1. Introduction
Oxidation reactions including low-cost and non-toxic transition metallic catalysts and green oxidants such as H2O2 have been extensively studied [1, 2, 3, 4]. We have shown previously that iron, manganese, copper, cobalt and nickel 1,3-bis(2′-Ar-imino)isoindoline complexes can mediate many types of redox reactions depending on their oxidation state, redox potential and the choice of oxidant [5]. Depending on the substrate, these reactions have been used as functional models of superoxide dismutase [6, 7, 8], catalase [9, 10, 11], phenoxazinone synthase, catechol oxidase [12], catechol and flavonol dioxygenase enzymes [13, 14]. Furthermore, their iron complexes can be used as catalysts for the oxidation of alcohols and sulfides [15, 16]. The activity of the hydrogen peroxide-based bleach reactions can also be increased by the use of transition metal-based catalysts [17, 18, 19]. These include mainly manganese and iron complexes with salen, macrocyclic tetraamido and other polypyridyl-type ligands [20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30]. Recently, we synthesized a series of divalent manganese complexes MnII(HL1−6) with the 1,3-bis(2′-Ar-imino)isoindolines and which are used as bleaching catalysts [31]. We have shown that the activity of the catalysts is significantly influenced by the Lewis acid and redox properties of the metal centers which can be controlled by varying the aryl substituent on the bis-iminoisoindoline moiety. The great variability of possible metal and ligand might result in a large variety of complexes with tunable catalytic properties. As a continuation of these studies, efforts have been made to evaluate the bleaching potential of our previously prepared [FeIII(L1−4)Cl2] complexes by the use of morin as a model compound for bleaching stain, to understand how the parameters influence the activity and how the catalytic activity can be optimized compared to our manganese system (Scheme 1). These kinds of catalytic systems are increasingly important for many applications such as pulp and paper bleach and waste water treatment.
2. Experimental section
2.1. Materials and methods
The ligands 1,3-bis(2′-Ar-imino)isoindolines (HLn, n = 1–4, Ar = pyridyl, thiazolyl, benzimidazolyl and N-methylbenzimidazolyl, respectively) and their complexes [FeIII(L1−4)Cl2] (1–4) were prepared according to published procedures [5]. UV/Vis spectra were recorded with an Agilent 8453 diode-array spectrophotometer with quartz cells.
2.2. Description of the catalytic bleaching
Bleaching experiments were carried out as described previously for 1,3-bis(2′-Ar-imino)isoindoline manganese complexes to allow comparison of the two systems. Kinetics of morin bleach were monitored on an Agilent 8453 diode-array spectrophotometer using thermostated quartz cells equipped with a magnetic stirring unit. The initial absorbance of morin in buffer solution was measured, and its bleaching reaction was followed as the decrease in absorbance at 410 nm. In a typical experiment, the cuvette (l = 1 cm) was filled with 3 mL buffer solution containing 0.62 μM catalyst, 160 μM morin, and the bleaching reaction initiated by adding 10 mM H2O2 into the reaction mixture (Tables 1 and 2). The pH was adjusted by 50 mM sodium carbonate–bicarbonate
Structure of the 1,3-bis(2′-Ar-imino)isoindoline ligands used for the synthesis of [FeIII(L1−4)Cl2] (1–4) catalysts involved in this study.
Summary of kinetic data for the catalytic oxidation of morin with [FeIII(L1)Cl2] (1) in sodium carbonate–bicarbonate
| [1] (10−7 M) | pH | [H2O2] (10−3 M) | [ | [Morin] (10−4 M) | kobs (10−3 s−1) | kox (107 M−3⋅s−1) |
|---|---|---|---|---|---|---|
| 6.2 | 8.5 | 10 | — | 1.6 | 0.691 | — |
| 6.2 | 10 | 10 | 50 | 1.6 | 3.20 | 1.03 ± 0.03 |
| 16 | 10 | 10 | 50 | 1.6 | 8.10 | 1.01 ± 0.04 |
| 25 | 10 | 10 | 50 | 1.6 | 12.63 | 1.01 ± 0.06 |
| 6.2 | 10 | 7.5 | 50 | 1.6 | 2.43 | 1.04 ± 0.03 |
| 6.2 | 10 | 5 | 50 | 1.6 | 1.64 | 1.05 ± 0.02 |
| 6.2 | 10 | 12 | 50 | 1.6 | 3.90 | 1.04 ± 0.04 |
| 6.2 | 10 | 10 | 100 | 1.6 | 6.41 | 1.03 ± 0.02 |
| 6.2 | 10 | 10 | 200 | 1.6 | 12.53 | 1.01 ± 0.04 |
| 6.2 | 10 | 10 | 300 | 1.6 | 18.76 | 1.01 ± 0.04 |
| 6.2 | 10 | 10 | 50 | 1.2 | 3.27 | 1.05 ± 0.02 |
| 6.2 | 10 | 10 | 50 | 0.8 | 3.23 | 1.04 ± 0.03 |
| 6.2 | 10 | 10 | 50 | 0.4 | 3.26 | 1.05 ± 0.01 |
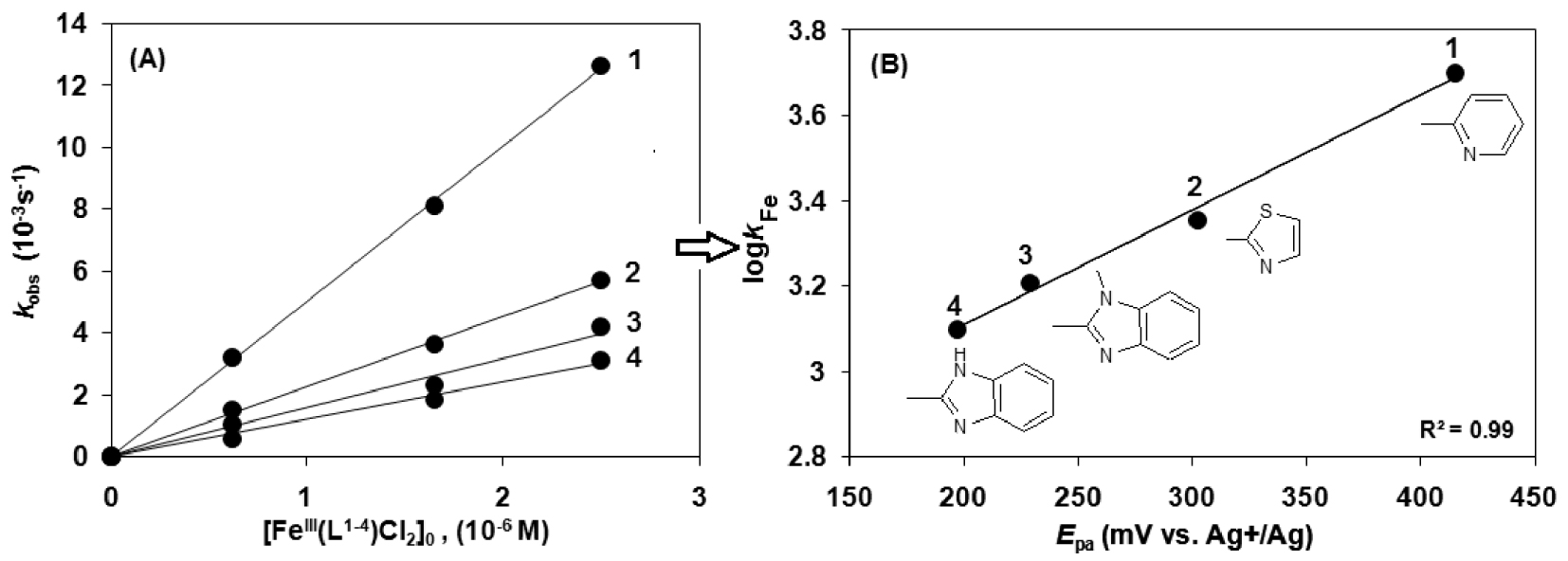
Summary of kinetic data for the catalytic oxidation of morin with [FeIII(L1−4)Cl2] (1–4) in sodium carbonate–bicarbonate
| Cat. | kFe (103 s−1) | kox (107 M−3⋅s−1) | Epa (FeIII∕FeII) (mV) |
|---|---|---|---|
| 1 | 5.011 | 1.03 | 415 |
| 2 | 2.253 | 0.45 | 302 |
| 3 | 1.615 | 0.32 | 229 |
| 4 | 1.255 | 0.25 | 197 |
(
2.3. Description of the catalase-like activity
Catalytic reactions were performed at 20 °C in a 30 mL thermostated reactor connected with a graduated burette filled with oil. To determine the time dependence of H2O2 decomposition, 0.211 mM (final concentration) of the complex dissolved in 1 mL DMF was added to 19 mL of a stirred sodium carbonate–bicarbonate
3. Results and discussion
Porphyrin and phthalocyanine metal complexes are known for many transition metals, but iron and manganese metals are the most suitable choices for a wide range of oxidation reactions due to their variable oxidation states and readily tunable properties such as Lewis acidity and redox potentials [32, 33, 34, 35]. The chemical modification of porphyrin led to the 1,3-bis(2′-Ar-imino)isoindolines, where the solubility, electronic structure and steric properties of the ligand as well as their complexes can be easily tuned, introducing various aryl groups on the bis-iminoisoindoline moiety [5]. This system resulted in several oxidation catalysts with high catalytic efficiencies and selectivities. Recently we reported a kinetic study of H2O2 and morin oxidation in aqueous solution by 1,3-bis(2′-Ar-imino)isoindoline manganese complexes, where both the catalase-like and the bleaching activity showed a linear correlation with the MnIII∕MnII redox potentials, and were remarkably influenced by the concentration of the bicarbonate ions [31]. We now report a kinetic study of the H2O2 and morin oxidation by 1,3-bis(2′-Ar-imino)isoindoline iron(III) complexes and compare the reactions of the manganese and iron catalysts.
3.1. Catalytic oxidation of morin
The bleaching activity of [FeIII(L1−4)Cl2] (1–4) was investigated in the oxidation of morin dye as a model marker for wine stains, utilizing H2O2 as the cooxidant and bicarbonate as additive at 25 °C (Scheme 2). It is well known that H2O2 reacts with bicarbonate anion to generate the peroxymonocarbonate which is a promising oxidant for bleaching [36].
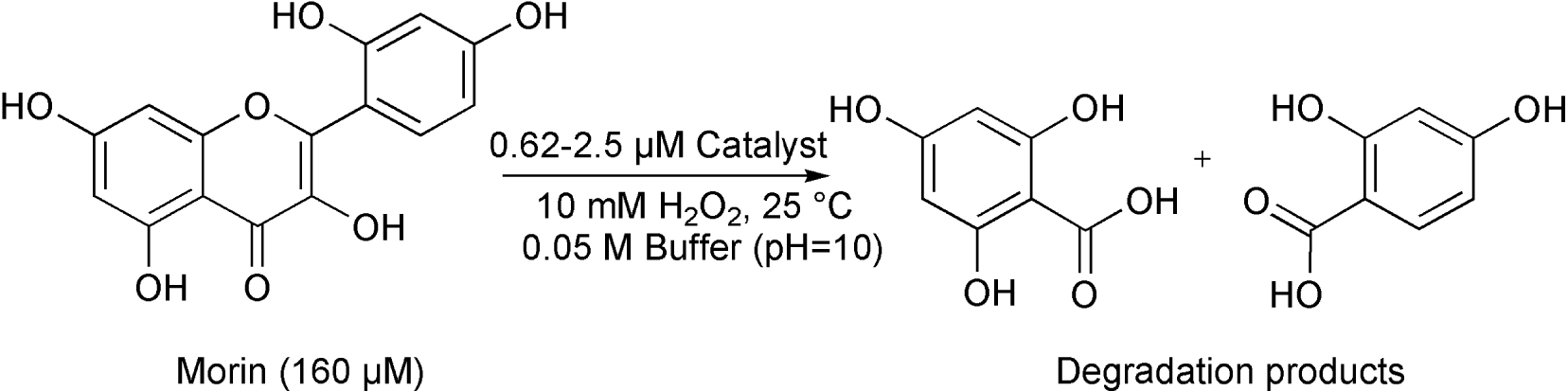
Catalytic oxidation of morin by [FeIII(L1−4)Cl2] (1–4) complexes.
Comparison of the [FeIII(L1−4)Cl2] (1–4) catalyzed disproportionation reaction of H2O2 in sodium carbonate–bicarbonate
| Cat. | Yield (%) | TONa | TOFb (h−1) | V0 (10−4 M⋅s−1) | |
|---|---|---|---|---|---|
| 1 | 415 | 21 | 451 | 9216 | 5.80 |
| 2 | 302 | 17 | 376 | 7164 | 3.95 |
| 3 | 229 | 11 | 239 | 5292 | 2.81 |
| 4 | 197 | 9 | 199 | 4428 | 2.30 |
| FeCl3 | — | 3.5 | 75 | — | — |
([Fe] = 0.211 mM and [H2O2] = 0.447 M).
a Turnover number = moles of converted substrate (H2O2)/moles of catalyst.
b Turnover frequency = TON/h.
Bicarbonate dependence of the [FeIII(L1)Cl2] (1) catalyzed disproportionation reaction of H2O2 in sodium carbonate–bicarbonate (
| Cat. | Yield (%) | V0 (10−4 M⋅s−1) | |
|---|---|---|---|
| 1 | 0.05 | 21 | 5.80 |
| 1 | 0.1 | 22 | 6.41 |
| 1 | 0.3 | 24 | 9.44 |
| 1 | 0.5 | 25 | 11.1 |
| 1 | — | 3.5 | — |
([Fe] = 0.211 mM and [H2O2] = 0.447 M).
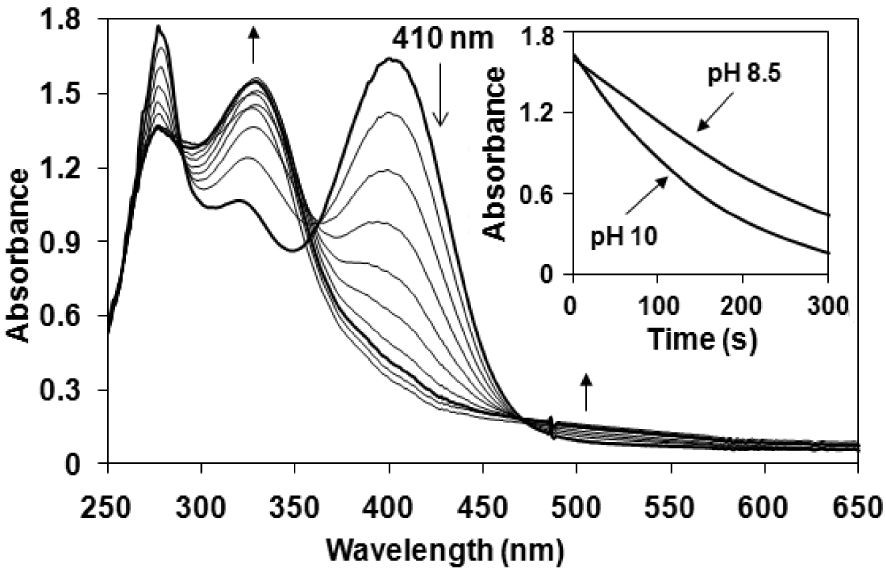
Time-dependent UV–Vis spectra of a 0.16 mM solution of morin at pH 10 in the presence of 10 mM H2O2 and 0.62 μM [FeIII(L1)Cl2] (1) at 25 °C. (Inset) Time course of the bleaching activity at pH 10 and pH 8.5.
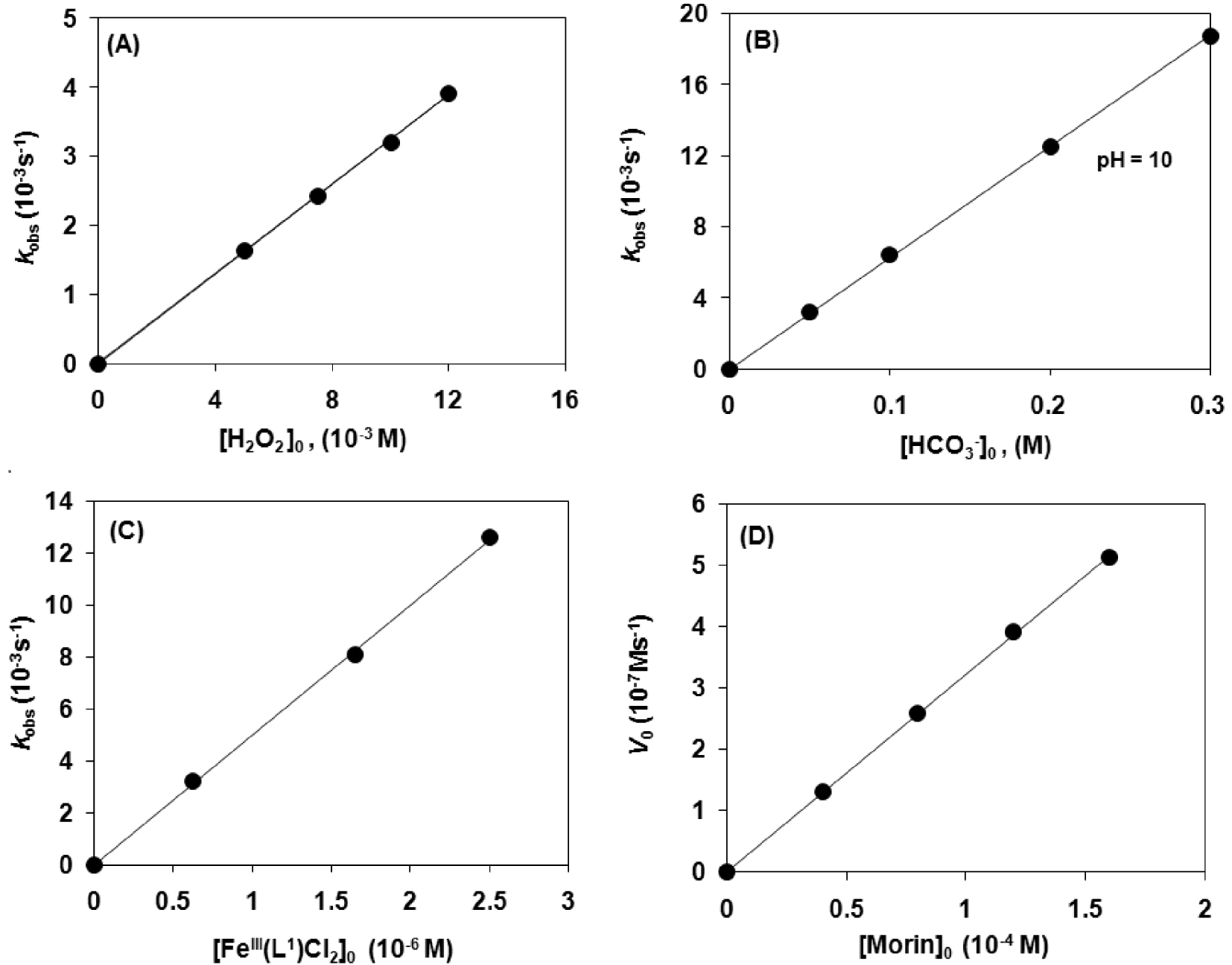
Catalytic oxidation of morin with [FeIII(L1)Cl2] (1) in sodium carbonate–bicarbonate
First we investigated the effect of the pH value of the aqueous solution and found that the best activity was observed at pH 10 for 1 (Figure 1). Lowering the pH resulted in a significant drop in the observed reaction rates (kobs = 3.2 × 10−3 s−1 at pH 10, and 0.691 × 10−3 s−1 at pH 8.5). This finding is consistent with the protonation state of the substrate (pKa values of morin are 3.5 and 8.1) and H2O2 (the concentration of HOO− is high at pH 10), factors that determine the reactivity [19]. Under these conditions the bleaching of morin was completed within 5 min with approximately 45 catalytic cycles per minute (Figure 1). A similar, but slightly smaller value (∼20) was observed for the analogous manganese(II) complex [MnII(L1)Cl2] [31]. However, the bleaching activity of the 1,3-bis(2′-Py-imino)isoindoline iron(III) complex is at least 15–20 times smaller than that of manganese terpyridine (TOF ∼ 800/min), and one order of magnitude smaller than that of iron phthalocyanine complexes [22].
To get more insight into the mechanism of this important reaction, detailed kinetic measurements were carried out. In order to determine the rate dependence on the various reactants, oxidation runs were performed with only the initial concentration of one reactant being varied and the other three initial concentrations remaining the same (pseudo-first-order conditions). The concentrations related to the investigation of catalyst dose variations were as follows; [1]:[H2O2]:[
Based on these results, the rate of morin decomposition can be described by the following equation,
Similarly to our system, the non-innocent role of the phosphate and sodium carbonate–bicarbonate

Proposed mechanism of bleaching reaction and H2O2 disproportionation.
The increase in activity in the presence of
The beneficial effect of
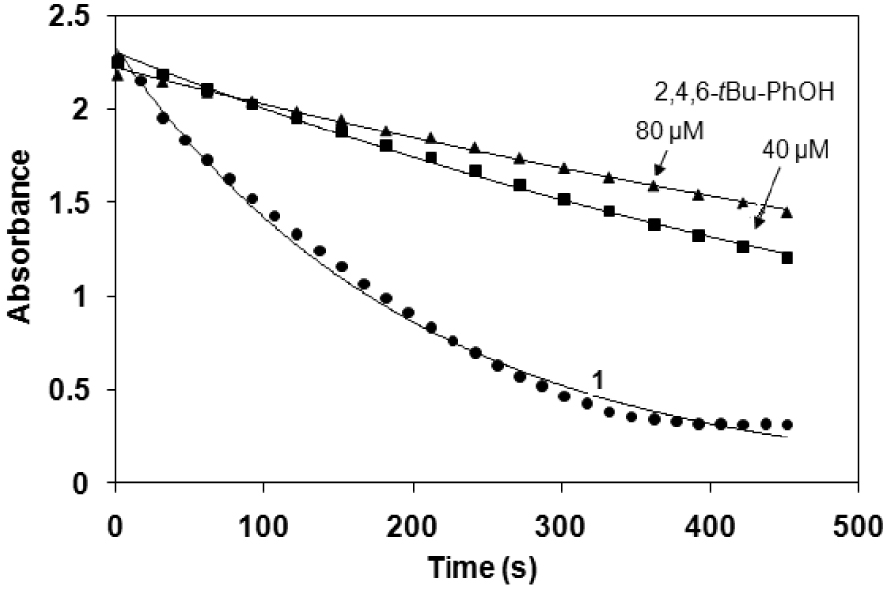
Time course of the bleaching activity in the absence and in the presence of 2,4,6-tBu-PhOH at pH 10 and 25 °C ([1] = 0.62 μM and [H2O2] = 10 mM).
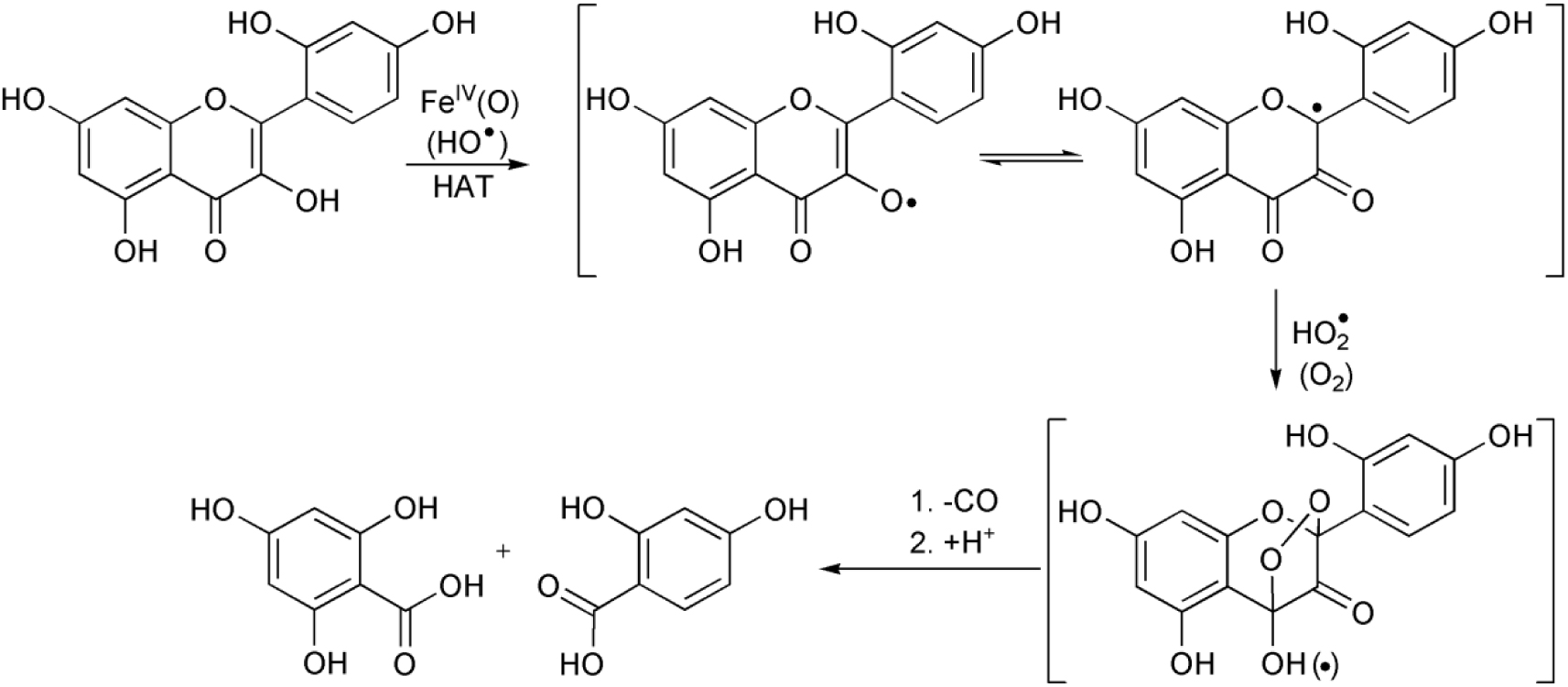
Proposed mechanism of the morin oxidation.
The modification of the ligand in the model catalysts (pyridyl (1), thiazolyl (2), benzimidazolyl (3) and N-methylbenzimidazolyl (4) show a decreasing reaction rate in the listed order (Figure 4A). The cyclic voltammograms of the catalysts 1–4 exhibited a decreasing oxidation potential in DMF, 1 = 415, 2 = 302, 3 = 222 and 4 = 197 mV (100 mV⋅s−1 scan rate, Fc/Fc+ internal standard, 0.1 M) (Table 3) [13, 44]. The decrease in the redox potential stabilizes the Fe(III) state, while the increase in it makes the Fe(III) center more easily reducible, stabilizing the iron(II) form. This is in agreement with the assumption that a faster reaction rate is observed with higher oxidation potential of the catalyst (1) (Figure 4B). Figure 4B shows that complex 1 with pyridine arms is the best oxidant and has the highest kFe among these complexes.
Catalytic oxidation of morin with [FeIII(L1−4)Cl2] (1–4) in sodium carbonate–bicarbonate
3.2. Catalytic oxidation of H2 O2
Our model experiments with morin revealed that the complexes are relatively stable under aqueous alkaline conditions in the presence of hydrogen peroxide and bicarbonate ions, and the optimal pH for bleaching activity is around 10, where the reactivity values are slightly higher than those of analogous manganese complexes. To get high bleach (or oxidation) performance, one of the most important criteria of the catalysts, is that it must possess a low activity to catalyze the non-productive decomposition of H2O2. This process is the major side reaction of the transition metal catalysts. To test the catalase-like activity of the 1,3-bis(2′-Ar-imino)isoindoline-iron(III) complexes the reactions were performed in alkaline aqueous solution at pH 9.6 (sodium carbonate–bicarbonate
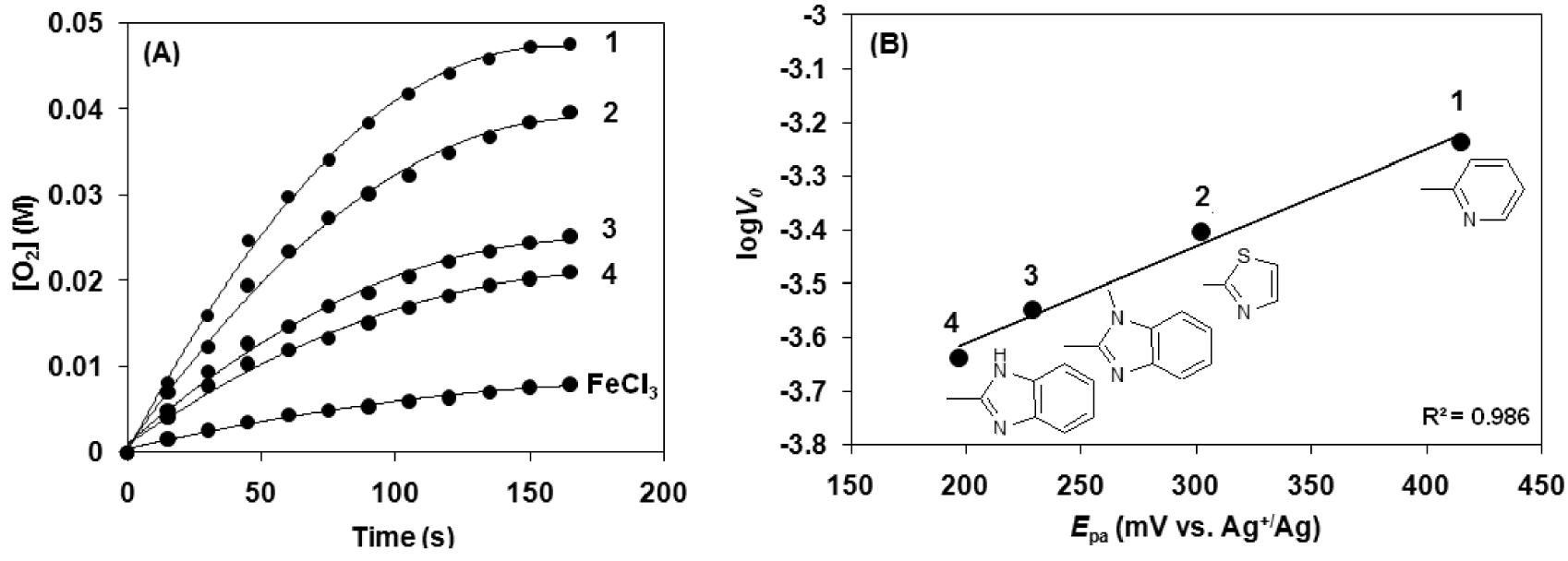
Kinetic analysis for the hydrogen peroxide disproportionation catalyzed by [FeIII(L1−4)Cl2] (1–4) complexes in sodium carbonate–bicarbonate
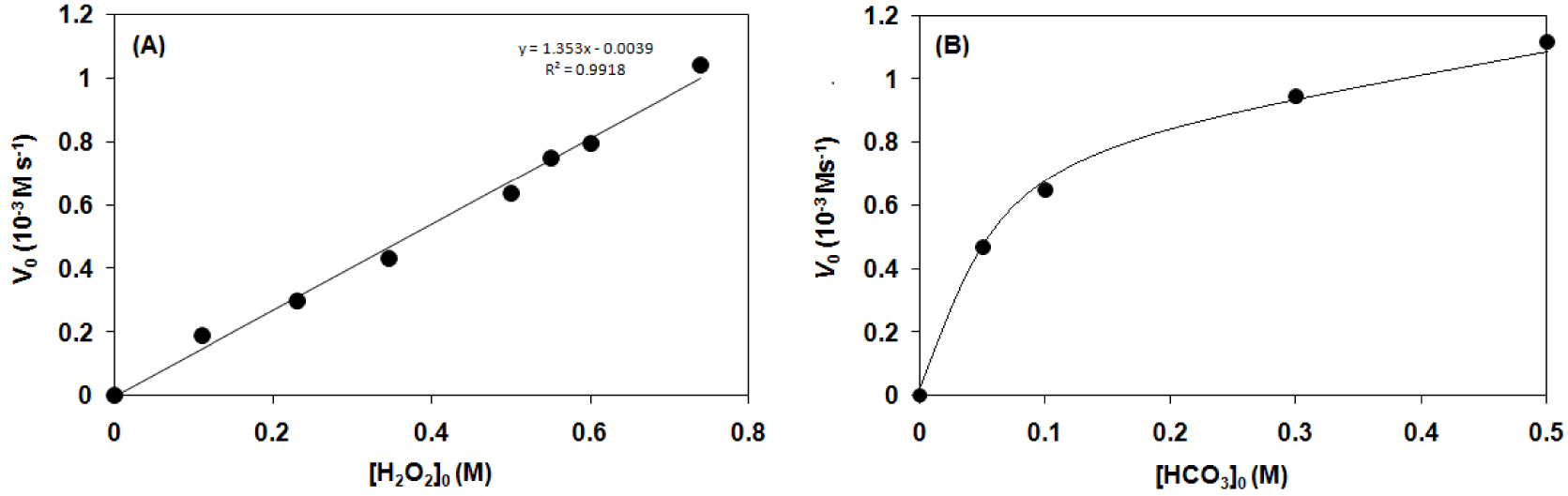
Kinetic analysis for the hydrogen peroxide disproportionation catalyzed by [FeIII(L1)Cl2] (1–4) complexes in sodium carbonate–bicarbonate
Based on kinetic studies, the reaction rate is directly proportional to the concentration of H2O2 and shows Michaelis–Menten-type saturation kinetics on
4. Conclusion
We have found that our simple 1,3-bis(2′-Ar-imino)isoindoline iron(III) complexes effectively oxidize the morin in aqueous solution under mild conditions in the presence of bicarbonate ions. We have also found that increasing the Lewis acidity and oxidation potentials (Epa) of the catalyst by introducing pyridine arms on the bis-iminoisoindoline moiety significantly improves the bleach performance in model experiments under European washing conditions at 25 °C. The optimal pH for bleaching activity is about 10, and our catalysts possess a relatively low activity for the disproportionation of hydrogen peroxide. Based on detailed kinetic measurements the bleaching rates depend on the initial concentrations of each reactant. Since the sterically hindered phenols inhibited the reaction, the radical chain mechanism cannot be excluded. The beneficial effect of bicarbonate ions might be explained by the reduction of iron(III) to iron(II) complexes, and the formation of the peroxymonocarbonate monoanion which is a much stronger oxidant than H2O2, and could easily form a catalytically active high-valent oxoiron species with the iron(II) form. Similar results, trends, and mechanisms were observed for the analogous 1,3-bis(2′-Ar-imino)isoindoline manganese(II) complexes, but with lower bleaching and slightly higher catalase-like activities.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support of the Hungarian National Research Fund (OTKA K108489), TKP2020-IKA-07 and GINOP-2.3.2-15-2016-00049 are gratefully acknowledged.




 CC-BY 4.0
CC-BY 4.0
Vous devez vous connecter pour continuer.
S'authentifier