1. Introduction
Perhaps most of us have forgotten what a unique figure of a scientist Alessandro Volta was in the XVIII century, an individual who dominated the horizon in its second half. In 1775 he had already invented the electrophor, a discovery that, when communicated to the English chemist Joseph Priestley, generated a genuine enthusiasm in the scientific world. A year later, in 1776, he discovered methane gas (dubbed by him “inflammable air”). In 1778, in a letter written to de Saussure, by the title “Sulla capacità dei conduttori elettrici”, he introduced for the first time the concept of electrical tension (today named potential differential).
By all means, though, his major and unique contribution to science was the discovery of the “pila” (dubbed by him “apparato elettromotore” or “apparato a Colonna”), indeed the very first power supply generating a continuous current and a potential differential. He communicated his invention to the president of the Royal Society, Sir Joseph Banks, in a letter dated 20 March 1800 that was published in the “Philosophical Transactions” with the title “On the Electricity excited by the mere Contact of conducting Substances of different Kinds”. He also named his invention “organo elettrico artificiale” in comparison with the natural electric organ of Raja Torpedo. It did not go unnoticed. An engraving of 1820 shows Hans Christian Oersted performing the first experiment demonstrating the correlation between electric current and magnetic fields. In the foreground, Volta’s “pila”. Immediately afterwards, Ampère repeated his experiments and derived his first law. The same occurred with Michael Faraday in 1821. Appreciation for his invention flocked in. François Arago: “Je disais, Messieurs, tout à l’heure avec quelque timidité, que la pile est le plus merveilleux instrument qu’ait jamais créé l’intelligence humaine”. Albert Einstein in 1927, the first centenary of Volta’s death: “the pila is the fundamental base of all modern inventions” [1, 2].
The Istituto Lombardo di Scienze e Lettere (ILSL, in Milano), of which Napoleon appointed him as president in 1802, stores a vast collection of Volta’s scripts, numbering a few thousands. We were allowed to inspect a number of these documents and noted that these writings are composed by very dense ink and display a very intense blackish–reddish tinge. We have been wondering if, in addition to all his inventions illustrated above, Volta might have manufactured his own personal ink of secret composition.
The non-invasive approach to study cultural heritage objects, precious artworks and ancient manuscripts is always preferred [3, 4, 5]. Thus, with the help of the EVA technology (ethylene vinyl acetate diskettes studded with strong cation and anion exchangers, as well as with C8 and C18 hydrophobic resins) [6, 7] we have been able to harvest ultra-minute amounts of ink and analyse it via GCXGC/TOFMS (gas chromatography coupled to time-of-flight mass spectrometry). The results are illustrated below.
2. Materials and methods
2.1. Archive material
The following pages from the Volta’s archive have been analysed by application of EVA diskettes (1 h contact): H40 folder pages 6 and 12; J34 folder pages 3 and 6 and a large ink spot on page 5. All these documents were written in the year 1796.
2.2. Diskette’s elution and sample derivatization
After EVA application on the surface of the four pages, the captured molecules were eluted from the film and then prepared by using the same protocol reported by Barberis et al. [8]. Basically, elution was implemented by using 1 mL of ethanol for 30 min under sonication. Then, the strips were removed and the metabolites were subjected to derivatization, which was accomplished by adding 20 μL of methoxamine hydrochloride in pyridine (20 mg/mL) and 50 μL of N,O-Bis(trimethylsilyl) trifluoroacetamide (BSTFA). The samples were incubated at 80 °C for 20 min, and then centrifuged for 15 min at 14,500g. Tridecanoic acid (1 ppm) and hexadecane (0.1 ppm) standard solutions were added as internal standards before derivatization and GCxGC–MS analyses, respectively.
2.3. Analyses via GCXGC/TOFMS
A LECO Pegasus 4D GCXGC/TOFMS instrument (Leco Corp., St. Josef, MI, USA) equipped with a LECO dual stage quad jet thermal modulator was used to carry out the analyses. The GC part of the instrument was an Agilent 7890 gas chromatograph (Agilent Technologies, Palo Alto, CA), equipped with a split/splitless injector. The first column was a 30 m Rxi-5Sil (Restek Corp., Bellefonte, PA) MS capillary column with an internal diameter of 0.25 mm and a stationary phase film thickness of 0.25 μm, and the second dimension chromatographic column was a 2 m Rxi-17Sil MS (Restek Corp., Bellefonte, PA) with a diameter of 0.25 mm and a film thickness of 0.25 μm. The carrier gas (Helium) was used with a flow rate of 1.4 mL/min. One μL of sample was injected in splitless mode with the following program: initial temperature 40 °C, 5 min isothermal, 8 °C/min up to 300 °C, 20 min isothermal. The secondary column was maintained at +5 °C relative to the GC oven temperature of the first column. The MS parameters were: electron impact ionization source temperature (EI, 70 eV) at 250 °C; scan range from 40 to 630 m/z, with an extraction frequency of 32 kHz. The acquisition rate was 200 spectra/s and the modulation period was maintained at 4 s for the entire run. The modulator temperature offset was set at +15 °C relative to the secondary oven temperature, while the transfer line was set at 280 °C. The chromatograms were acquired in TIC (total ion current) mode. The mass spectral assignment was performed by matching with NIST MS Search 2.3. libraries, implemented with the MoNa Fiehns libraries. During data processing method, the similarity score calculation in library searching was set at >700 (index of identification reliability), in order to select only the higher library hits which were correctly assigned with high confidence [9].
The left side shows the entire page 6 of the H40 folder, whereas the right side shows a detail of the area on which the EVA diskette was applied (number 6, smudged) with a red arrow indicating the covered zone.
In order to confirm the vegetable origin of Volta’s ink, natural colophony resin from Portugal, madder root from Rubia tinctorum, iron gall ink and logwood ink, all obtained and prepared by the producer (Zecchi Belle Arti, Firenze, Italy) according to ancient recipes, were also analysed. 20 mg of madder root and colophony resin and 5 μL of iron gall and logwood inks were eluted or diluted in 1 mL of ethanol for 30 min and analysed with the same protocols and conditions previously described for the EVA strips.
3. Results
Figure 1 shows page 6 of the H40 folder while the right panel details the area onto which the EVA was applied (it is the number 6, indicated by a red arrow, together with smudges on its top and left sides). Figure 2 displays page 3 of folder J34; Figure 3 exhibits page 12 of the H40 folder whereas Figure 4 shows a large, diffuse ink spot at the bottom of page 5. In all these figures, wherever present, the red arrow indicates the area of application of EVA film.
Page 3 of the J34 folder. The red arrow indicates the written line to which the EVA diskette was applied.
Page 12 of the J34 folder. The red arrow indicates the numbers to which the EVA diskette was affixed.
A large ink spot at the bottom of page 6 of the J34 folder. The red arrow indicates the zone of application of the EVA diskette.
The metabolomic analysis performed on molecules captured from the surface of the above pages reported the presence of more than 1800 unique molecules. The complete list is available in Supplementary Table 1. This table lists only the identified molecules detected on all four EVA diskettes analysed, all of them in duplicate. The first column displays the names of the various molecules, while the second one gives the retention times (RT) in the first and second dimensions, followed by their area, similarity, base mass, retention index, signal to noise (SN) ratio of each peak and quantitative mass. Note that this massive table (50 pages long) lists not only the 1892 compounds in Volta’s ink, but also those identified in Rubia (from p. 24), followed by Logwood ink (from p. 30), iron gall ink (from p. 37), colophony (from p. 42) and finally from the blank (a void EVA diskette, from p. 47). The metabolomic profiles were compared to ink production techniques and materials from the same period. The analysis showed the presence of small molecules associated with the use of plant extracts. In particular, Volta’s manuscripts were written with a combination of different ingredients, mainly consisting of tannins, vegetable oils and resins together with root and wood dyes. This complex recipe suggests that Volta had the excellent ability to combine organic materials (assuming that he produced the ink by himself, of which he was capable due to his extensive knowledge of chemistry together with physics). The main compound classes, molecules and markers identified in the ink are reported in Table 1.
List of the main classes of compounds, detected in the four EVA samples, with the associated molecular markers
| Class of compound | Detected organic molecules |
|---|---|
| Tannins | Gallic acid, 4TMS derivative |
| Hydroxy and dihydroxyanthraquinones, natural quinoids | Alizarin; 9, 10-Anthracenedione; Anthracene, 9, 10-dihydro-2-methyl-; Hydroquinone; Chrysophanol; Anthrone; |
| Saturated and unsaturated mono and dicarboxylic acids; monounsaturated fatty acids | Stearic acid; Palmitic acid; Oleic acid; Capric acid; Suberic acid; Pelargonic acid; Lauric acid; Enanthic acid; Arachidic acid; |
| Dioncophyllaceae-type alkaloids | Dioncophyllin A; Dioncophyllin B |
| Benzopyranones | 2H-1-Benzopyran-2-one, 7-(diethylamino)-; 6H-Dibenzo(b, d)pyran-1-ol, 3-hexyl-7, 8, 9, 10-tetrahydro-6, 6, 9-trimethyl- |
| Benzofuranone derivatives | 1(3H)-Isobenzofuranone; 1(3H)-Isobenzofuranone, 3, 3-dimethyl-; 1(3H)-Isobenzofuranone, 5-methyl-; 2(3H)- |
| Flavonoids | Pinostrobin; 5-hydroxy-7-methoxyflavanone, tert.-butyldimethylsilyl ether |
| Alkaloids | Weberine, 5-desmethoxy-; Yohimban-17-one |
| Azulene derivatives | 1-Acetyl-4, 6, 8-trimethylazulene; Chamazulene |
| Cyclic monosaccharides | Arabinitol; D-Glucopyranose; 𝛼-D-Mannopyranose; 𝛽-D-(+)-Talopyranose; |
| Phenols | 4-Cumylphenol; Dihydrochalcone |
| Terpenoids, terpenoid alcohols, terpenic phenols | Cuminyl alcohol; Kauren-19-oic acid; Dihydromyrcenol; Thymol; trans-Calamenene; 𝛼-Terpineol; Carvone; |
| Dihydro-furanones | Furanone, 5-butyldihydro-; 5-Methyl-2-(2-methyl-2-tetrahydrofuryl)tetrahydrofuran; 2(3H)-Furanone, dihydro-5-pentyl-; 2(3H)-Furanone, dihydro-5-tetradecyl- |
| Plant sterols | Stigmasterols; 𝛽-Sitosterol; |
| Abietanes | Methyl dehydroabietate; 1-Methyl-10, 18-bisnorabieta-8, 11, 13-triene; 10, 18-Bisnorabieta-5, 7, 9(10), 11, 13-pentaene; Abietic acid; 10, 18-Bisnorabieta-8, 11, 13-triene; Dehydroabietic acid; |
| Others | Artedouglasia oxide A; Estragole; Diftalone; |
Figure 5 reproduces a two-dimensional visualization of total ion chromatogram of the EVA film analysed with GCxGC–TOFMS. In this image the magnification of some markers identified in Volta’s inks are reported with relative m/z assignments. Hydroquinone, Cinnamic acid, 4-Cumylphenol, 9-10-Anthracenedione, 10, 18-Bisnorabieta-8, 11, 13-triene, Gallic acid, Alizarin, Chrysophanol and Dioncophyllin A are clearly identified. The assignments of other main molecules discussed in the paper are reported in Supplementary Figures 1S.
Two-dimensional visualization of total ion chromatogram of the EVA film analysed via GCxGC–TOFMS. In the composite image the magnification of some of markers identified in Volta’s ink is reported with the relative m/z assignment. Hydroquinone, Cinnamic acid, 4-Cumylphenol, 9-10-Anthracenedione, 10, 18-Bisnorabieta-8, 11, 13-triene, Gallic acid, Alizarin, Chrysophanol and Dioncophyllin A are clearly identified.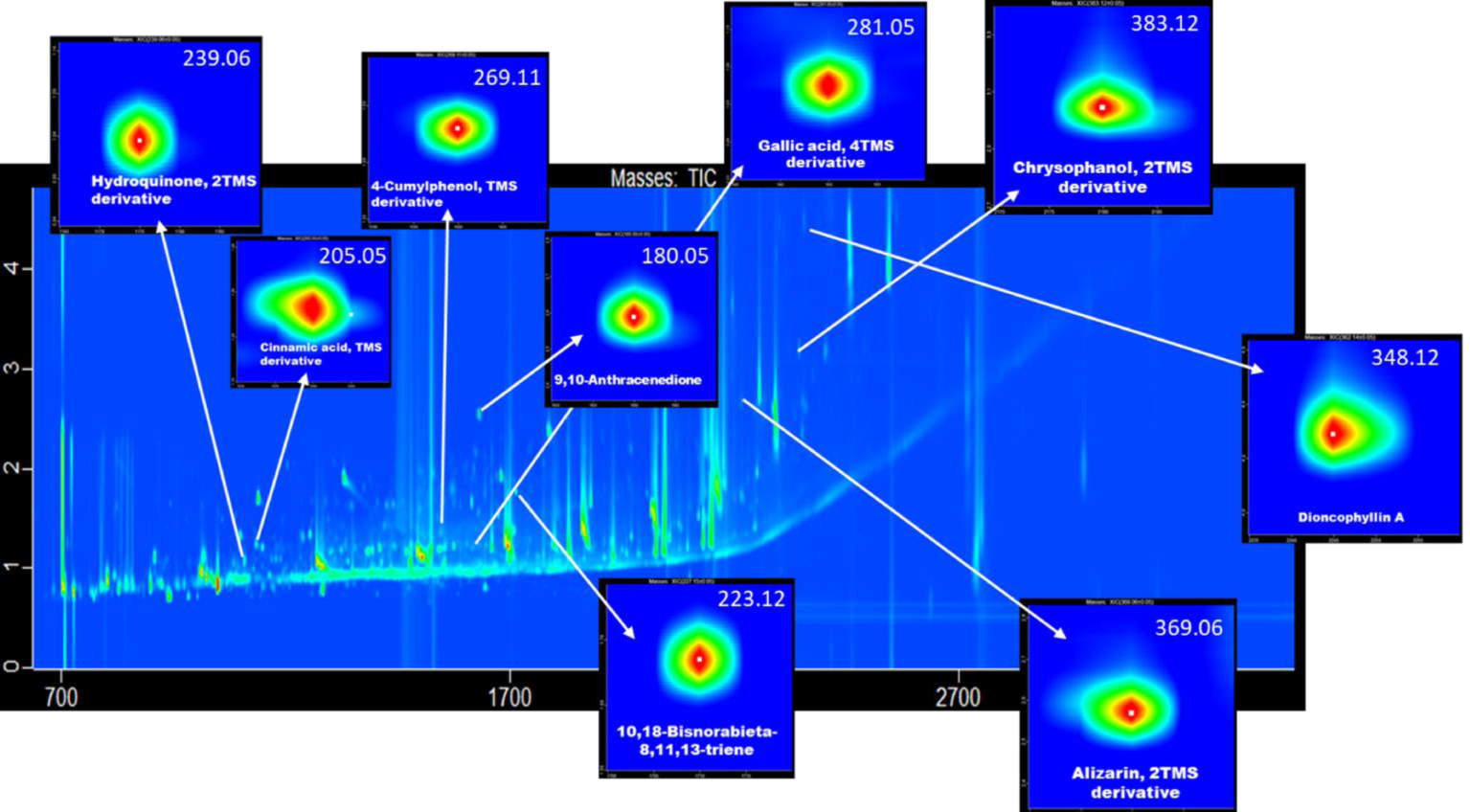
In order to obtain a better insight into Volta’s ink and detect the possible presence of other types of ink in use since the Middle Ages and the Renaissance, we have compared its composition with that of four other types of inks (or commonly used plant extracts), notably Rubia, logwood ink, iron gall ink and colophony. They are listed in Table 2, together with the number of chemicals identified. Their identity can be gleaned from Supplementary Table 1 that lists, after the composition of Volta’s ink, their formulations in the order in which they have been quoted above. In order to see which of them might have entered into Volta’s ink composition, we have compared them via a five-membered overlapping Venn diagram, as shown in Figure 6. Although, in binary comparisons, each of the four inks appeared to share ca. 50–55% of its composition with Volta’s ink, indeed Figure 6 shows that most of these chemicals are dispersed into binary, tertiary and quaternary mixtures, leaving, in common to all five inks, a narrow core of only 70 components. Each of the four inks retains a reduced number of chemicals specific to each individual, as follows: Rubia 102, logwood ink 138, iron gall 62 and colophony 193. Yet Volta’s ink stands out for a huge number of unshared compounds, as many as 1502, suggesting that quite a few more plant extracts must have been used to reach this outstanding complexity. The significance of these data will be discussed below.
List of the five inks analysed together with the number of compounds identified
| Name of the ink | Total number of compounds identified |
|---|---|
| Volta’s ink | 1892 |
| Rubia | 362 |
| Logwood ink | 462 |
| Iron gall ink | 321 |
| Colophony | 415 |
4. Discussion
4.1. An excursus on ink ingredients
As shown in the Supplementary Table 1 the ink composition appears to be extremely complex, to an extent never so far described in available literature. We will discuss here some of the most important components and their attribution to specific plants. To start with, we underline that the presence of gallic acid and the absence of ellagic acid does not necessarily imply the use of iron gall ink, although the identification of the exact plant species has not been quite possible so far, as already mentioned by Saez et al. [6] and confirmed by our GC–MS analyses of the standards. Gallic acid, as well as other tannins, could come from various parts of plants [10] and, in fact, are quite ubiquitous. Our analyses indeed reported the presence of gallic acid in both logwood ink and iron gall ink.
Five-membered overlapping Venn diagrams showing the extent of sharing of various chemicals of plant origin among the five inks analysed.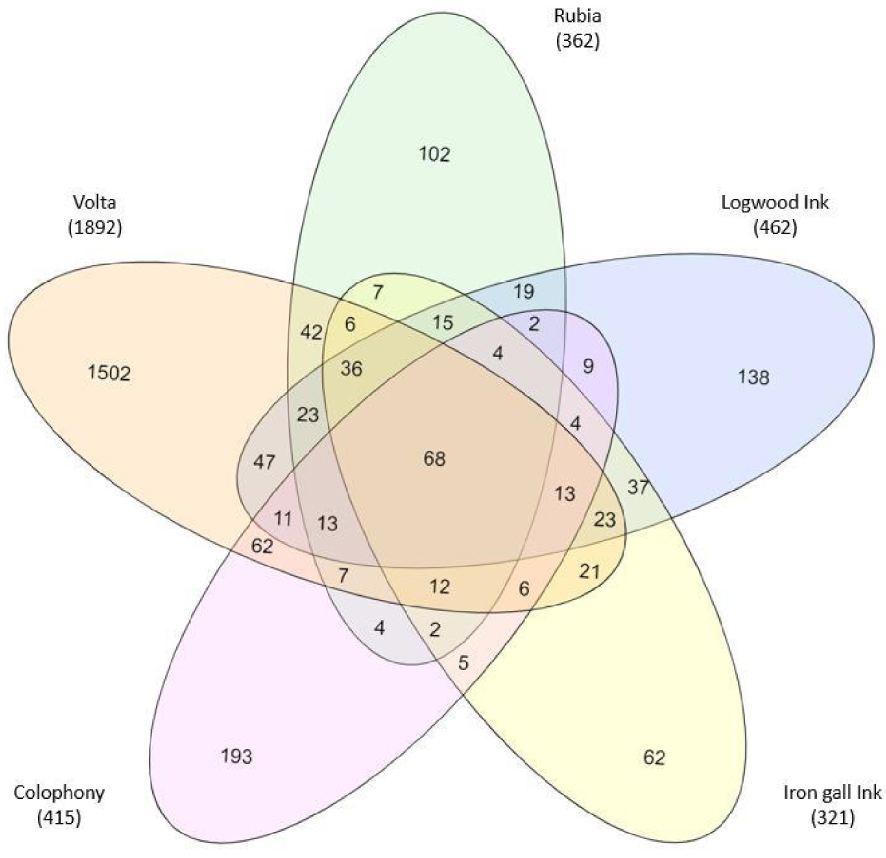
However, the presence of hydroxy and dihydroxyanthraquinones, as well as natural quinoids, confirmed the use of madder dyes from Rubiaceae as an important component of Volta’s ink. Indeed, natural quinoids based on 9, 10-anthraquinone skeleton, hydroquinone and anthrone derivatives, and even the specific marker of alizarin, strongly suggest the use of the precious Rubia tinctorum, probably used as an additional dye imparting a red tinge to the ink. This notion is further reinforced by inspecting Figure 4: as the wet ink spot spread and was chromatographed on the page surface, the red component clearly separated from the brown-blackish material lagging behind. The specific attribution of these signals is also supported by our results obtained through the analysis of natural dye extracted from madder root (Supplementary Table 1). The addition of dyes to the iron gall ink was a particular practice sometimes used to give a more fluid product as well as a pleasant and bright colour [11].
The presence of several signals of fatty acids, saturated and unsaturated mono- and dicarboxylic acids, as well as the existence of the typical signals of Pinaceae resins, confirmed the use of a vegetable oil and colophony, as reported in one of the first organic manuals of typography at the end of the 17th century [12]. Joseph Moxon mentioned the “Dutch method” of making ink, believing it to be the best of its time, characterized by the addition of good hot linseed oil and a small amount of quality rosin, a vegetable resin residual from the distillation of turpentines (from conifers) also known as “Greek pitch” or “Colophony”. In particular, as already summarized in Table 1, the analysis of colophony resin allowed the correct attribution of the detected molecules of abietane (abietic acid, oxo and dehydroabietic acid derivatives) and colophony resins. Furthermore, the palmitic/stearic ratio at about 1 in all four EVA samples, suggested that linseed oil had been used as well, in agreement with historical manuals (Supplementary Table 1).
The presence of several signals of cyclic monosaccharides, also suggested the use of natural gum (Acacia from Senegal, better known as Arabic gum). Literature confirms that Arabic gum, as well as linseed oil, were often employed as thickeners to increase the viscosity of ink and to protect the ink from excess absorption of atmospheric oxygen [8]. In contrast to other scientific studies on brown or black dyes, our results excluded the use of Brazilwood. In fact, we did not detect its typical signals such as haematoxylin and urolithin [13, 14].
In Volta’s ink we also found singular and characteristic signals from Dioncophyllaceae-type alkaloids such as Dioncophyllin A and B, typical metabolites from tropical/exotic plants, closely related to tropical liana families, which could derive only from three monotypic genera: Triphyophyllum, Habropeltatum and Dioncophyllum [15]. To the best of our knowledge, no references to dioncophyllin have been made in previous literature relating to inks or dyes.
Signals common to Logwood were found in flavonoids, gallic acid and benzopyranone derivatives. Logwood is a member of the class of dyeing woods rich in neoflavonoids, but also in anthraquinones such as danthron [16]. It was introduced in Europe by the Spaniards from the beginning of 16th century. Under the name of “logwood” were often included exotic woods, which were also used by the dyeing industry, or as basic constituent for preparing brown vegetable inks, but only from the 18th century [17]. The most important logwood in Europe is the Haematoxylum campechianum, which belongs to the Leguminosae family.
The presence of azulene derivatives, chamazulene and other particular alkaloids such as Weberine, 5-desmethoxy and Yohimban-17-one, suggest the use of other plants extracts, mainly in the form of coloured essential oils or violet/blue dyes. In fact, Chamazulene can be found in a large variety of plants including German or Matricaria chamomile, Artemisia and Achillea millefolium [18], which are known for producing blue essential oil dyes. Weberine and Yohimban-17-one, instead, are alkaloids identified respectively in Lophophora and in the bark of Pausinystalia species [19]. Although the identification of these last metabolites is particularly suggestive, we cannot attribute them with certainty to a specific use. The detection of Artedouglasia oxide A, one of the most common metabolites associated with Artemisia [20], which is usually associated with the dyes’ palette from yellow to green, given by Wormwood, is also very interesting [21].
As reported by Ruggiero et al. [11], a very important contribution in the preparation of inks was given by the use of raw materials employed in different proportions, which were extremely variable from one recipe to another. This guaranteed the complete uniqueness of the final handmade product at least until the second half of the 17th century.
4.2. A brief survey of inks over the millenia
It is of interest here to offer a brief excursus on ink production over the centuries. We have recently investigated a very ancient ink used in the Dead Sea Scrolls (DSS) [22]. Indeed the ink pigment used for writing them is mainly composed of carbon soot, which however could not possibly adhere or be adsorbed by the supporting parchment; for that, one would need some gluing material. By applying the same EVA diskettes here presented on DSS fragments and analysing the captured material, we were able to determine the composition of this binder. Plant proteins (ribulose biphosphate carboxylase, rhamnogalacturonate lyase, 𝛼-galactosidase A, calmodulin, among those identified) as well as a few glycoproteins with different combinations of pentosyl and hexosyl units, together with plant acids (stearic, palmitic, oleic, linoleic and linolenic acids) and terpenes (triacontanol, catechin, lupeol), as typically found in acacia trees of the region, indicated Arabic gum as the ink’s binder. The evolution of inks over the centuries has been nicely surveyed by C. Pastena in a series of documents published on the internet in 2019 (Greek–Roman inks (part 1); Medieval inks (part 2) and Egyptian and Chinese inks (part 3)).
The first ones (Greek–Roman) were typically made with black pigments (carbon soot here too) supplemented with different types of binders: not only Arabic gum, but alternatively gelatin (from animal skins), egg white, different oils. Interestingly, the best soot was obtained by burning lamp oil or wooden torches (this kind of black was also named atramentum by Romans). However a variant was to take wine dregs and burn them in furnaces. This was a most appreciated variant since the ink colour was closer to indigo than to pitch black. Medieval inks were also dubbed metal-gall, since they were produced from plant extracts supplemented with metals. These extracts were obtained from oak gall, which in turn is a kind of tumour growth on the oak leafs and branches. They are rich in tannic acids, which readily complex with iron when supplemented with ferrous sulphate. With time, this ink turns from brownish to blackish. If one needed vermillion, a colour used in miniatures, this was obtained by mercury sulphate with egg white or Arabic gum as binding materials. In the third instance, Egyptians and Chinese also used soot (in fact their inks are the oldest ones and likely it was they who first invented this type of ink). Curiously, though, the binder used was quite peculiar: it appears that they used ground horns of deer, fallow deer, even rhinoceros, even though they did not disdain other animal binders, such as those derived from cow or donkey skins as well as from fishes.
A most interesting manual on inks was published in 1832 by Savage [23] (Volta could not possibly have had access to it, since he died in 1827). In his days this was a kind of encyclopaedia on printing inks. Its six chapters summarize all that was known up to his days. Not only in terms of black, but also of coloured inks. He lists any possible colouring agent known in his time, almost a rainbow of any possible pigment. Here is a list of orange and yellows (of which he explains also the proper use, stability, quality of the tint and its tone): Orange Lead, Burnt Terra do Sienna (sic!), Indian Yellow, Gall Stone, Gamboge, King’s Yellow, Patent Yellow, Roman Ochre, Yellow Ochre (that is something that would have made Van Gogh happy).
Here is a most interesting historical summary on printing inks: “Moon published the Dutch method of preparing Printing Ink in 1677; Fertel, a French printer at Saint Omers, published a practical work on printing in 1723. Breton, printer to the King of France, supplied the article on printing to the Encyclopaedia, in 1751. Dr. Lewis published, in 1763, his Philosophical Commerce of Arts, in which he details the results of some experiments in boiling different sorts of oils, to ascertain their qualities for making different types of varnish. Papilla, a celebrated French engraver on wood, published a Treatise on Engraving on Wood in 1766. Baskerville’s method, published by Mr. T. C. Mansard, in 1824, is short, general, and unsatisfactory, does not elicit any new fact. Nicholson, in his Dictionary of Chemistry, published in 1795, gives a vague article on this subject, taken from Lewis, whose authority was Breton. The Messrs. Akin, in their Dictionary of Chemistry, avowedly take this subject from Lewis, who copied Breton; the article on this subject in Reed’s Cyclopaedia is quoted from Lewis. It of course does not advance our knowledge. The Manual of Printing, a French work, published in 1817, on this subject, is nearly a copy of Breton’s article, and gives nothing new”.
Here are his disheartened conclusions: “it thus appears that all the French writers to the present day are little more than copies of Moon’s Dutch method, without once mentioning him and all the English writers, subsequent to Moon, have quoted Dr. Lewis, who avowedly took Breton for his authority”.
4.3. On the possible presence of contaminant molecules
Although not presented in the above text, this is an important aspect that deserves scrutinizing. If such contaminant molecules were present in our ink inventory, this would invalidate our data. EVA film could be the prime culprit. Yet, very early in the game, we published technical articles [6, 7] that categorically excluded leaching out of any chemicals from EVA foils, thus affirming the impossibility of contaminating any item under investigation. This was of course confirmed in all our investigations in Cultural Heritage. We additionally underline that EVA is a plastic foil (a polymer of vinyl acetate) and even all the various chromatographic beads embedded therein are polymeric. For instance the strongly acidic resin is a polymer of styrene-divinyl benzene boiled with sulphuric acid to covalently attach sulphate residues. Were this not enough, the EVA film, once laminated, is extensively washed untill removal of any residual contaminants. Moreover, prior to application onto the items under investigation, EVA diskettes are humidified in a large excess of distilled water. In any event, we also analysed a blank EVA disk, prepared with the same protocol conditions, and had no relevant signals in the GCXGC/TOFMS instrument (Supplementary Table 1).
Next comes the writing paper. We recall here that writing material obtained from the pulp of trees was commercially produced only after 1850, thus could not have been possibly available in Volta’s time. Instead for centuries it was produced from cotton rags, which were extensively washed, bleached with hypochlorite, treated with lime and finally macerated to pulp. The sheet thus produced were treated with animal binder so as to prevent their disintegration. In fact, in an investigation on a plague bout in Milano in 1630, when analysing the pages of the death registries of the lazaretto, we noticed that the EVA diskettes had eluted, from blank pages, collagen and keratin of animal origin (typically from cows and rabbits) [24]. A few more words should be said on control diskettes applied to regions of the pages devoid of any scripts (in general empty margins). As expected, no components of the ink, as presented in Supplementary Table 1, could be detected. The eluates, though, in this particular case, were also analysed not only for plant metabolites, via GC/TOFMS but also for proteins, via LC/MS, as was done in most of the articles published by our group in the domain of Cultural Heritage [25, 26]. As expected, a few dozen salivary proteins and human keratins were detected, due to handling of the pages either by Volta himself or by subsequent readers. In the case of Bulgakov though, analysis of metabolites and proteins present in the margins of his manuscript Master i Margarita allowed us to detect his use of drugs (morphine) [27] as well as to identify biomarkers of his renal pathology [28]. In the case of Orwell [29] we could detect, via proteomic analysis, traces of the Koch bacillus, since he had been infected by the M. tuberculosis bacterium when he was hospitalized in Barcelona during the Spanish civil war. Yet in the present case, the proteins detected would not have had any particular meaning, therefore these data have not been reported in this research report, whose main aim was the ink composition.
5. Conclusions
Our data suggest that Alessandro Volta illuminated his scripts with a special ink of very complex composition, mostly of plant origin, apparently containing more than 1800 different chemicals, some imparting to his brown–black aspect a reddish tinge, quite likely obtained from the root of Rubia tinctorum. He might have adopted such a complex ink composition to impart to it a particular brilliancy and a unique elegance that would distinguish his writing from those of the other scientists he was corresponding with. Whether he devised this concoction and produced it by himself, or bought it from other sources, however, is not known to us.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We are much indebted to the Istituto Lombardo di Scienze e Lettere, to its president Professor Stefano Maiorana and his staff for granting us access to the collection of Volta’s manuscripts.
Supplementary data
Supporting information for this article is available on the journals website under https://doi.org/10.5802/crchim.128 or from the author.




 CC-BY 4.0
CC-BY 4.0