluorine atoms mainly in those parts. In the mild fluorination conditions in terms of duration (less than 20 min for silver fir) or fluorine quantity (3 h for wood flour but with addition of small quantity of F2 molecules in comparison with the wood flour weight, 5 g), CHF, CF2, and CF3 groups are mainly located in the outer parts of the cell maintaining the inner ones nonmodified. SEM image tends to corroborate such a mechanism. In mild conditions, fluorination is spatially located in the outer surface of the cells. Maintaining of the mechanical properties is then expected. On the contrary, thermal treatments usually used to render wood hydrophobic are known for their mechanically detrimental effect [5].
3.2 Mechanical properties
Tensile and flexural properties are given in Table 3. The tensile modulus of elasticity and the strength are similar before and after the wood treatment taking into account standard deviation measurement. As for the tensile tests, the flexural property values before and after fluorination are similar for silver fir. The modulus of elasticity conservation proves that wood samples have not been subject to volumetric mechanical damage, whereas similar strength confirms that the mechanical surface properties (e.g., no crack appears on the surface) have been maintained. The similitude between pristine and treated samples prove that the fluorination does not result in a loss of mechanical properties in the wood contrary to conventional physical treatment.
Tensile and flexural properties of untreated and fluorinated silver fir (5 min at room temperature).
| Sample | Silver fir | ||
| Untreated | Fluorinated 5 min at room temperature | ||
| Tensile | Modulus of elasticity (MPa) | 699 ± 276 | 599 ± 214 |
| Strength (MPa) | 3.5 ± 0.9 | 3.6 ± 0.5 | |
| Flexural | Modulus of elasticity (MPa) | 332 ± 106 | 342 ± 94 |
| Strength (MPa) | 6.2 ± 1.0 | 6.6 ± 1.0 |
Table 4 also reports the tensile stress–strain data of the composites from untreated and fluorinated wood flour. The mean tensile strength and the composite stiffness were improved by the treatment with different amplitude, +29% and +8%, respectively. On the contrary, the elongation at break was slightly decreased (−6%) but this reduction is negligible because of the high standard deviations observed for this property (10–20%). Such an enhancement in the tensile properties proves that the fiber/matrix interface is improved by both the better chemical compatibility (less difference in hydrophobicities for the two components) and the weakened porosity that are provided by the fluorination treatment. The effect of the fluorination on the flexural mechanical properties of the composites was also studied (Table 4). As for the tensile tests, the composites appeared to be stiffer after the direct fluorination (+25%). Because the composites were processed in exactly the same way, this observation could only be attributed to the grafting of fluorine atoms. For composite with raw and treated wood flour, failure occurred for similar values of strain. The strength was also increased by the wood fluorination (+27%). Compared with other treatments, the direct fluorination enables an improvement in the composite tensile and flexural properties at least comparable to conventional chemical treatments using maleic anhydride or silane [34,35]. The gain in flexural properties is even higher after fluorination than for the conventional method [36,37].
Tensile and flexural properties of the composites from untreated or fluorinated wood flours. Flexural properties of the composites from untreated or fluorinated wood flours stored 20 days under a relative humidity of 80%.
| Sample | Composite | Aged composite | |||
| Untreated | Fluorinated | Untreated | Fluorinated | ||
| Tensile | Modulus of elasticity (GPa) | 4.4 ± 0.2 | 4.8 ± 0.2 | – | – |
| Strength (MPa) | 32.4 ± 1.5 | 41.7 ± 2.4 | – | – | |
| Ultimate strain (%) | 1.4 ± 0.3 | 1.3 ± 0.1 | – | – | |
| Flexural | Modulus of elasticity (GPa) | 4.5 ± 0.3 | 5.6 ± 0.6 | 5.8 ± 0.5 | 7.8 ± 0.6 |
| Strength (MPa) | 50.1 ± 1.8 | 63.8 ± 5.7 | 50.6 ± 0.4 | 59.2 ± 3.4 | |
| Ultimate strain (%) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 |
The contact angle and the total absorption time of a water drop were measured on a composite made from either untreated wood flour or fluorinated wood flour. The composite from the nonfluorinated wood flour exhibited the lowest contact angle of the two composites (θc = 78°), whereas this angle was significantly higher in the case of the fluorinated flour (θc = 101°). These values were comparable to those obtained for the fluorinated wood flour (Table 2). The water behavior on the surface of the wood pellet (with fluorinated or nonfluorinated wood) was similar to that on the surface of the composite containing the corresponding wood flour. The affinity of the water with the composite appeared to stem from the wood composite reinforcements.
To prove the less dependence of composite with water adsorption, mechanical tests were conducted with samples aged in high relative humidity (80%) for 20 days (at constant temperature of 20 °C). Fluorination increases the flexural properties of wood polymer composites (Table 4). This tends to prove that, even after exposure to water, the fluorinated wood/polyester composites keep good flexural properties.
The improvement in mechanical properties may be explained by the better compatibility between the polymer matrix and the wood flour surface chemistries after the fluorination. The difference in hydrophobicity is decreased as revealed by the water contact angles that are closer after the fluorination (95° and 87° for fluorinated wood flour and polyester, respectively). It may enhance the compatibility between wood particles and polyester, limiting the volume available for air and water and hindering the formation of bubbles when the counter mold is pressed on the materials.
4 Conclusions
Fluorination, a surface treatment based on a heterogeneous gas/solid reaction was successfully applied to either massive silver fir or wood flour (mixture of spruce and Douglas fir) to sustainably increase its hydrophobicity. In both the cases, fluorination results in the substitution of COH groups by covalent CF bonds as proved by FTIR and 19F NMR spectroscopies. Using mild fluorination conditions, either short duration (less than 20 min) or very small F2 quantity in comparison with total wood weight, the treated wood exhibits a lower affinity for water. Indeed, the water contact angles were higher for treated wood than for the pristine sample. SEM underlined the integrity of the wood's superficial structure for mild treatment conditions. Fluorination in a surface treatment and only the middle lamella and primary wall seem to be affected. Those regions exhibit the highest content of lignin, which favor the fluorination. Durable hydrophobic character is reached by fluorine covalent grafting for silver fir and wood flour, with no significant structural modifications. Measurements of water contact angles 2 years after the fluorination, without precaution or storage of the samples, give the same values that prove the durability of the treatment. Strong covalent CF bonds are formed that allow durable hydrophobicity to be achieved. Fluorination would be extrapolated to other lignocellulosic species, in the form of massive piece, fibers, or powder.
Acknowledgments
The authors wish to thank the photochemistry team of the “Institut de chimie de Clermont-Ferrand” (ICCF) for their help during the experimental phase. We express our gratitude to the “Conseil régional d'Auvergne” for funding this project.


