1. Introduction
Selective oxidation of hydrocarbons is a serious challenge in the field of catalysts because of their potential use as a feed for producing suitable chemical substances [1, 2, 3]. The oxidation of olefins to α, β-unsaturated ketones is a fundamental reaction in synthetic organic chemistry [4]. Among the olefins, cyclohexene is one of the most important compounds that has been the subject of intense research in the past decades. Selective oxidation of cyclohexene is known as an important reaction in the chemical industry for producing 2-cyclohexene-1-ol, 2-cyclohexene-1-one, and cyclohexene oxides as an intermediary for producing some polymers, drugs, agrochemicals, and surfactants. In this compound, not only the allylic C–H bonds but also the double bond C=C are the active sites that could be oxidized [5, 6, 7]. Some oxidants including potassium dichromate, potassium bromate, or potassium permanganate were applied for this reaction [8, 9]. The use of these reagents involves some disadvantages such as high temperature and high pressure along with the use of some environmentally harmful solvents (i.e., chloroform and carbon tetrachloride) [10, 11, 12].
Therefore, to reduce environmental concerns, it is very important to use heterogeneous catalytic systems under solvent-free conditions with environmentally compatible oxidants such as molecular oxygen and hydrogen peroxide. The use of H2O2 as an oxidant is a suitable method in this regard because the only product is water; however, its high cost prevents its widespread industrial applications [13]. In comparison, using molecular oxygen as an oxidant is simple and inexpensive [14, 15] but requires highly active catalytic systems.
So far, many heterogeneous catalytic systems have been reported based on transition metals for oxidation reactions in the presence of molecular oxygen [16, 17, 18]. Compared with metal catalysts such as Ru, Pt, Pd, and Ag that are applied in this reaction [19, 20, 21, 22, 23], Au nanoparticles (NPs) with high stability showed the best catalytic activity and selectivity [24]. Another catalyst parameter that affects the activity and selectivity of catalytic systems is the support, which forms the main body of the catalyst. The benchmarking methodology in selecting appropriate support is its ease of use, availability, low cost, and high surface area [25]. Among the common supports, carbon-based materials such as graphene have the highest surface area and accelerate the penetration stage and adsorption of the organic substrate onto the surface because of their organic nature [26, 27]. Graphene has a layered structure with special electronic properties and a tendency to agglomerate due to van der Waals interactions. Therefore, an increase in distance between graphene layers, especially during modification, occurred with covalent linking of organic fragment [28, 29, 30]. To deal with this issue, it is recommended modifying the surface of graphene using thiol, thioester, amine, or carboxylate groups in order to increase strong interaction with Au metal as the capping agent [31].
Herein, in continuation of our previous works [32, 33, 34, 35, 36], a novel approach is employed to fabricate rGO-dithiooxamide/Au hybrid nanocatalyst. Graphene oxide (GO) was synthesized using graphite and chemically converted to reduced graphene oxide (rGO). Using rGO as support compared to GO in our approach effectively accelerates the functionalization steps. The rGO has a smooth surface and high affinity in organic reactions. Then, target functional groups were assembled on the surface of rGO and this support was decorated with Au NPs. The catalytic activities of prepared rGO-dithiooxamide/Au nanocatalyst were tested in the aerobic oxidation of cyclohexene by molecular oxygen in solvent-free system. The results showed a suitable conversion and high selectivity.
2. Experimental section
2.1. Preparation of reduced graphene oxide (rGO)
Graphene oxide (GO) was synthesized in accordance with the Hummers method using the commercially obtained graphite [34, 35]. Next, prepared GO was converted to reduced graphene oxide (rGO) through a chemical procedure. In a typical reaction, 1.25 mL hydrazine hydrate (50%) was added into 150 mL aqueous suspension of GO (1.0 mg⋅mL−1) at pH = 9. Then, the reaction mixture was stirred at 100 °C for 2 h until the suspension color turned to brown-black, which is indicative of the formation of rGO. The obtained rGO was washed with deionized water several times and then freeze-dried.
2.2. Functionalization of reduced graphene oxide (rGO-dithiooxamide)
Preparation of functionalized rGO includes several steps. Initially, 4 mmol p-aminophenylacetic acid (0.6 g) was dissolved in 40 mL HCl (1.0 M) in an ice bath. Sodium nitrite (1.0 g) was separately dissolved in 20 mL distilled water and added dropwise to the first solution. As soon as nitrogen bubbles are completely released from the solution, rGO (0.3 g) was dispersed in 20 mL distilled water and added slowly to the reaction mixture. The obtained suspension was stirred in an ice bath for 12 h. Then, the modified rGO with p-aminophenylacetic acid (rGO-COOH) was separated through centrifugation, washed and dried. Afterward, the rGO-COOH (0.3 mg) was suspended in SOCl2 (30 mL) and refluxed at 70 °C for 48 h under nitrogen atmosphere. After completing the reaction, the excess SOCl2 was removed, the reaction mixture was distilled under reduced pressure, and the remaining solid was washed with dry dimethylformamide (DMF) several times (rGO-COCl). Subsequently, 4.0 mmol dithiooxamide and 4.0 mmol triethylamine were added to 300 mL rGO-COCl suspension (1.0 mg/mL anhydrous DMF), and the reaction mixture was refluxed at 60 °C for 24 h. Finally, the reaction was cooled to room temperature and the rGO-dithiooxamide precipitate was collected by centrifugation, washed with dried DMF three times (GO-melamine), and dried under vacuum.
2.3. Synthesis of Au nanoparticle-grafted rGO-dithiooxamide support (rGO-dithiooxamide /Au)
The rGO-dithiooxamide (300 mg) was completely suspended in 300 mL deionized water with ultrasound probes. Afterward, 2.0 mL HAuCl4 solution (1.0 wt.%) was added to the reaction mixture and stirred at a constant speed for 24 h at ambient temperature. Eventually, gold nanoparticles (AuNPs) were immobilized on rGO-dithiooxamide, according to the typical sodium citrate reduction method. Briefly, 150 mL of obtained suspension (1 mg⋅mL−1) from the previous step was brought to a boil in a 250 mL flask under vigorous stirring. Then 5 mL of aqueous sodium citrate (1%) was added and allowed to react for 1 h. Finally, rGO-dithiooxamide/Au hybrid catalyst was centrifuged and washed several times with deionized water and dried at room temperature.
2.4. General procedure for oxidation of cyclohexene
In order to perform cyclohexene oxidation, rGO-dithiooxamide/Au nanocatalyst (10 mg) along with cyclohexene (5 mL) was transferred to a steel reactor and exposed to oxygen pressure under different conditions in the absence of any solvent and any initiator. Next, the reaction mixture was diluted by adding methanol and the products were characterized by GC-MS analysis.
3. Results and discussion
To synthesize rGO-dithiooxamide/Au hybrid catalyst, GO was synthesized from graphite powder using modified Hummers method and reduced to rGO based on the reaction with hydrazine as a reducing agent via a chemical method. Modification of the surface of rGO was then performed by p-aminophenylacetic acid via a well-documented method of forming diazonium in the presence of nitrous acid [36]. Then, for further modification, carboxylic acid groups were activated with SOCl2 on the surface of modified rGO and subsequently reacted with dithiooxamide. This active bifunctional reagent has two amine groups that are capable of reacting with very active sites such as acyl chloride by intra- and intermolecular reactions. The prepared support was dispersed in deionized water and Au NPs were decorated on the surface of modified support by reducing Au ions with a citric acid solution. The specific surface area of the rGO-dithiooxamide/Au, as determined by N2 adsorption (single point method), was 465.22 m2∕g. The steps in the synthesis of rGO-dithiooxamide/Au hybrid catalyst are shown in Scheme 1.
A schematic illustration of the preparation of rGO-dithiooxamide/Au hybrid catalyst.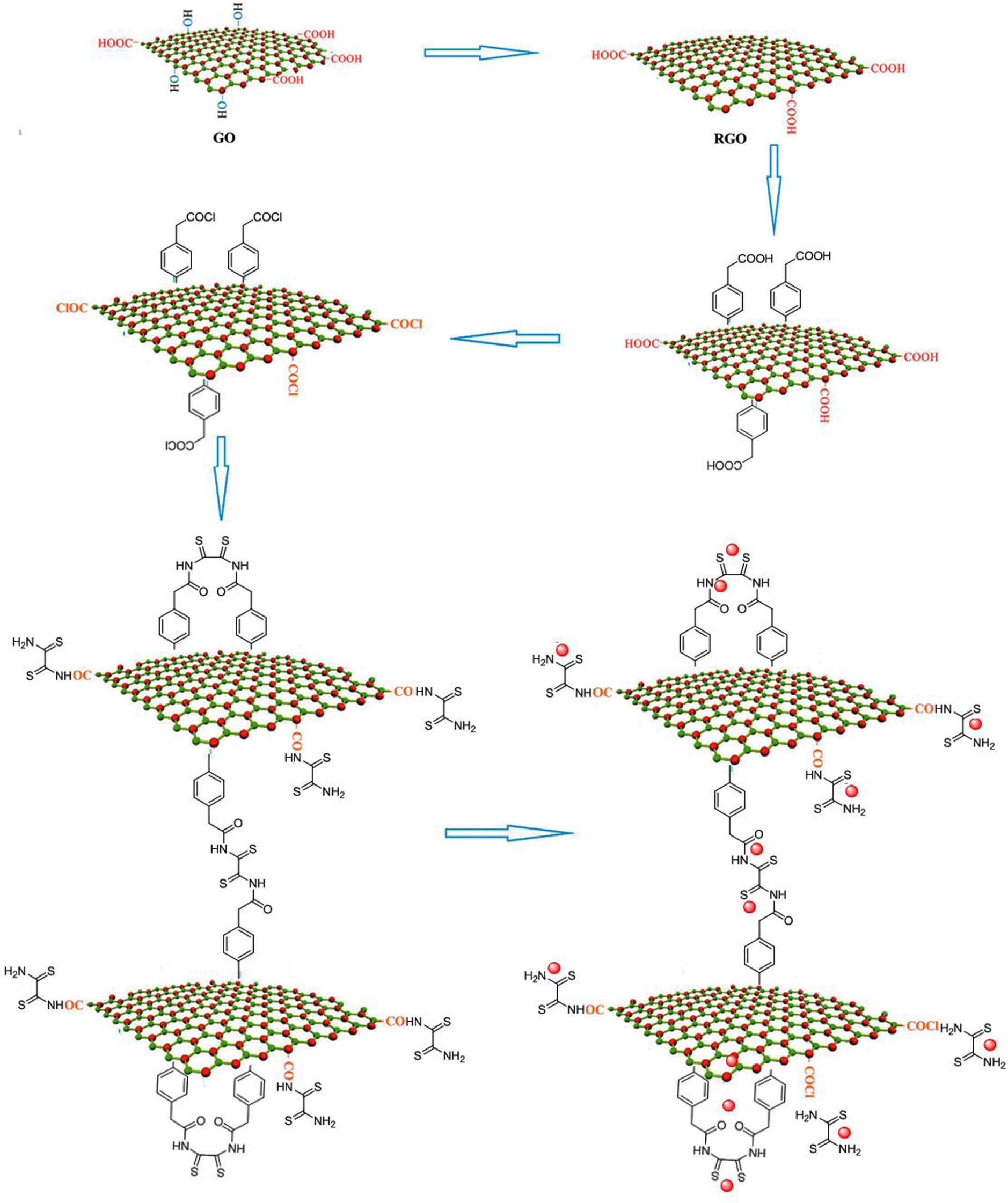
3.1. Catalyst characterization
3.1.1. Fourier transform-infrared spectroscopy
To determine the functional groups on the surface of GO in different stages of catalyst synthesis, the Fourier transform-infrared (FTIR) results were taken at ambient temperature within the range of 400 cm−1 to 4000 cm−1 (FT-IR; Bruker, Germany, RT-DLATGS detector). According to FTIR spectra, GO (Figure 1a) generally exhibits absorption peaks at 3383 cm−1 and 1041 cm−1, which is indexed to the stretching vibrations of O–H and C–O groups of carboxylic acids of the edge of GO. The fundamental mode of vibration around 1220 cm−1 corresponds to stretching vibrating of C–O–C groups of the epoxy. Moreover, the peaks at 1617 cm−1 and 1716 cm−1 suggest the existence of C=C and C=O in the GO, respectively. Overall, all peaks confirm the structure of the GO [37]. IR spectra of rGO (Figure 1b) show the degree of removal of the oxygen-containing functional groups. As can be seen, the peak at 1730 cm−1 disappeared in rGO because of the removal of C=O groups. This proves that many oxygen groups were removed during the transformation from GO to rGO. The new peaks at 2925 and 2865 cm−1, which can be attributed to methylene stretch, represent the existence of CH2 or CH groups. After modification of rGO surface by p-aminophenylacetic acid (Figure 1c), and radical coupling of rGO surface and aryl groups, the intensity of appeared peak at 3444 cm−1 increased, suggesting the formation of OH groups. In addition, the new peak at 1617 cm−1 corresponds to the carbonyl group. These two peaks are consistent with the formation of carboxylic acid. In the FTIR spectra obtained from the last stage of chemical modification (Figure 1d), new peaks at 1564 cm−1 and 1693 cm−1 can be indexed to the C=S and C=N vibrations respectively. Moreover, the peak intensity of stretching vibrations of O–H groups decreased due to their conversion to amide groups. As shown in Figure 1e, the IR scanning patterns of rGO dithiooxamide/Au hybrid catalyst are almost similar, indicating that the structure of amide bonded to modified GO preserved the process of coordination and reduction.
Fourier transform-infrared (FT-IR) spectra of (a) graphene oxide (GO), (b) reduced graphene oxide (rGO), (c) rGO-pPAPA and (d) rGO-dithiooxamide, (e) rGO-dithiooxamide/ Au.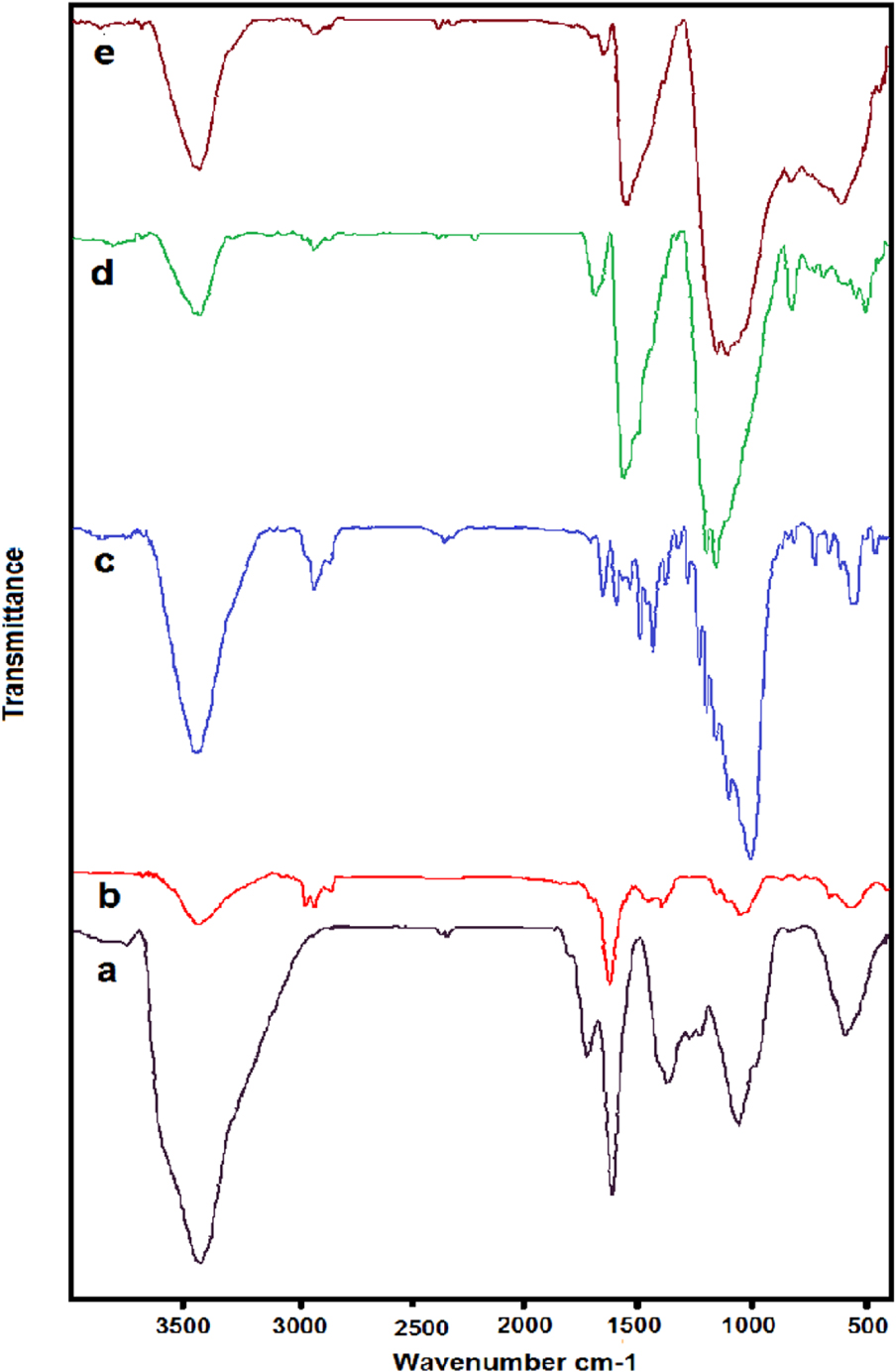
3.1.2. X-ray diffraction analysis
To characterize the chemical composition and crystallographic structure of GO, rGO, and rGO-dithiooxamide/Au hybrid catalyst, X-ray diffraction (XRD) patterns were measured using an X’Pert Pro diffractometer operating with scanning angles (2θ) ranging from 10° to 80°. The XRD pattern of GO, rGO, and rGO-dithiooxamide/Au hybrid catalyst are shown in Figure 2. In the case of GO, the sharp diffraction peak at 11.2° with the Miller index (001) demonstrates the crystalline nature of the sample. This diffraction peak is sufficient to characterize GO with an inter-planar distance of 7.4 Å [38]. After the chemical reduction of GO, the GO characteristic peak disappeared and the new peak at 24.5° appeared. The intense peak was assigned to the plane (002), in which the inter-planar distance is reduced to 3.66 Å due to disappearing oxygen-containing functional groups [39]. As can be seen from the XRD pattern of rGO-dithiooxamide/Au hybrid catalyst, there are additional peaks correlating to Au NPs at 38.20°, 44.48°, 64.58°, and 77.60° with miller indices of (111), (200), (220), and (311), respectively [39].
X-ray diffraction (XRD) patterns of graphene oxide (GO), reduced graphene oxide (rGO), and rGO-dithiooxamide/Au.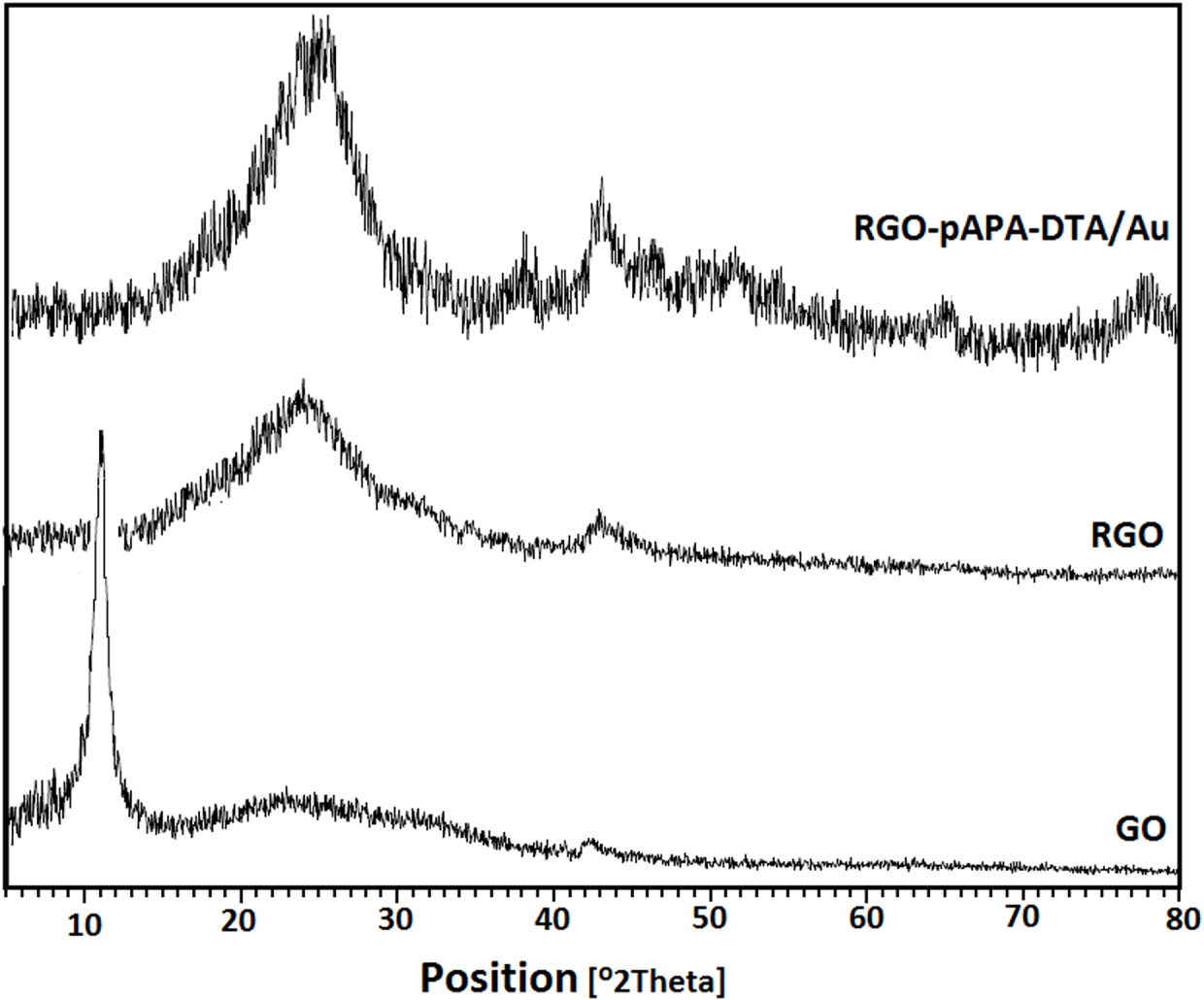
3.1.3. Scanning electron microscopy & Energy- dispersive X-ray analysis (EDX)
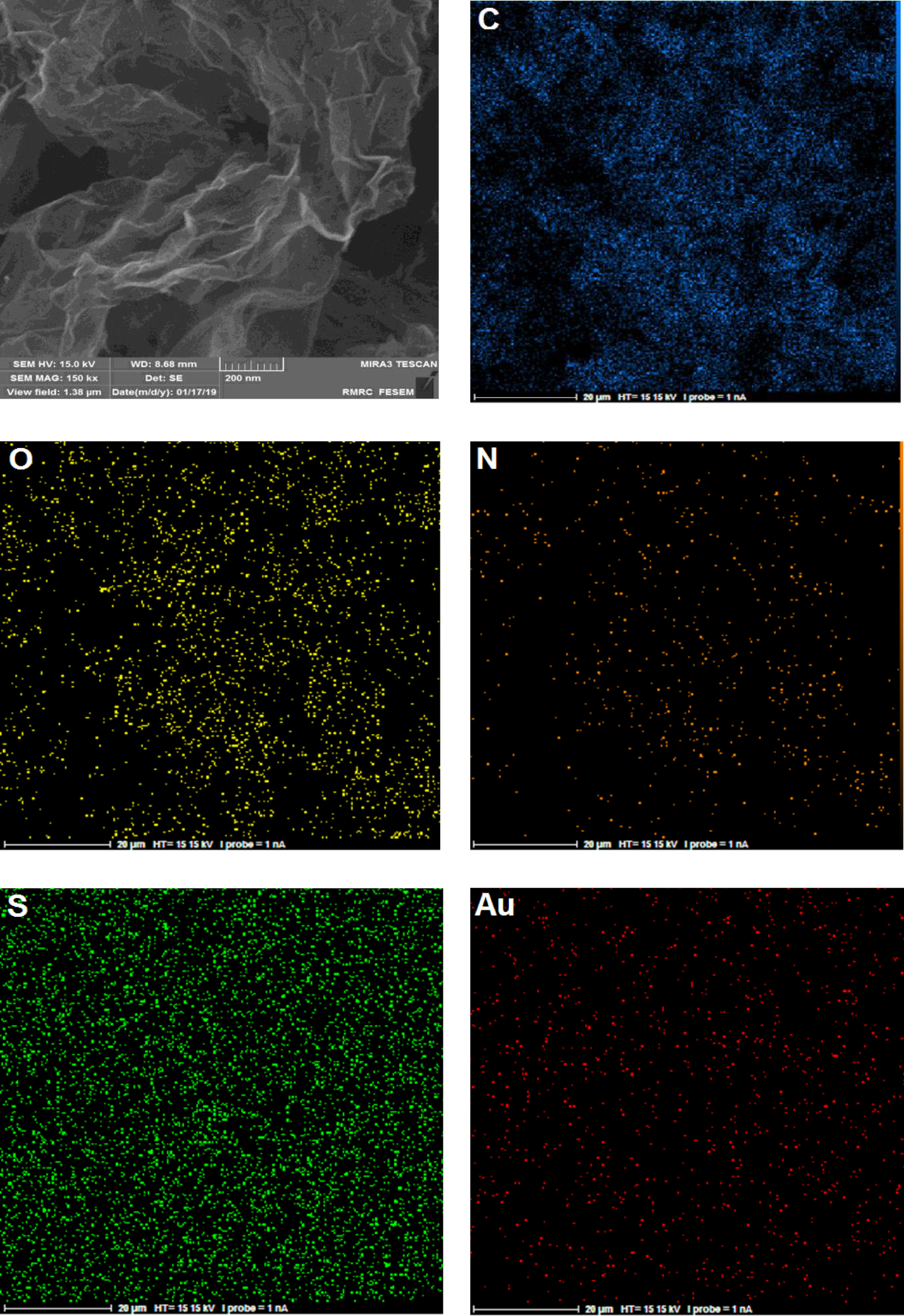
The typical surface morphology of the rGO-dithiooxamide/Au was studied by scanning electron microscopy (SEM; FESEM-TESCAN-MIRA3) and energy dispersive X-ray (EDX; FESEM-TESCAN MIRA3). The SEM images of rGO-dithiooxamide/Au revealed a large irregular and folding structure (Figures 3a and 3b). Moreover, they are entangled with each other, their inter-planar distance being increased successfully. Subsequently, EDX mapping was carried out to analyze the chemical composition and formation of rGO-dithiooxamide/Au (Figure 3c). The results showed that the positions of EDX peaks were consistent with dithiooxamide/Au hybrid catalyst and the presence of Au NPs in the final catalyst was proven in this analysis. Also, EDX mapping showed the uniform distribution of elements on the dithiooxamide/Au hybrid catalyst (Figure 4).
Scanning electron microscopy (SEM) images (3a and 3b) and energy-dispersive X-ray (EDX) pattern (3c) of rGO-dithiooxamide/Au.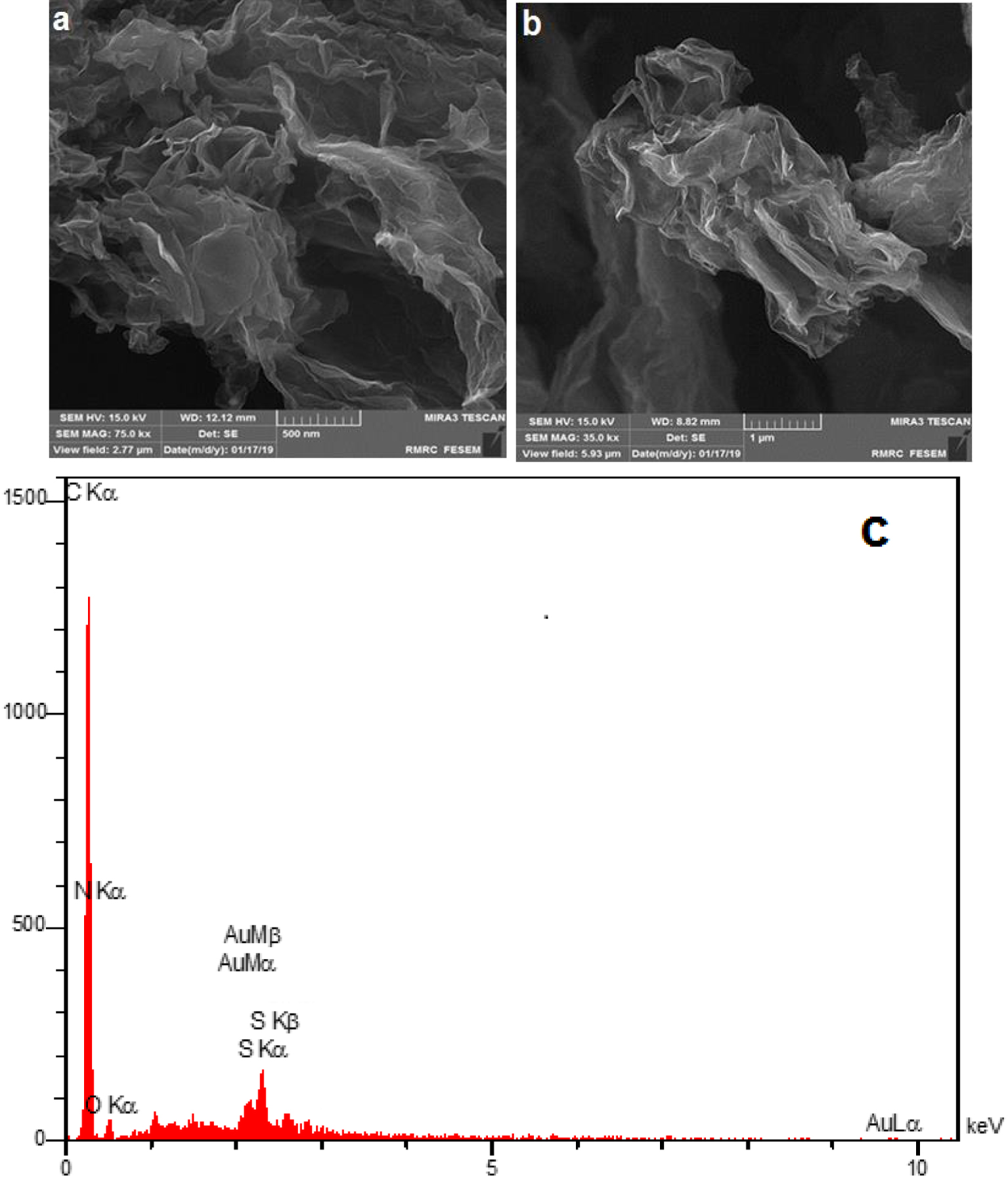
3.1.4. Transmission electron microscopy
TEM images (Figure 5) of the rGO-dithio- oxamide/Au were studied using transmission electron microscope (TEM; EM10c-100 kV). The modified rGO exhibited a typically wrinkled structure and ripped surface. Such an appearance may be partly attributed to their intrinsic nature and their 2D membrane structure. In addition, Au NPs were successfully coated on the surface of the modified rGO to form rGO-dithiooxamide/Au NPs. It is of note that Au NPs are spherical in shape with sizes between 6 and 30 nm.
3.2. Catalytic studies
The hybrid catalysis of rGO-dithiooxamide/Au was prepared via decoration of Au NPs on the surface of modified rGO as the support. The prepared catalyst was used in the oxidation of cyclohexene as an alkene with C=C and allylic positions. In this research, molecular oxygen was used as a green and economic oxidant that provided a superior alternative to the other mentioned oxidants because of its solvent-free conditions. Several products were obtained after cyclohexene oxidation reaction with molecular oxygen (Scheme 2). Considering that oxidation of allylic positions is more important because it produces 2-cyclohexene-1-ol and 2-cyclohexene-1-one, it was the aim of the present study. The product distribution was independent of temperature, O2 pressure, reaction time, and the amount of catalyst.
3.2.1. Reaction optimization
The prepared rGO-dithiooxamide/Au hybrid catalyst was used for the oxidation of cyclohexene, and this system was further optimized. To obtain the optimized reaction condition, the reaction was performed within a temperature rate of 60 to 90 °C, with O2 pressures ranging from 3 bar to 9 bar, and reaction times from 6 h up to 24 h (Table 1). First, the reaction proceeded under different temperatures (Figure 6). With increase in temperature, the reaction conversion and selectivity of 2-cyclohexene-1-one increased and reached 49% and 75% at 80 °C respectively. Considering the cyclohexene boiling point of 83 °C, the expected reaction conversion decreases over 90 °C due to substrate vaporization which diminishes substrate adsorption on the catalyst surface.
Based on the results of the present study, there was no conversion decrease at this temperature but 2-cyclohexene-1-one selectivity was reduced, leading to further oxidation of reaction mixture. Therefore, like in our previous work on the oxidation of cyclohexene [30, 31], 80 °C was chosen as the optimum temperature.
The conversion and selectivity of a cyclohexene oxidation reaction in rGO-dithiooxamide/Au catalytic system are plotted in Figure 7 as a function of pressure at 80 °C and 12 h.
Transmission electron microscopy (TEM) images of rGO-dithiooxamide/Au.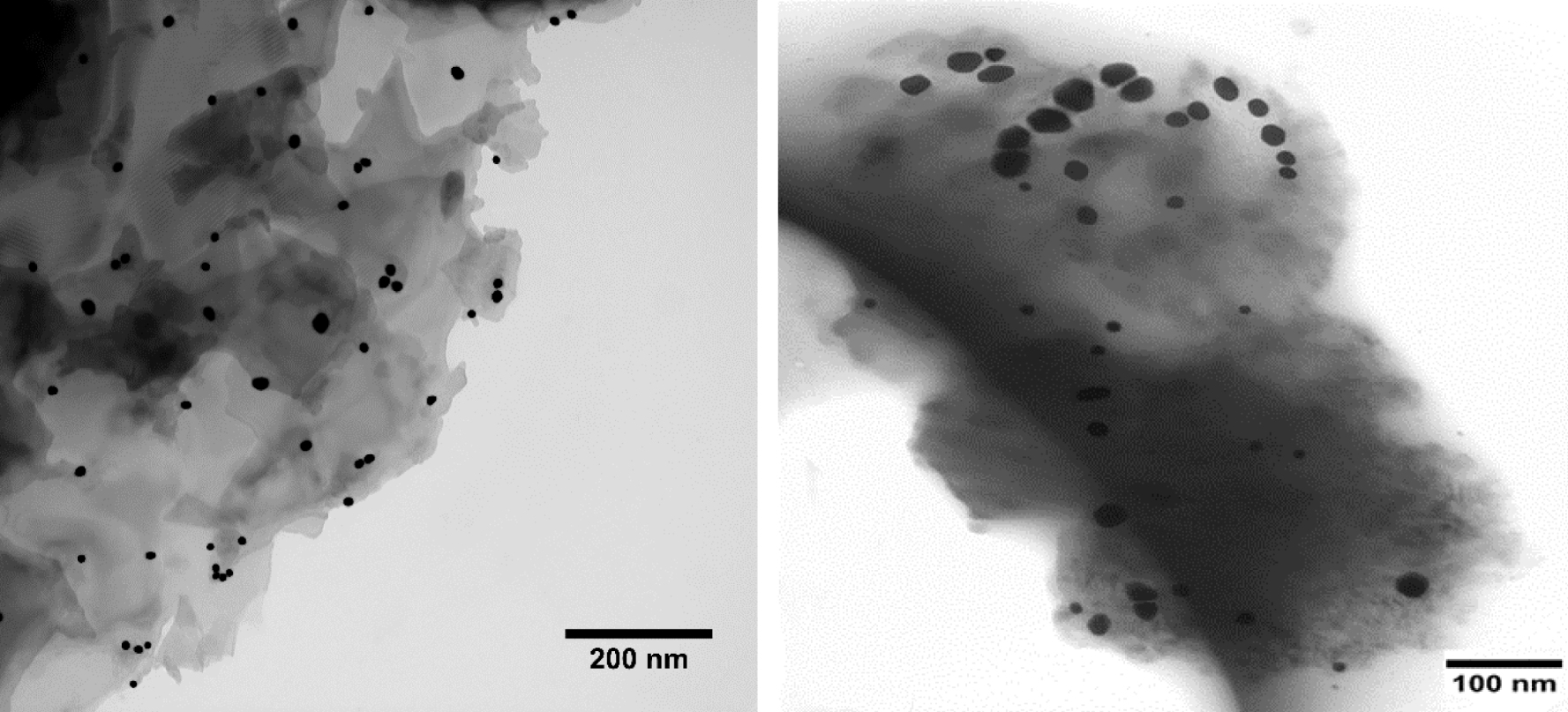
Selectivity of cyclohexenone and conversion of cyclohexene over rGO-dithio- oxamide/Au (15 mg), cyclohexene (5 mL), 5 bars O2, 12 h.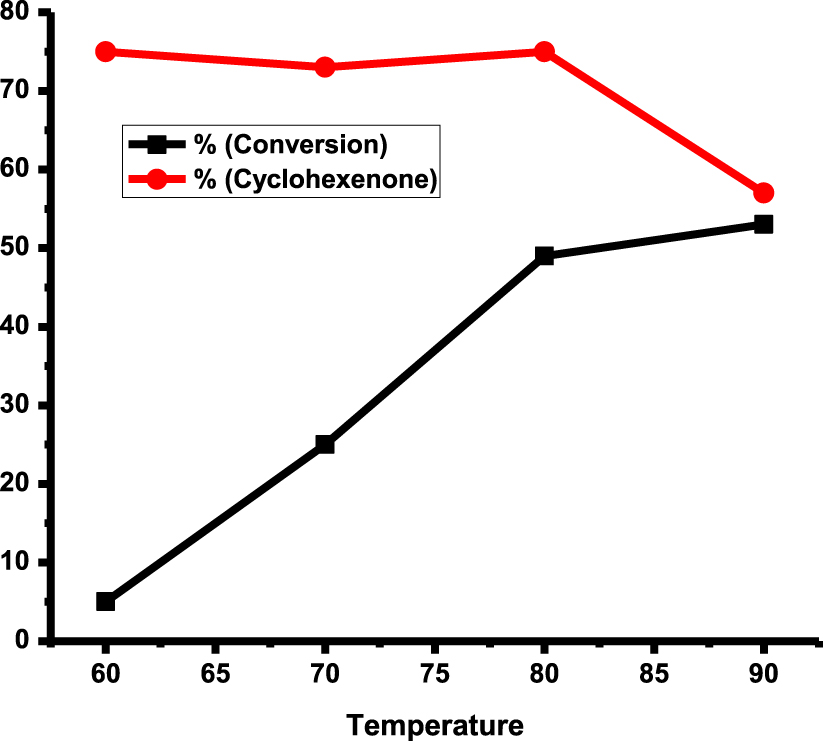
As can be seen, the best O2 pressure was 7 bar. By increasing the pressure to 9 bar, the conversion was decreased probably due to saturating the catalyst surface by oxygen. Here, there would be fewer sites for substrate and low selectivity in comparison to 7 bar, because of oxidation in an unsaturated position.
Selectivity of cyclohexenone and conversion of cyclohexene over rGO-dithio- oxamide/Au (15 mg), cyclohexene (5 mL), 80 °C, 12 h.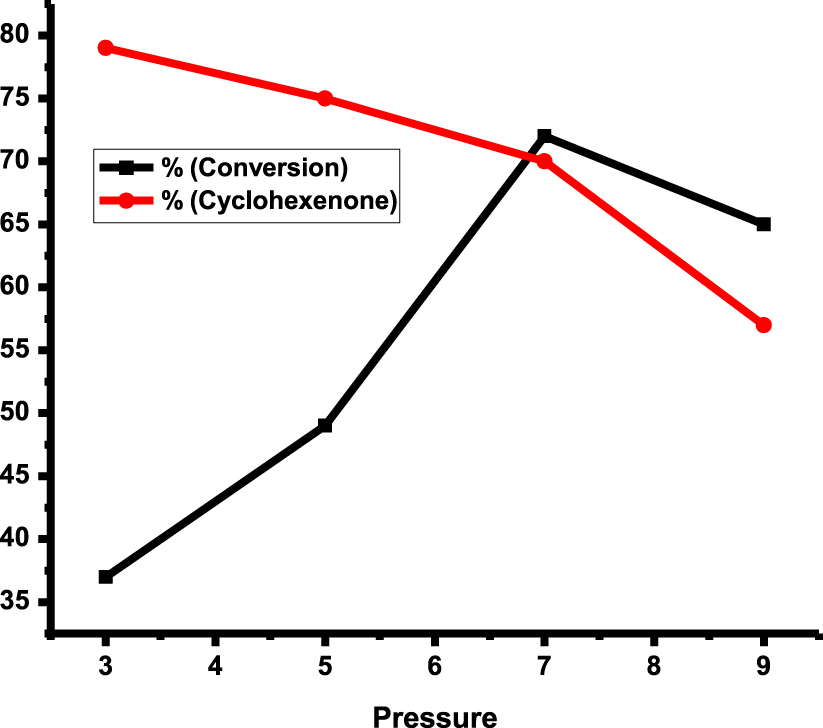
Next, the catalytic activity was studied as a function of time at 80 °C and 7 bar pressure (Figure 8). After 24 h, the substrate was almost fully transformed into the product, despite observing a large decrease in selectivity because of further oxidation of cyclohexenone to other products.
Selectivity of cyclohexenone and conversion of cyclohexene over rGO-dithio- oxamide/Au (15 mg), cyclohexene (5 mL), 80 °C, 7 bar O2.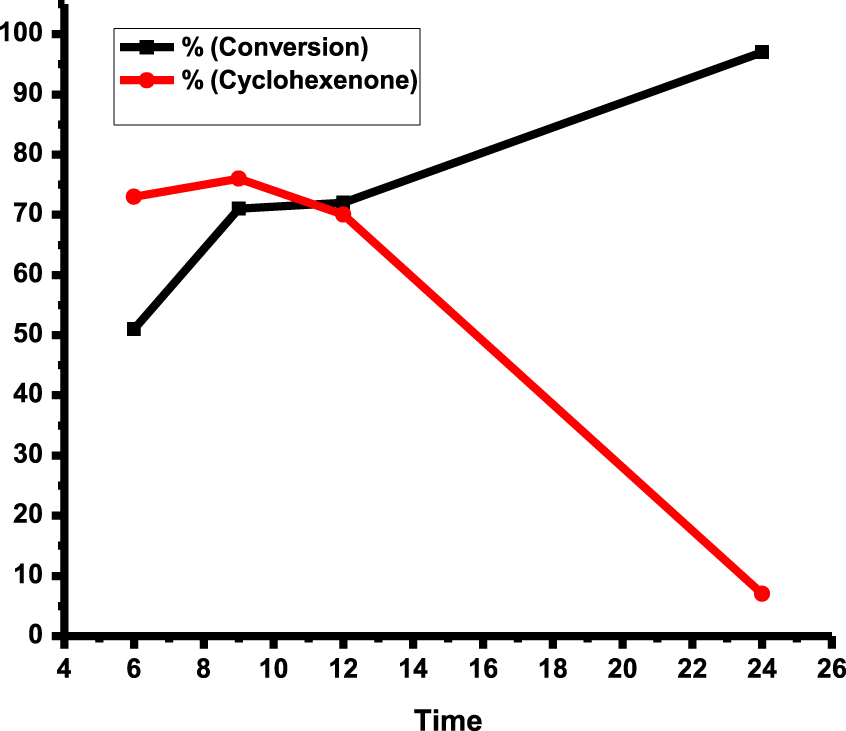
Oxidation of cyclohexene with molecular oxygen.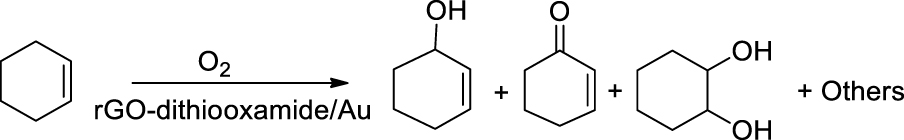
Oxidation of cyclohexene with rGO-dithiooxamide/Au hybrid catalyst under various reaction conditions
| Temperature | Pressure | Time | Amount of | Conversion | Selectivity (%) | |||
|---|---|---|---|---|---|---|---|---|
| Entry | (°C) | (bar) | (h) | catalyst (mg) | (%) | Cy-ol | Cy-one | Others |
| 1 | 60 | 5 | 12 | 15 | 5 | 25 | 75 | 0 |
| 2 | 70 | 5 | 12 | 15 | 25 | 27 | 73 | 0 |
| 3 | 80 | 5 | 12 | 15 | 49 | 25 | 75 | 02 |
| 4 | 90 | 5 | 12 | 15 | 53 | 28 | 57 | 15 |
| 5 | 80 | 3 | 12 | 15 | 37 | 21 | 79 | 0 |
| 6 | 80 | 7 | 12 | 15 | 72 | 21 | 70 | 9 |
| 7 | 80 | 9 | 12 | 15 | 71 | 20 | 64 | 15 |
| 8 | 80 | 7 | 24 | 15 | 97 | 26 | 7 | 67 |
| 9 | 80 | 7 | 9 | 15 | 71 | 20 | 76 | 4 |
| 10 | 80 | 7 | 6 | 15 | 51 | 24 | 73 | 3 |
| 11 | 80 | 7 | 9 | 20 | 69 | 23 | 67 | 7 |
| 12 | 80 | 7 | 9 | 10 | 85 | 15 | 85 | 0 |
| 13 | 80 | 7 | 9 | 5 | 61 | 31 | 69 | 0 |
To evaluate the effect of the amount of catalyst, this reaction was performed with different amounts of catalyst at 80 °C, 7 bar O2, and 9 h (Figure 9). The results revealed that the best conversion and selectivity were obtained using 10 mg catalyst. Finally, the optimum reaction conditions were selected in the oxidation of cyclohexene as follows: 80 °C, 8 h, and 7 bar O2 pressure, and 10 mg rGO-dithiooxamide/Au hybrid catalyst.
Selectivity of cyclohexenone and conversion of cyclohexene over, cyclohexene (5 mL), 80 °C, 7 bar O2, and 9h.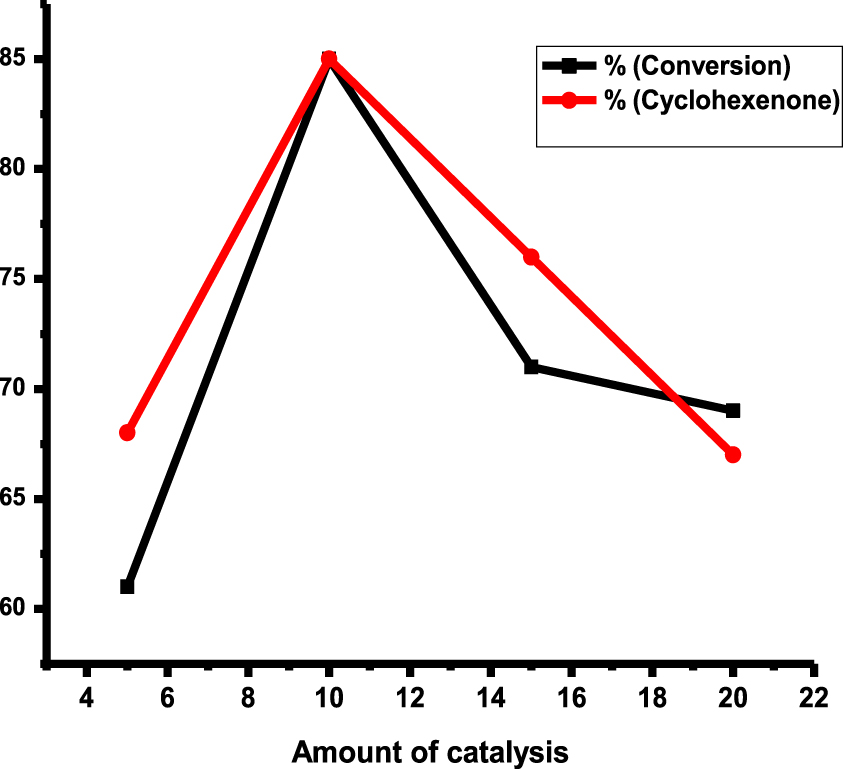
Catalyst activities of rGO-dithiooxamide/Au and some previously reported catalysts for cyclohexene oxidation
| Catalyst | Time | Temperature | Pressure | Cyclohexene | Conversion | Selectivity (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst | amount (mg) | (h) | (°C) | (bar) | amount (mL) | (%) | Cy-OH | Cy-C = O | Others | Ref |
| P-S–Co-Na | 1.5 | 12 | 70 | 1 | 2 | 91.4 | 51.3 | 41.4 | 7.3 | [40 ] |
| Co-resinate | 2000 | 7 | 70 | - | 25 | 94.5 | 40.2 | 44.4 | 15.4 | [41 ] |
| Au-OMS | 200 | 24 | 80 | 4 | 20 | 39.9 | 35.5 | 51.2 | 13.3 | [42 ] |
| PS-DD-Mn | 2 | 10 | 70 | 1 | 2 | 30 | 34 | 22.7 | 43.3 | [43 ] |
| PS-DD-Cu | 2 | 10 | 70 | 1 | 2 | 43 | 22.6 | 28.7 | 51.3 | [43 ] |
| PS-DD-Co | 2 | 10 | 70 | 1 | 2 | 29.7 | 37.8 | 32.1 | 30.1 | [43 ] |
| PS-DD-Ni | 2 | 10 | 70 | 1 | 2 | 24.2 | 26 | 11.4 | 37.4 | [43 ] |
| Au- HNTS | 200 | 12 | 80 | 4 | 20 | 29.5 | 35.3 | 49 | 15.5 | [44 ] |
| Au-C | 200 | 24 | 80 | 3 | 20 | 24 | 23.6 | 28.4 | 48 | [45 ] |
| Au-Bentonite | 50 | 10 | 80 | 8 | 10 | 53 | 34 | 63 | 3 | [15 ] |
| GNPs/TChD | 50 | 8 | 80 | 17 | 20 | 87 | - | 70 | 30 | [46 ] |
| rGO-dithiooxamide/Au | 10 | 9 | 80 | 7 | 5 | 85 | 15 | 85 | - | This study |
Catalytic activity of rGO-dithiooxamide/Au compared to other previously reported catalysts for oxidation of cyclohexene has been reported in Table 2. The rGO-dithiooxamide/Au exhibit higher activity compared to supported different Schiff-base metal complexes on polymeric materials [40, 41, 42, 43] and other nanogold-based catalysts [44, 45, 46, 15].
3.3. Catalytic stability and reusability
One of the main advantages of heterogeneous catalysts over homogeneous is that the former can be recovered and reused. The catalyst was recovered from the reaction mixture after each cycle using centrifugation. Then, the surface of the catalyst was washed three times with methanol. The catalyst was sonicated and isolated by centrifugation after each use, and finally dried at ambient temperature. The recovered catalyst was used in the oxidation reaction of cyclohexene under optimum reaction conditions. The results showed that the conversion and selectivity reaction remained unchanged after four cycles (Figure 10). According to ICP analysis, the content of Au was 0.84 wt% in the fresh catalyst and 0.80 wt% in the 5-times reused recovered catalyst. This result indicates that Au NPs were linked with functional groups on the surface of rGO and were not easily separated from it or agglomerated.
Recycling of rGO-dithiooxamide/ Au hybrid catalyst. Reaction condition: cyclohexene 5 mL; catalyst 10 mg; pressure 7 bar; time 9 h; and temperature 80 °C.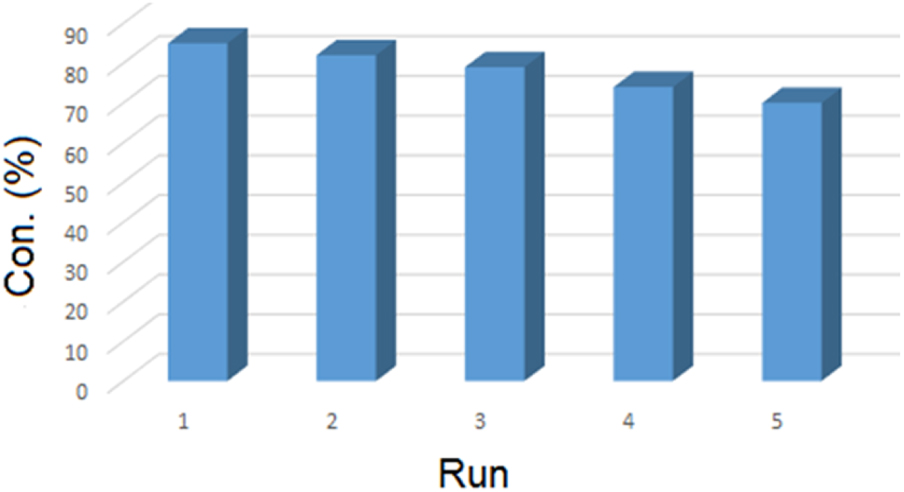
4. Conclusion
In this work, GO and rGO were synthesized based on a modified Hummers method and a chemical process respectively. Next, the surface of rGO was promoted with an organic agent and used as a support for immobilizing the Au NPs. The size of Au NPs on rGO-dithiooxamide/Au hybrid catalyst was in the range of 6 to 14 nm. This nanocatalyst was applied in the oxidation of cyclohexene under solvent-free conditions. The results showed its good catalytic activity (i.e., 85% conversion and 85% selectivity to 2-cyclohexene-1-one). Moreover, this catalyst was successfully recovered and reused for four runs with a negligible loss of activity (Figure 10).




 CC-BY 4.0
CC-BY 4.0