1. Introduction
Pharmacologically active spiroindolepiperidines.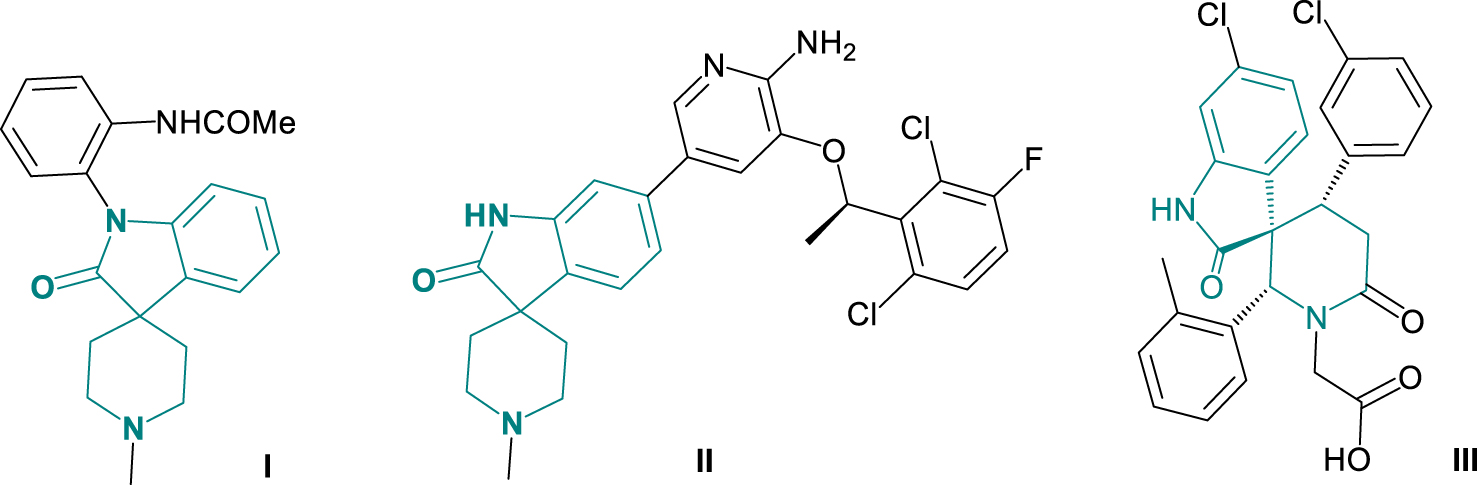
The selective and efficient assembling of pharmacologically active structures in domino reactions is one of the main aims of modern organic synthesis. The domino approach makes organic synthesis more elegant and leads to the formation of complex organic compounds more easily [1]. This approach is advantageous due to high atom economy and, therefore, due to reduction of generated waste [2, 3]. At the same time, it is more beneficial and more complex when the reaction involves five molecules at once.
Among other complex heterocycles, spiro derivatives of oxindole and piperidine show antidepressant, anxiolytic and analgesic activities [4]. Spiroindolepiperidine I (Figure 1) has antidepressant activity [4]. Spiroindolepiperidine II (Figure 1) is known as an orally bioavailable gastric tumor inhibitor with single-digit nanomolar activity [5]. 3-Spiroindole-2,4-diaryl-piperidine-6-one III (Figure 1) shows anticancer activity [6]. Generally, spiro derivatives of piperidine have been also claimed to be prospective neurokinin receptor antagonists [7].
The first four-molecule domino synthesis of piperidines was carried out by condensation of ketone, ethyl cyanoacetate and ammonia (Scheme 1. A) [8, 9].
Another four-molecule synthesis of piperidines has been carried out by the reaction of nitrostyrene, aromatic aldehyde, malonic ester and formamide (stirring in EtOH at 0–63 °C for 40 h, Scheme 1. B) [10]. Later, the modification with Meldrum’s acid and ammonium acetate was also described (stirring in EtOH at 45 °C for 20 h, Scheme 1. C) [11]; up until now, the latter is the only known domino synthesis of spiropiperidines.
Known domino approaches to piperidines.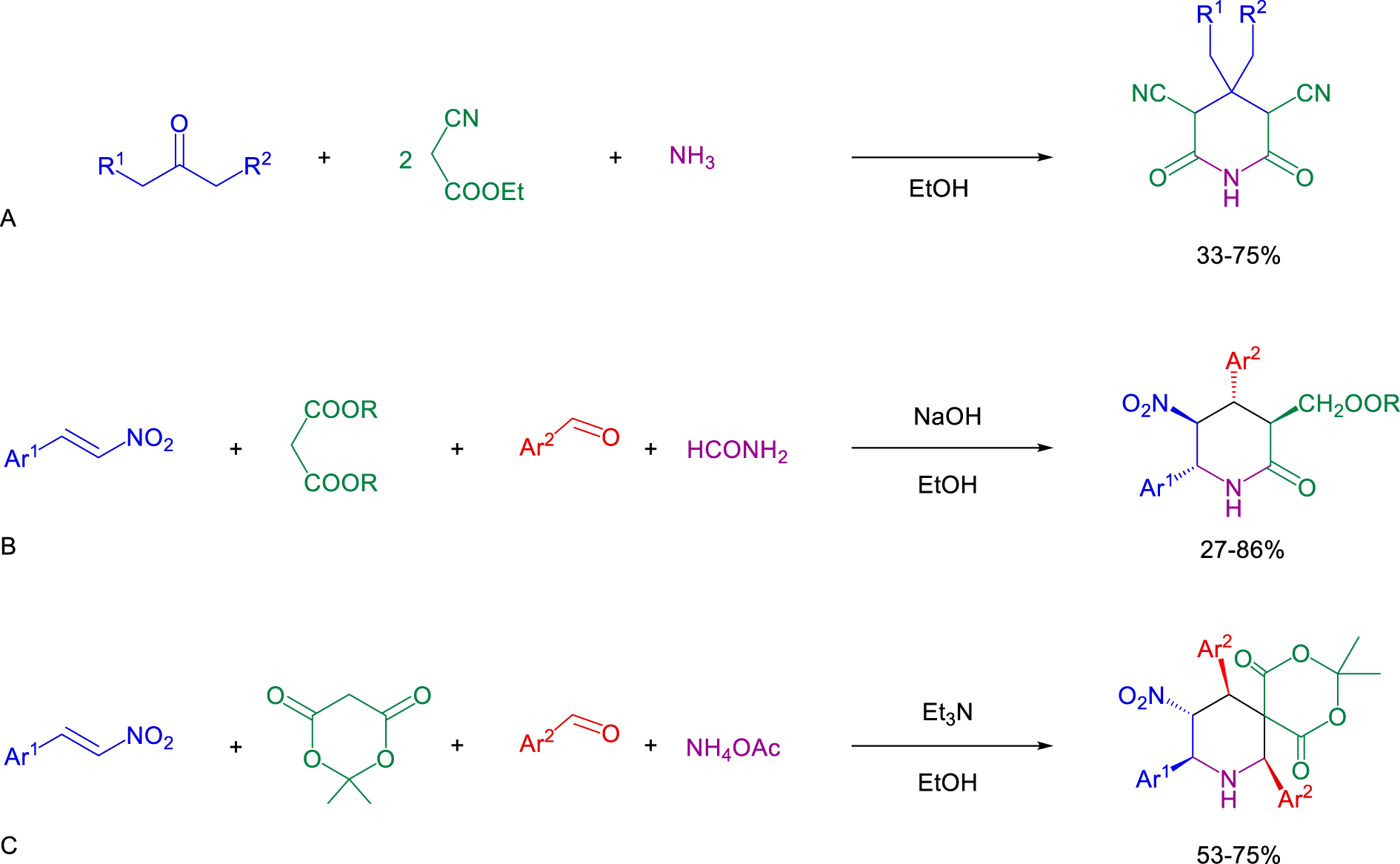
Thus, domino syntheses of piperidines have been known earlier, but the domino approach, or any other way to 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridines], however, has never been described.
In the past years, we accomplished new types of domino reactions of carbonyl compounds and different CH acids [12, 13, 14, 15, 16].
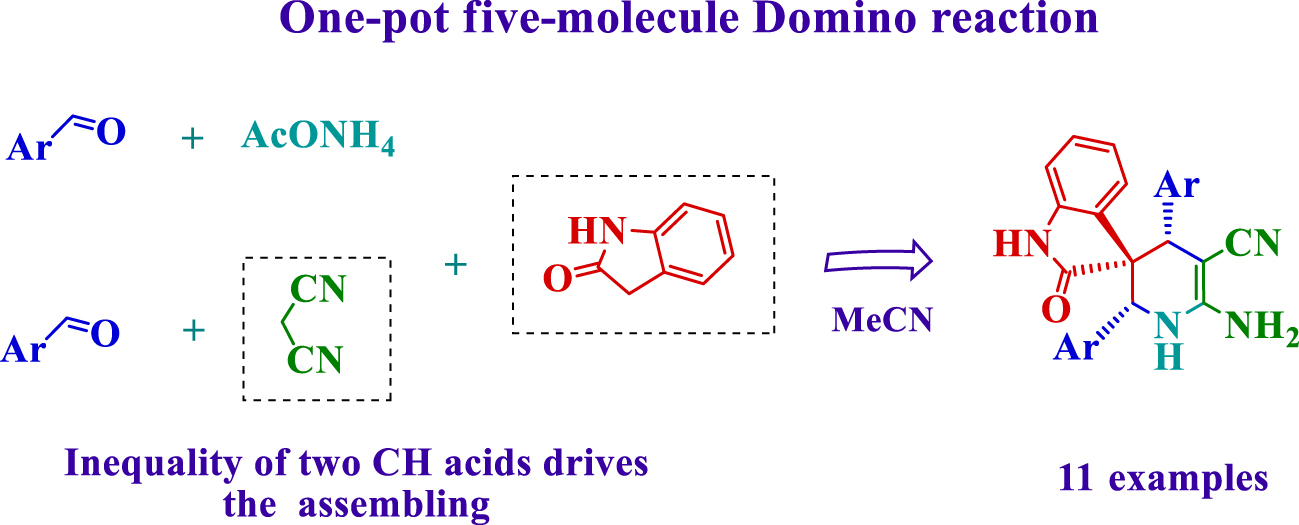
In the present study, we were motivated to develop the five-molecule domino assembling of two equivalents of benzaldehyde, malononitrile, oxindole and ammonium acetate.
2. Results and discussion
Thus, in this report, we present results on five-molecule stereoselective domino assembling of (2′ R*,3 S*,4′ R*)-6′-amino-2-oxo-2′,4′-diaryl-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitriles 2a-k (Table 2).
Stereoselective domino reaction of benzaldehyde 1a (two equivalents), malononitrile, oxindole and ammonium acetate.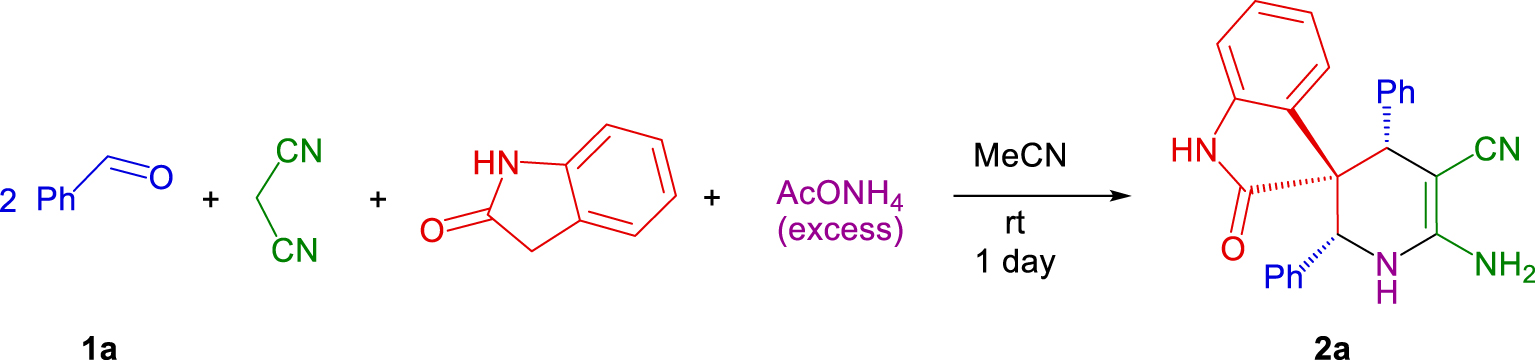
The work was started by stirring two equivalents of benzaldehyde 1a, malononitrile, oxindole and excess of ammonium hydroxide in MeOH (Table 1, Entry 1). The reaction resulted in the formation of benzylidenemalononitrile in 78% yield, but the 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a was not detected under these conditions (Table 1, Entry 1).
Taking into consideration that excess water could block the formation of a required intermediate, the next experiment was accomplished with ammonium acetate in MeOH. Stirring of two equivalents of benzaldehyde 1a, malononitrile, oxindole and ammonium acetate at ambient temperature for 1 and 3 days resulted in the formation of 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a in 27% and 28% yields (Table 1, Entries 2 and 3).
An increase in the amount of solvent up to 6 ml and 10 ml raised yields up to 33% and 49%, respectively (Table 1, Entries 4 and 5). In 20 ml of MeOH, the yield of 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a was slightly decreased to 45% (Table 1, Entry 6).
To reduce the possible protonation of a crucial intermediate, MeCN was utilized as the solvent instead of alcohol. In MeCN, the 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a was formed in 57% yields (Table 1, Entry 8).
Domino synthesis of 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a
| Entry | Solvent, mla | Time, days | Temperature, °C | Yield of 2a, %b |
|---|---|---|---|---|
| MeOH,4 | ||||
| ml + NH3∙H2O, | ||||
| 1 | 1 ml, 25% water | 1 | 25 | nd |
| solution) | ||||
| 2 | MeOH (4 ml) | 1 | 25 | 27 |
| 3 | MeOH (4 ml) | 3 | 25 | 28 |
| 4 | MeOH (6 ml) | 1 | 25 | 33 |
| 5 | MeOH (10 ml) | 1 | 25 | 49 |
| 6 | MeOH (20 ml) | 1 | 25 | 45 |
| 7 | EtOH (10 ml) | 1 | 25 | 38 |
| 8 | MeCN (10 ml) | 1 | 25 | 57 |
| 9 | MeCN (10 ml) | 1 | 82 | nd |
aReaction conditions: benzaldehyde 1a (10 mmol), malononitrile (5 mmol), oxindole (5 mmol) and ammonium acetate (10 mmol) stirred in solvent.
bIsolated yield. nd: Not detected.
Ten other variously substituted derivatives of 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridines] 2b-k were obtained in MeCN (10 ml) by stirring at ambient temperature for 1 day in 35–57% yields (Table 2).
Several reactions were carried out to investigate the mechanism of the process. The reaction of one equivalent of benzaldehyde, benzylidenemalononitrile, oxindole and ammonium acetate resulted in 60% yield of the 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a (Scheme 3. A). Therefore, benzylidenemalononitrile, which is formed rapidly at the initial stage of the reaction, essentially does not affect subsequent transformations. The reaction of benzaldehyde, malononitrile and benzylideneoxindole 3 in the presence of excess of ammonium acetate did not result in the formation of 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a (Scheme 3.B). At the same time, benzylideneoxindole 3, as well as benzylidenemalononitrile, was found as a by-product of the main reaction. However, benzylideneoxindole 3 remained inactive and could not be used to obtain 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a.
Hence, CH acidity of initial components is crucial for the domino process; the more active malononitrile (pKa DMSO = 11.1) [17] reacts more rapidly than oxindole (pKa DMSO = 18.2) [18]. This feature makes domino assembling with two different CH acids feasible (it allows us ‘to program’ reaction).
Taking into consideration the above results and the data on the multicomponent transformations of benzaldehyde, malononitrile, aldehyde and ammonium acetate [19, 20, 21], the following mechanism for the 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2a formation was proposed (Scheme 4).
The domino reaction begins with two initial parallel processes: imine 4 and benzylidenemalononitrile 5 formations. Later, in the presence of ammonium acetate, the Michael addition of oxindole to benzylidenemalononitrile 5 results in the formation of anion B. The Mannich type reaction of anion B with imine 4 and further Pinner type cyclization lead to anion C. The latter, after protonation and isomerization, forms the final (2′ R*,3 S*,4′ R*) 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine] 2.
The structure of spiro[indoline-3,3′-pyridine] 2a (XRD).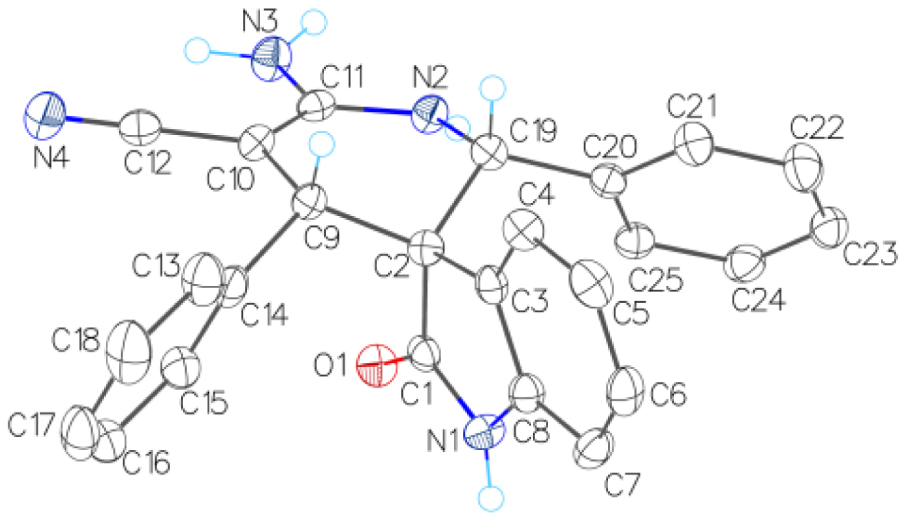
1H and 13C NMR spectra of compounds 2a-k show only one set of signals, which provides evidence of stereoselectivity. The X-ray diffraction (XRD) data unambiguously supports a (2′ R*,3 S*,4′ R*) configuration for 6′-amino-2-oxo-2′,4′-diphenyl-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile 2a (Figure 2).
The study of the mechanism of the main reaction.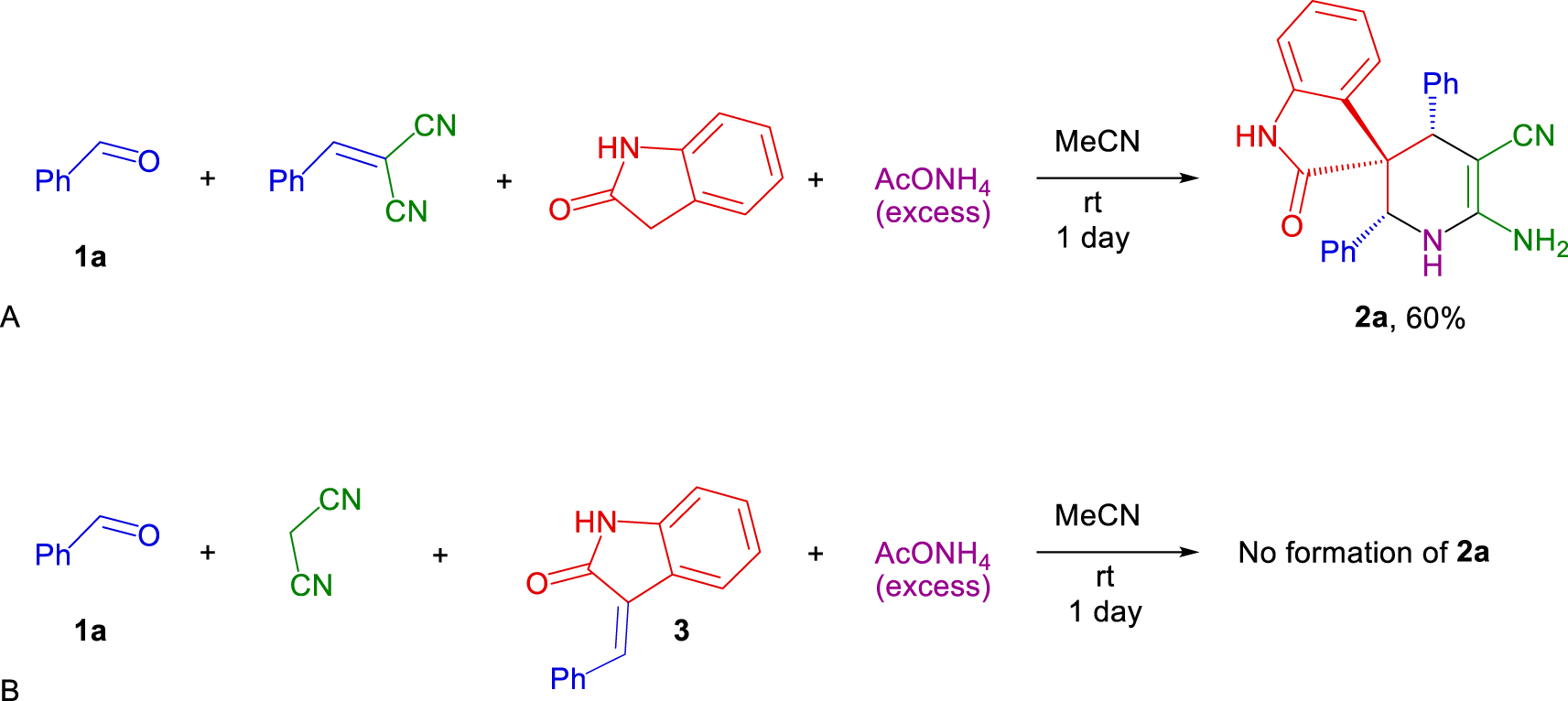
Proposed mechanism for domino reaction of benzaldehyde 1 (two equivalents), malononitrile, oxindole and ammonium acetate.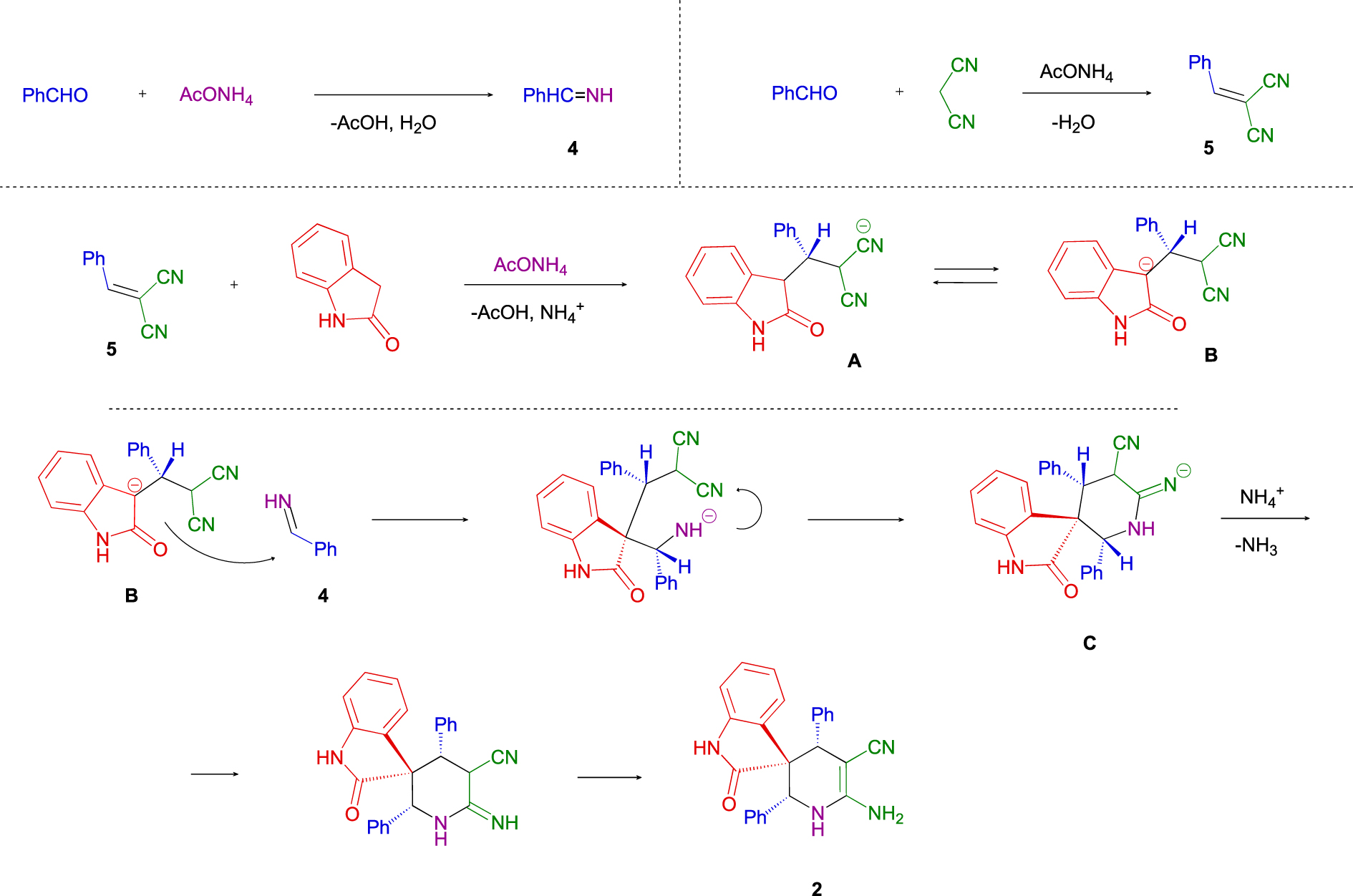
Synthesis of substituted 1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridines] 2a-k
 |
Reaction conditions: benzaldehyde 1a-k (10 mmol), malononitrile (5 mmol), oxindole (5 mmol) and ammonium acetate (10 mmol) stirred in 10 mL of MeCN for 1 day. Then precipitate was filtered out and washed with MeOH.
Taking into account the proposed mechanism, stereoselectivity of the process is the result of significant steric hindrance caused by aromatic rings. Hence a maximum distance between the oxindole ring and aryl moiety at each stage of the reaction is favorable.
3. Conclusion
In conclusion, differences in acidity between malononitrile and oxindole allow assembling of previously unknown 6′-amino-2-oxo-2′,4′-diaryl-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitriles (structure proven with XRD). The stereoselective domino reaction occurs under mild conditions by the five-molecule transformation of benzaldehydes, malononitrile, oxindole and ammonium acetate in one-pot process. The synthesis is easy to perform; it utilizes simple equipment, and work-up is carried out without column chromatography or recrystallization. This new approach opens the way to a consistent and convenient synthesis of spiropiperidines with high complexity per transformation.
4. Experimental section
4.1. General remarks
The solvents and reagents were purchased from commercial sources (Sigma-Aldrich, Alfa Aesar, Acros) and used as received. All melting points were measured with a Gallenkamp melting point apparatus and are uncorrected. IR spectra were registered with a Bruker ALPHA-T FT-IR spectrometer in KBr pellets. 1H and 13C NMR spectra were recorded with a Bruker AM-300 (300 and 75 MHz, respectively) at ambient temperature in DMSO-d6 solutions. Chemical shift values are given in δ/ppm scale relative to Me4Si. Mass spectra (EI = 70 eV) were obtained directly with a Finnigan MAT INCOS 50 spectrometer. For elemental analysis, PerkinElmer 2400 Series II and multi EA 5000 were used.
4.2. General procedure for synthesis of spiro[indoline-3,3′-pyridines] 2
A mixture of benzaldehyde 1 (10 mmol), malononitrile (0.33 g, 5 mmol), oxindole (0.67 g, 5 mmol) and ammonium acetate (0, 77 g, 10 mmol) was stirred in 10 mL of acetonitrile in a flask for 1 day. When the reaction was completed, the reaction mixture was cooled to − 10 °C for 15 min. Then the precipitate was filtered out, washed with MeOH (2 × 2 cm3) and dried under reduced pressure to isolate pure 2.
4.2.1. 6′-Amino-2-oxo-2′,4′-diphenyl-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2a: yield 1.12 g (57%). White solid, mp 276–278 °C. IR (KBr): 𝜈 = 3377, 3313, 2161, 1703, 1615, 1578, 1547, 1488, 749, 700 cm−1. lH NMR (300 MHz, DMSO-d6): 4.40 (s, 1H, CH), 5.01 (s, 1H, CH), 5.37 (s, 2H, NH2), 6.12 (d, J = 7.3 Hz, 1H, Ar), 6.58 (s, 1H, NH), 6.83–7.12 (m, 12H, Ar), 7.58 (d, J = 7.3 Hz, 1H, Ar), 9.65 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 47.9, 51.9, 54.3, 60.6, 108.1, 120.6, 123.6, 123.9, 126.6, 127.0 (2C), 127.6 (4C), 127.9, 128.1 (2C), 129.3 (2C), 137.0, 138.5, 141.4, 158.0, 174.5 ppm. MS (EI, 70 eV) m/z (%) = 392 (M, 53), 222 (17), 221 (100), 220 (17), 193 (13), 172 (6), 171 (4), 170 (11), 144 (10). Anal. Calcd. for C25H20N4O (392.16): C, 76.51%; H, 5.14%; N, 14.28%, found C, 76.49%; H, 5.19%; N, 14.31%. Crystal Data for 2a: C34H41N7O4 (M = 611.74 g∕mol): triclinic, space group P-1 (no. 2), a = 10.1746(15) Å, b = 13.395(2) Å, c = 13.840(2) Å, 𝛼 = 114.396(3)°, 𝛽 = 93.763(4)°, 𝛾 = 107.333(3)°, V = 1600.7(4) Å3, Z = 2, T = 120 K, 𝜇(MoK𝛼 = 0.085 mm−1, Dcalc = 1.269 g∕cm3, 19004 reflections measured (3.6°⩽2𝛩⩽56.56°), 7946 unique (Rint = 0.0616, Rsigma = 0.0886), which were used in all calculations. The final R1 was 0.0568 (>2sigma(I)) and wR2 was 0.1382 (all data).
4.2.2. 6′-Amino-2-oxo-2′,4′-di-m-tolyl-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2b: yield 0.80 g (38%). Pale yellow solid, mp 274–276 °C. IR (KBr): 𝜈 = 3491, 3375, 3200, 2847, 2162, 1708, 1614, 1592, 1539, 702 cm−1. lH NMR (300 MHz, DMSO-d6): 2.07 (s, 3H, CH3), 2.11 (s, 3H,CH3), 4.36 (s, 1H, CH), 4.96 (s, 1H, CH), 5.38 (s, 2H, NH2), 6.14 (d, J = 7.2 Hz, 1H, Ar), 6.55 (s, 1H, NH), 6.62–7.11 (m, 10H, Ar), 7.57 (d, J = 7.2 Hz, 1H, Ar), 9.68 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 21.0 (2C), 47.8, 52.1, 54.2, 60.6, 108.0, 120.4, 121.1, 123.6, 123.9, 125.3, 126.5, 126.8, 127.4 (2C), 128.5, 128.6, 129.6, 130.1, 135.5, 136.5, 137.0, 138.4, 141.5, 158.0, 174.6 ppm. MS (EI, 70 eV) m/z (%) = 420 (M, 9), 236 (19), 235 (100), 234 (40), 220 (11), 207 (16), 184 (18), 144 (18), 118 (11), 91 (12). Anal. Calcd. for C27H24N4O (420.20): C, 77.12%; H, 5.75%; N, 13.32%, found C, 77.11%; H, 5.70%; N, 13.38%.
4.2.3. 6′-Amino-2-oxo-2′,4′-di-p-tolyl-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2c: yield 0.97 g (46%). Pale yellow solid, mp 199–200 °C. IR (KBr): 𝜈 = 3349, 2165, 1694, 1590, 1544, 1476, 1190, 749, 703, 550 cm−1. lH NMR (300 MHz, DMSO-d6): 2.12 (s, 3H, CH3), 2.13 (s, 3H,CH3), 4.34 (s, 1H, CH), 4.94 (s, 1H, CH), 5.34 (s, 2H, NH2), 6.13 (d, J = 6.3 Hz, 1H, Ar), 6.48 (s, 1H, NH), 6.75–7.04 (m, 10H, Ar), 7.55 (d, J = 6.3 Hz, 1H, Ar), 9.62 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 20.5 (2C), 47.5, 52.1, 54.3, 60.4, 108.1, 120.5, 123.6, 123.8, 127.6, 127.7 (2C), 128.0 (2C), 128.2 (2C), 129.2 (2C), 129.8, 134.1, 135.4, 135.5, 137.0, 141.5, 158.0, 174.6 ppm. MS (EI, 70 eV) m/z (%) = 420 (M, 2), 236 (19), 235 (100), 234 (42), 220 (7), 207 (20), 206 (14), 184 (25), 178 (6), 144 (14). Anal. Calcd. for C27H24N4O (420.20): C, 77.12%; H, 5.75%; N, 13.32%, found C, 77.09%; H, 5.74%; N, 13.40%.
4.2.4. 6′-Amino-2′,4′-bis(4-ethylphenyl)-2-oxo-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2d: yield 1.14 g (51%). Yellow solid, mp 179–181 °C. IR (KBr): 𝜈 = 3348, 3231, 2966, 2167, 1694, 1622, 1594, 1546, 1474, 752 cm−1. lH NMR (300 MHz, DMSO-d6): 1.05 (t, J = 7.5 Hz, 3H, CH3), 1.07 (t, J = 7.5 Hz, 3H, CH3), 2.44 (q, J = 7.4 Hz, 4H, CH2), 4.35 (s, 1H, CH), 4.94 (s, 1H, CH), 5.33 (s, 2H, NH2), 6.14 (d, J = 7.2 Hz, 1H, Ar), 6.53 (s, 1H, NH), 6.79–7.04 (m, 10H, Ar), 7.54 (d, J = 7.2 Hz, 1H, Ar), 9.61 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 15.0, 15.3, 27.6 (2C), 47.5, 52.1, 54.3, 60.4, 108.0, 120.5, 123.7, 123.8, 126.3 (2C), 127.0 (2C), 127.5, 128.1 (2C), 129.2 (2C), 129.8, 134.4, 135.7, 141.5, 141.6, 143.3, 157.9, 174.6 ppm. MS (EI, 70 eV) m/z (%) = 448 (M, 4), 250 (21), 249 (10), 248 (3). Anal. Calcd. for C29H28N4O (448.23): C, 77.65%; H, 6.29%; N, 12.49%, found C, 77.60%; H, 6.33%; N, 12.55%.
4.2.5. 6′-Amino-2′,4′-bis(2-bromophenyl)-2-oxo-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2e: yield 1.46 g (53%). Yellow solid, mp 251–253 °C. IR (KBr): 𝜈 = 3343, 2154, 1688, 1589, 1541, 1472, 1024, 749, 558 cm−1. lH NMR (300 MHz, DMSO-d6): 5.10 (s, 1H, CH), 5.37 (s, 2H, NH2), 5.54 (s, 1H, CH), 6.21 (d, J = 7.5 Hz, 1H, Ar), 6.62 (s, 1H, NH), 6.76 (t, J = 7.2 Hz, 1H, Ar), 6.86 (t, J = 7.5 Hz, 1H, Ar), 6.98 (t, J = 7.5 Hz, 1H, Ar), 7.12 (t, J = 7.2 Hz, 1H, Ar), 7.19–7.60 (m, 6H, Ar), 10.31 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 46.1, 52.3, 53.38, 59.5, 107.9, 119.8, 122.8, 124.5, 125.1, 125.8, 126.7, 127.2, 127.5, 127.8, 128.2, 129.0, 130.3, 130.8, 132.2, 132.6, 135.7, 137.7, 140.8, 157.9, 175.1 ppm. MS (EI, 70 eV) m/z (%) = 552 (M, 81Br, 81Br, 1), 550 (M, 81Br, 79Br, 2), 548 (M, 79Br, 79Br, 1), 301 (6) , 299 (6), 250 (8), 221 (18), 220 (100), 219 (10), 165 (9). Anal. Calcd. for C25H18Br2N4O (550.25): C, 54.57%; H, 3.30%; Br, 29.04%; N, 10.18%, found C, 54.50%; H, 3.23%; Br, 28.99%; N, 10.24%.
4.2.6. 6′-Amino-2′,4′-bis(3-bromophenyl)-2-oxo-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2f: yield 0.96 g (35%). Yellow solid, mp 267–269 °C. IR (KBr): 𝜈 = 3343, 2165, 1694, 1590, 1544, 1476, 1190, 749, 703, 550 cm−1. lH NMR (300 MHz, DMSO-d6): 4.41 (s, 1H, CH), 5.02 (s, 1H, CH), 5.47 (s, 2H, NH2), 6.20 (d, J = 6.3 Hz, 1H, Ar), 6.78–7.31 (m, 11H, Ar+NH), 7.60 (d, J = 6.3 Hz, 1H, Ar), 9.86 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 46.1, 52.3, 53.4, 59.5, 107.9, 119.8, 122.8, 124.5, 125.1, 125.8, 126.7, 127.2, 127.8, 128.2, 128.8, 129.0, 130.3, 130.8, 132.2, 132.6, 135.7, 137.7, 140.8, 157.9, 175.1 ppm. MS (EI, 70 eV) m/z (%) = 550 (M, 81Br, 79Br, 8), 89 (48), 77 (63), 76 (93), 63 (60), 51 (57), 50 (64), 44 (64), 43 (100), 28 (63). Anal. Calcd. for C25H18Br2N4O (550.25): C, 54.57%; H, 3.30%; Br, 29.04%; N, 10.18%, found C, 54.47%; H, 3.29%; Br, 29.0%; N, 10.27%.
4.2.7. 6′-Amino-2′,4′-bis(4-bromophenyl)-2-oxo-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2g: yield 1.32 g (48%). Yellow solid, mp 281–283 °C. IR (KBr): 𝜈 = 3407, 2165, 1704, 1622, 1598, 1538, 1487, 1010, 751, 552 cm−1. lH NMR (300 MHz, DMSO-d6): 4.41 (s, 1H, CH), 5.03 (s, 1H, CH), 5.43 (s, 2H, NH2), 6.22 (br s, 1H, Ar), 6.67 (s, 1H, NH), 6.87 (d, J = 7.7 Hz, 2H, Ar), 6.93 (m, 2H, Ar), 7.02 (d, J = 7.7 Hz, 2H, Ar), 7.22 (d, J = 7.7 Hz, 2H, Ar), 7.33 (d, J = 7.7 Hz, 2H, Ar), 7.60 (br s, 1H, Ar), 9.75 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 47.1, 51.5, 54.1, 59.8, 108.3, 119.9, 120.9, 121.1, 123.2, 123.9, 128.0, 128.9, 130.0 (2C), 130.2 (2C), 130.5 (2C), 131.4 (2C), 136.2, 137.9, 141.3, 158.0, 174.2 ppm. MS (EI, 70 eV) m/z (%) = 550 (M, 81Br, 79Br, 3), 302 (10), 301 (76), 300 (40), 299 (100), 298 (26), 274 (10), 273 (43), 272 (6), 271 (42). Anal. Calcd. for C25H18Br2N4O (550.25): C, 54.57%; H, 3.30%; Br, 29.04%; N, 10.18%, found C, 54.47%; H, 3.29%; Br, 29.0%; N, 10.27%.
4.2.8. 6′-Amino-2′,4′-bis(4-(tert-butyl)phenyl)-2-oxo-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2h: yield 1.39 g (55%). White solid, mp 260–261 °C. IR (KBr): 𝜈 = 3381, 2963, 2178, 1700, 1622, 1599, 1525, 1515, 1473, 572 cm−1. lH NMR (300 MHz, DMSO-d6): 1.15 (s, 18H, CH3), 4.35 (s, 1H, CH), 4.95 (s, 1H, CH), 5.31 (s, 2H, NH2), 6.14 (d, J = 7.1 Hz, 1H, Ar), 6.53 (s, 1H, NH), 6.83 (d, J = 8.1 Hz, 2H, Ar), 6.91 (m, 6H, Ar), 7.14 (d, J = 8.1 Hz, 2H, Ar), 7.56 (d, J = 7.1 Hz, 1H, Ar), 9.65 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 31.0 (3C), 31.1 (3C), 34.0 (2C), 47.5, 51.9, 54.0, 60.3, 107.9, 120.6, 123.6 (2C), 123.7, 123.8, 124.4 (2C), 127.6, 127.9 (2C), 129.0 (2C), 129.8, 134.3, 135.3, 141.6, 148.4, 150.1, 157.9, 174.6 ppm. MS (EI, 70 eV) m/z (%) = 505 ([M+H]+, 5), 448 (1), 386 (1), 328 (3), 277 (100), 226 (9), 170 (8), 132 (3), 77 (1), 28 (7). Anal. Calcd. for C33H36N4O (504.29): C, 78.54%; H, 7.19%; N, 11.10%, found C, 78.47%; H, 7.20%; N, 11.20%.
4.2.9. 6′-Amino-2′,4′-bis(3-pyridyl)-2-oxo-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2i: yield 0.77 g (39%). White solid, mp 289–290 °C. IR (KBr): 𝜈 = 3400, 3238, 3074, 2163, 1702, 1616, 1554, 1473, 1021, 715 cm−1. lH NMR (300 MHz, DMSO-d6): 4.48 (s, 1H, CH), 5.11 (s, 1H, CH), 5.48 (s, 2H, NH2), 6.17 (d, J = 7.2 Hz, 1H, Ar), 6.85 (s, 1H, NH), 6.92–7.02 (m, 2H, Ar), 7.07–7.22 (m, 2H, Ar), 7.36 (d, J = 7.5 Hz, 1H, Ar), 7.46 (d, J = 7.5 Hz, 1H, Ar), 7.65 (d, J = 6.7 Hz, 1H, Ar), 7.99 (s, 1H, Ar), 8.19 (s, 1H, Ar), 8.23 (d, J = 4.6 Hz, 1H, Ar), 8.31 (d, J = 4.6 Hz, 1H, Ar), 9.85 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 44.9, 50.8, 54.2, 58.1, 108.4, 121.1, 122.5, 122.9, 123.2, 124.1, 128.3, 128.4, 132.3, 133.8, 135.4, 136.6, 141.2, 148.1, 149.3, 149.4, 150.3, 158.3, 174.1 ppm. MS (EI, 70 eV) m/z (%) = 394 (M, 4), 329 (1), 318 (1), 262 (1), 222 (100), 194 (24), 144 (24), 104 (5), 89 (17), 43 (17). Anal. Calcd. for C23H18N6O (394.15): C, 70.04%; H, 4.60%; N, 21.31%, found C, 70.01%; H, 4.65%; N, 21.43%.
4.2.10. Methyl 4-(2′-(4-acetoxyphenyl)-6′-amino-5′- cyano-2-oxo-1′,4′-dihydro-2′ H- spiro[indoline-3,3′-pyridine]-4′-yl)benzoate
2j: yield 1.32 g (52%). White solid, mp 272–273 °C. IR (KBr): 𝜈 = 34448, 3346, 2162, 1702, 1599, 1542, 1436, 1285, 1107, 740 cm−1. lH NMR (300 MHz, DMSO-d6): 3.76 (s, 6H, OMe), 4.55 (s, 1H, CH), 5.16 (s, 1H, CH), 5.48 (s, 2H, NH2), 6.12 (d, J = 7.4 Hz, 1H, Ar), 6.79 (s, 1H, NH), 6.87–7.00 (m, 2H,Ar), 7.06 (d, J = 7.4 Hz, 2H, Ar), 7.21 (d, J = 8.1 Hz, 2H, Ar), 7.60 (d, J = 8.1 Hz, 2H, Ar), 7.64–7.70 (m, 3H, Ar), 9.71 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 47.5, 51.3, 51.9, 52.0, 54.2, 60.1, 108.2, 120.9, 123.2, 124.1, 127.9 (2C), 128.0, 128.1, 128.3 (2C), 128.5 (2C), 128.6, 129.2, 129.7 (2C), 141.2, 142.1, 144.3, 158.0, 165.7, 166.0, 174.0 ppm. MS (EI, 70 eV) m/z (%) = 508 (M, 9), 438 (1), 391 (1), 345 (1), 279 (100), 220 (16), 192 (7), 144 (8), 104 (1), 44(1). Anal. Calcd. for C29H24N4O5 (508.17): C, 68.49%; H, 4.76%; N, 11.02%, found C, 68.40%; H, 4.80%; N, 11.10%.
4.2.11. 6′-Amino-2′,4′-di(furan-2-yl)-2-oxo-1′,4′-dihydro-2′ H-spiro[indoline-3,3′-pyridine]-5′-carbonitrile
2k: yield 0.80 (43%). Pale brown solid, mp 248–249 °C. IR (KBr): 𝜈 = 3442, 3331, 3180, 2180, 1703, 1620, 1591, 1473, 1016, 744 cm−1. lH NMR (300 MHz, DMSO-d6): 4.52 (s, 1H, CH), 5.10 (s, 1H, CH), 5.38 (s, 2H, NH2), 5.85 (d, J = 3 Hz, 1H, fur), 5.95 (d, J = 3 Hz, 1H, fur), 6.09–6.20 (m, 2H, fur), 6.37 (d, J = 7.4 Hz, 1H, Ar), 6.45 (s, 1H, NH), 6.87 (t, J = 7.3 Hz, 1H, fur), 6.97 (t, J = 7.3 Hz, 1H, fur), 7.21–7.40 (m, 3H, Ar), 9.92 (s, 1H, NH) ppm. 13C NMR (75 MHz, DMSO-d6): 41.8, 50.7, 51.0, 54.7, 107.2, 107.8, 108.0, 110.0, 110.2, 120.9, 122.9, 124.5, 127.8, 129.6, 141.4, 141.8, 142.4, 150.3, 152.6, 157.3, 174.4 ppm. MS (EI, 70 eV) m/z (%) = 372 (M, 7), 328 (1), 298 (1), 240 (1), 211 (100), 183 (6), 154 (16), 107 (4), 77 (2), 39 (4). Anal. Calcd. for C21H16N4O3 (372.12): C, 67.73%; H, 4.33%; N, 15.05%, found C, 67.63 %; H, 4.33%; N, 15.18%.
Supplementary data
CCDC 1901210 contains the supplementary crystallographic data for this paper, which can be obtained from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk.




 CC-BY 4.0
CC-BY 4.0