1. Introduction
Schematic representation of crystalline pathologies; we can distinguish two different families according to the origin of the abnormal deposit.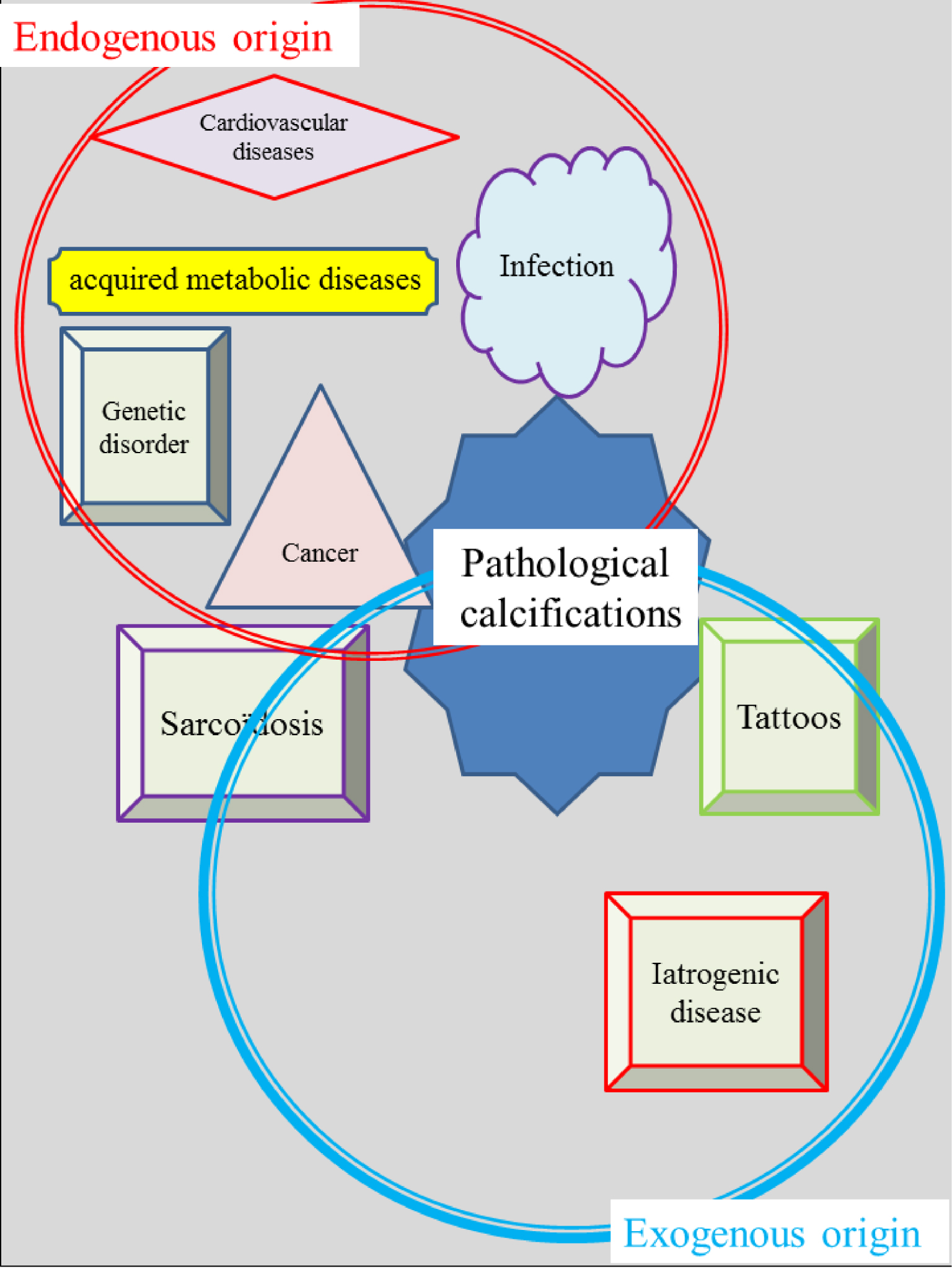
Among crystalline pathologies, urinary lithiasis (from the Greek lithos,) was widespread in ancient populations [1, 2]. In fact, crystalline pathologies [3, 4, 5, 6, 7, 8, 9, 10, 11] (Figure 1) can affect all human organs and encompass several major diseases such as cancer [12, 13, 14, 15, 16, 17, 18, 19], cardiovascular [20, 21, 22, 23], infection [24, 25, 26, 27, 28, 29, 30, 31], genetic [32, 33, 34, 35, 36, 37] and neurodegenerative disorders [38, 39] and rheumatological diseases [40]. The pathogenesis of calcifications can be observed in the case of new public health problems such as the one related to Zika virus, in which the presence of cerebral calcifications has been emphasized [41, 42, 43].
At this point, it is worth underlining that some of these pathologies are highly prevalent; urolithiasis affects 10% of the population [44] while the prevalence of osteoarthritis is approximately 10% in men and 13% in women among adults 60 years of age or older [40].
The complexity of such research also arises from the multiple origins of the pathologies. Figure 1 gives a schematic representation of some crystalline pathologies. We distinguish the ones in which the abnormal deposits are generated inside the body with physiological participation, while in the second family it is the introduction of foreign chemical compounds which initiate the disease. For example, primary hyperoxaluria leads to the formation of calcium oxalate kidney stones from endogenous calcium and oxalate in the human body [45, 46] while in some cases of sarcoidosis, it is the insertion of silica in the skin which induces the formation of cutaneous granuloma [47, 48, 49, 50, 51, 52, 53, 54]. Note that in sarcoidosis the presence of calcium carbonate calcifications from endogenous calcium and carbonate has also been emphasised [53]. Recently, we have considered the case of tattoos [55] for which the number of organic and inorganic compounds inserted in the skin is huge [56, 57]. Iatrogenic disease is the third component leading to abnormal deposits of exogeneous origin that we have considered [58, 59, 60, 61, 62, 63, 64, 65, 66]. In fact, exogenous and endogenous factors often may be intertwined in a calcification process (for example, in the case of JJ stent encrustation, the foreign body promotes heterogeneous nucleation in patients with hypercalciuria and/or urinary tract infection). The endogenous as well as the exogenous origin of the pathological calcifications explains their broad chemical diversity.
What is the exact relationship between the calcification and the pathology? The answer is quite complex. Sometimes the calcification is classified as benign by the clinician, as in the case of breast calcifications composed of calcium oxalate dihydrate. Such type I microcalcifications are found only in benign cysts and are not associated with carcinoma or epithelial hyperplasia [67]. By contrast, breast calcifications made of calcium phosphate may be, but are not always, associated with tumor processes [67, 68, 69]. Regarding the urinary tract, there is evidence linking the formation of kidney stones containing struvite to urinary tract infection [70, 71, 72, 73, 74]. In contrast, the calcification processes of some bacteria induced by a serum calcification factor may be a component of the vertebrate immunological response against bacterial infections [75]. Finally, recent publications indicate that ectopic calcification is an active biological process and may induce a modification of cellular phenotype [76].
In this contribution, we would like to discuss and highlight the different kinds of research we have performed around the processes of biological mineralization and, more generally, on the presence of abnormal deposits in tissues. Some well-chosen examples will show that, despite a number of significant publications in this field, many experiments still remain to be performed to describe their pathogenesis precisely.
2. On the definition of crystalline pathologies
A classic definition of the chemical composition of abnormal deposits in the human body is “the accumulation of calcium salts in a tissue”. Such a definition is completely inadequate. In the kidney for instance many chemical compounds without calcium have been identified (Figure 2). Field Emission Scanning Electron Microscopy (FE-SEM) offers the opportunity to observe them at the submicrometer scale [77, 78, 79, 80].
Examples of chemical compounds without calcium identified in the kidney. (A) 2,8-dihydroxyadenine (6-Amino-1H-purine-2,8(7H,9H)-dione, C5H5N5O2; (B) Uric acid (7,9-dihydro-1H-purine-2,6,8(3H)-trione, C5H4N4O3) (C) L-cystine (2-amino-3-(2-amino-2-carboxy-ethyl)disulfanyl-propanoic acid, L–C6H12N2O4S2); (D) Struvite (Magnesium ammonium phosphate, MgNH4PO4 ⋅ 6H2O); (E) kidney stone containing a mixture of calcium oxalate monohydrate, calcium oxalate dihydrate and carbonated apatite.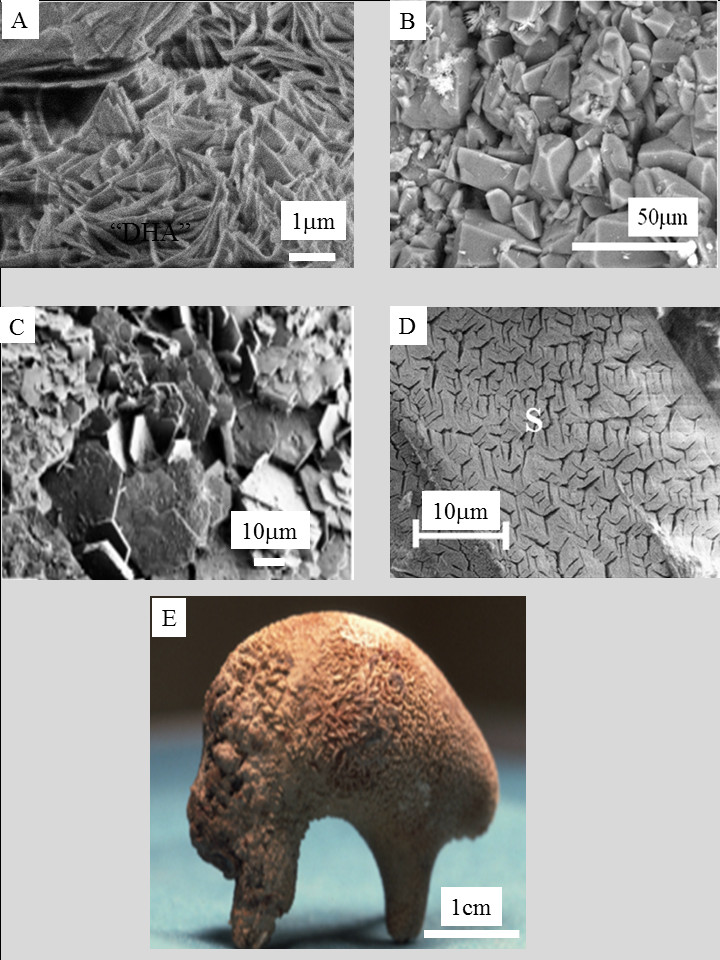
Figure 2 exemplifies some of them more precisely: dihydroxyadenine crystals (Figure 2A) related to the genetic disease adenine phosphoribosyltransferase (APRT) deficiency [81, 82, 83, 84, 85, 86], uric acid (Figure 2B) as a consequence of metabolic syndrome and derangement of renal acid–base metabolism, resulting in a lower urine pH and an increased risk of uric acid stone disease [87, 88, 89, 90, 91], cystine (Figure 2C) accounting for 1–2% of all cases of urolithiasis [92, 93, 94, 95, 96] and related to cystinuria, an autosomal recessive disorder resulting in a transport defect of dibasic aminoacids through the tubular membrane of the nephron, and struvite (Figure 2D) considered as a marker of urinary tract infection by urea-splitting micro-organisms [25, 70, 71, 72, 73, 74]. Furthermore, in examples of iatrogenic pathologies, several compounds corresponding to drugs and their metabolites have been reported [58, 59, 60, 61, 62, 63, 64, 65, 66].
Other compounds without calcium have been identified in other organs as well [97, 98, 99, 100, 101, 102]. Among them, we can mention cholesterol in gall bladder [98, 99], or monosodium urate causative for gout in joints [101, 102]. Finally, some chemical compounds contain calcium but are amorphous, as in the case of amorphous carbonated calcium phosphate which has been identified in different organs like breast [13, 103], thyroid [104, 105, 106] and kidney [3, 107]. “Crystalline pathology” is thus not really an appropriate definition and “pathologies related to abnormal deposits” may be a better one. However, it is a fact that numerous pathological deposits are composed of calcium salts. Today, as illustrated in Figure 2E, the majority of kidney stones is made of a mixture of calcium oxalates and calcium phosphates [108, 109, 110].
3. Characterization by physicochemical techniques
Chemical compounds may be present in abnormal deposits as different crystalline phases that may be related to different biological conditions [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. Moreover, as well demonstrated for urinary stones [111, 112, 113, 114, 115, 116, 117, 118, 119, 120], the morphological characteristics of these concretions may be a marker of specific pathological conditions regardless of the crystalline phase. Finally, a large diversity of molecules may be involved in crystalline pathologies. For example, more than 105 compounds have been identified in kidney stones [121] and more than 25 compounds have been identified by μFTIR spectroscopy in kidney biopsies [64, 122, 123].
Taking into account this broad chemical diversity of abnormal tissue deposits, conventional hospital staining procedures cannot be considered to precisely determine their chemical composition [124]. In fact, however, characterization by techniques such as FT-IR spectroscopy has been routinely used at the hospital for several decades for determination of kidney stone composition [125, 126, 127, 128, 129]. Figure 3 shows the four experimental devices routinely used at the “Service des Explorations Fonctionnelles” at the Tenon Hospital.
Using this platform, more than 85,000 kidney stones have been analysed by FTIR spectroscopy, as well as more than 2000 biological tissues, including more than 1600 kidney biopsies. Such investigations, involving several public or private analytical laboratories, are accepted by the French social security system. Surprisingly, this is discussed as being only a future reality in a recent article on the subject “is infrared spectroscopy ready for the clinic?” in a journal dedicated to analytical chemistry [130].
Experimental devices used at the service des explorations fonctionnelles at the Tenon Hospital. (A) Stereomicroscopy for morphological analysis of stones, (B) optical microscope with polarized light for examination of crystals in tissue, (C) and (D) Fourier transform infrared spectrometer Vector 22 (C) and Invenio (D) from Bruker Optics for identifying crystalline phases of stones, (E) and (F) μFTIR Spotlight 400 imager from Perkin–Elmer for identifying crystals observed in tissue biopsy slices.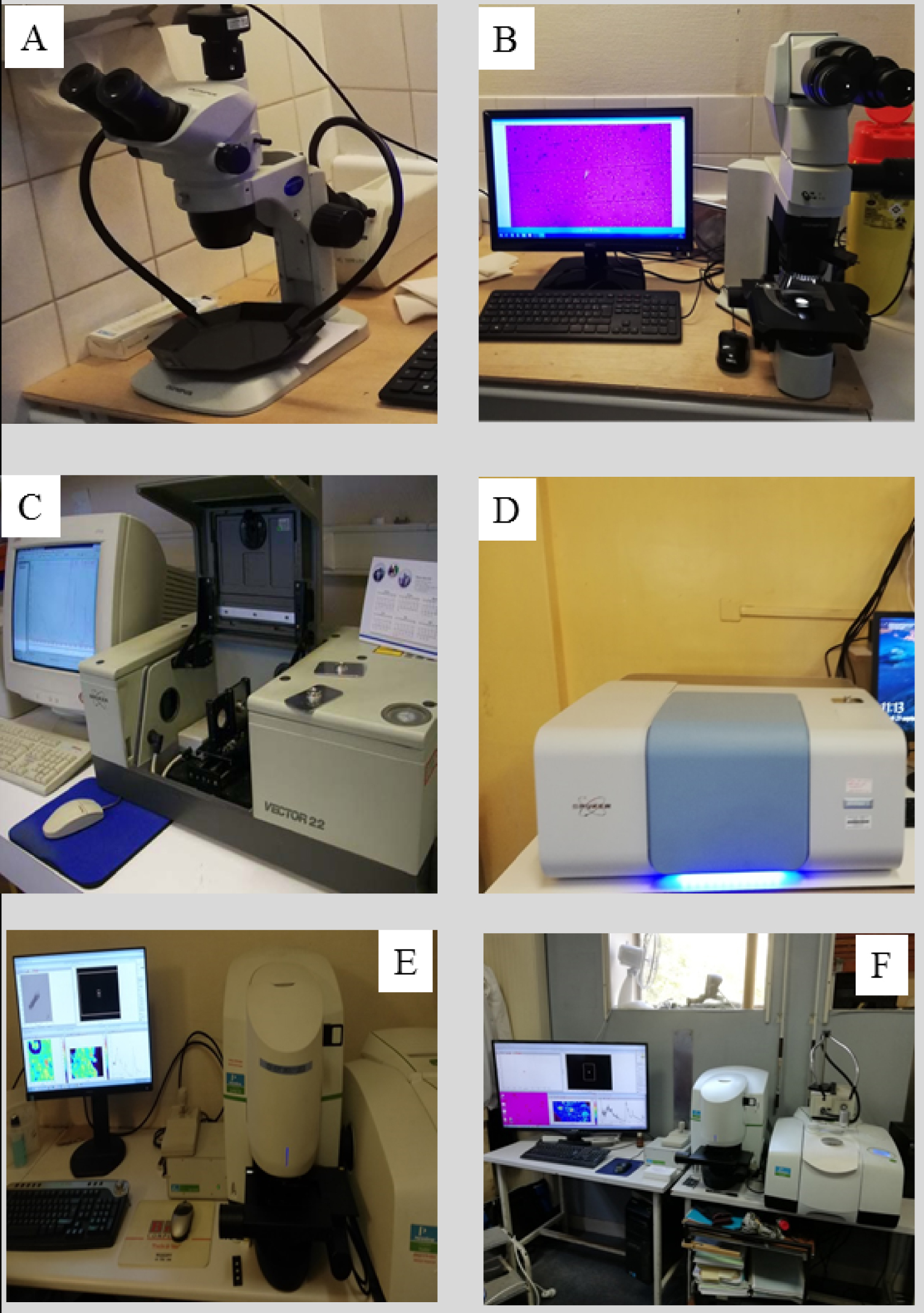
Figure 4 shows different techniques used in our research on predominantly human samples [131, 132, 133, 134, 135]. Some of them are “classics” i.e. Raman and Infra-Red spectroscopies [136, 137, 138, 139], Second Harmonic Generation (SHG), Scanning and Transmission Electron Microscopy (SEM and TEM) [80, 140, 141, 142], μX-ray fluorescence [143, 144, 145, 146, 147], μX-ray diffraction [148, 149, 150, 151, 152, 153] or Nuclear Magnetic Resonance (NMR) [154, 155, 156, 157, 158, 159].
Schematic of our pathological calcification research axis. While some in-lab characterization techniques can be considered as diagnostic tools, access to those associated with large scale instruments is governed by committee which makes rapid clinical applications difficult.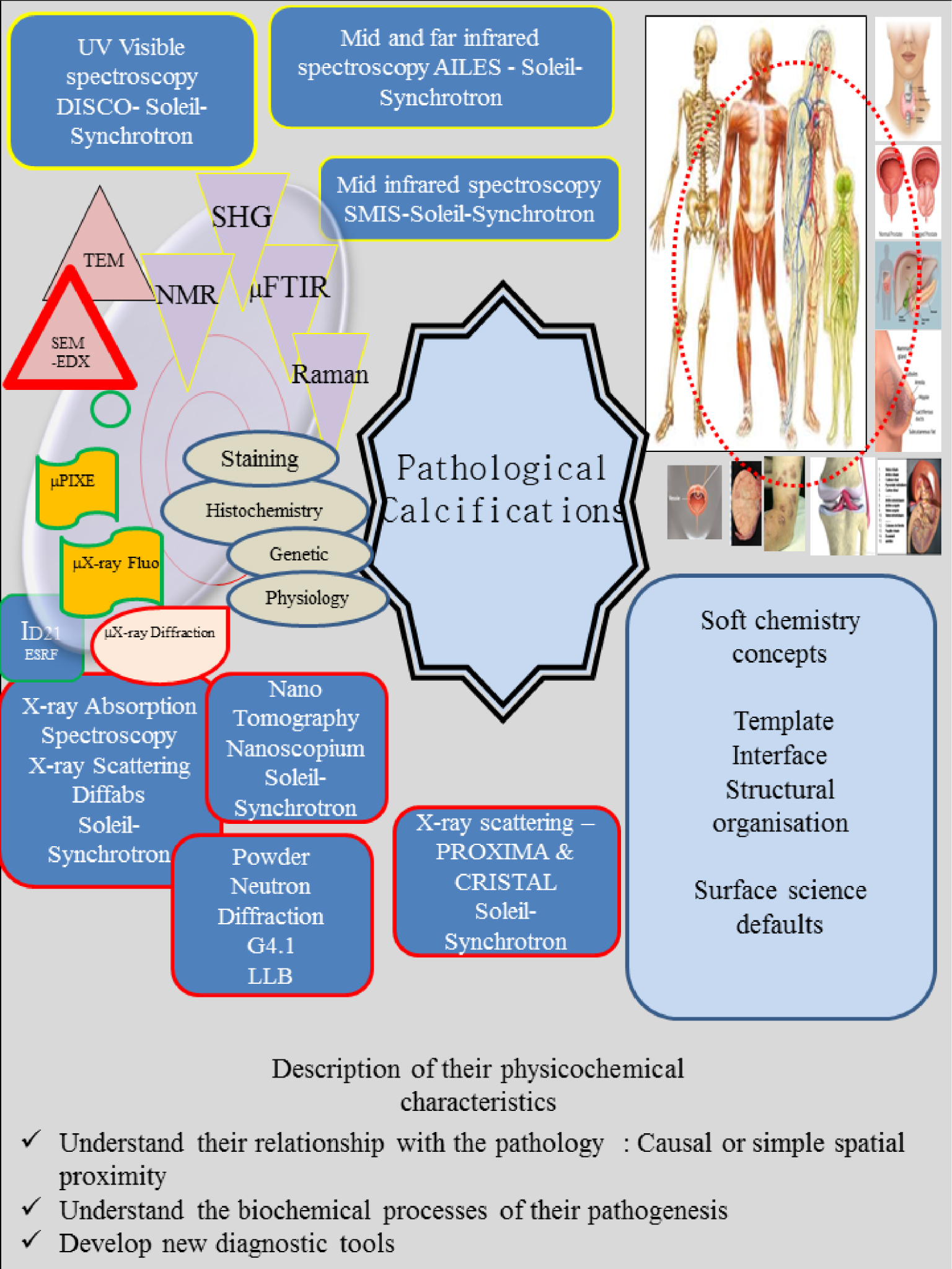
Other techniques, implemented in a synchrotron radiation centre [131, 132, 133, 134, 135], include X-ray absorption spectroscopy (Diffabs beamline) [160, 161, 162, 163, 164, 165], μX-ray diffraction (Proxima and Cristal beamlines) [166, 167], nanotomography (Nanoscopium beamline) [168], and μFTIR (SMIS beamline) [123]. We have also performed experiments on location at a nuclear research reactor [90, 93, 131, 169, 170], as well as at the European Synchrotron Radiation Facility [147]. All these physicochemical techniques complement the conventional ones implemented at the hospital such as histochemistry, staining and genetic analysis.
We may distinguish several stages in this research (Figure 5). The starting point (Figure 5A) is based on optical microscopic and FTIR spectroscopic observations, leading to a morphoconstitutional model [111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122].
Different stages in pathological calcification characterization. (A) Morphoconstitutionnal analysis of kidney stones by optical microscopy and FTIR spectroscopy (B) Micrometer scale analysis, (B1) SEM imaging, (B2) 3D micrometer scale tomography, (B3) Chemical mapping via μFTIR spectroscopy, (C) Nanometer scale analysis, (C1) TEM observations, (C2) X-ray fluorescence mapping (data collected on the Synchrotron Soleil Nanoscopium beamline), (C3) Nanometer scale IR data collected in AFMIR configuration (Spatial resolution of 50 nm).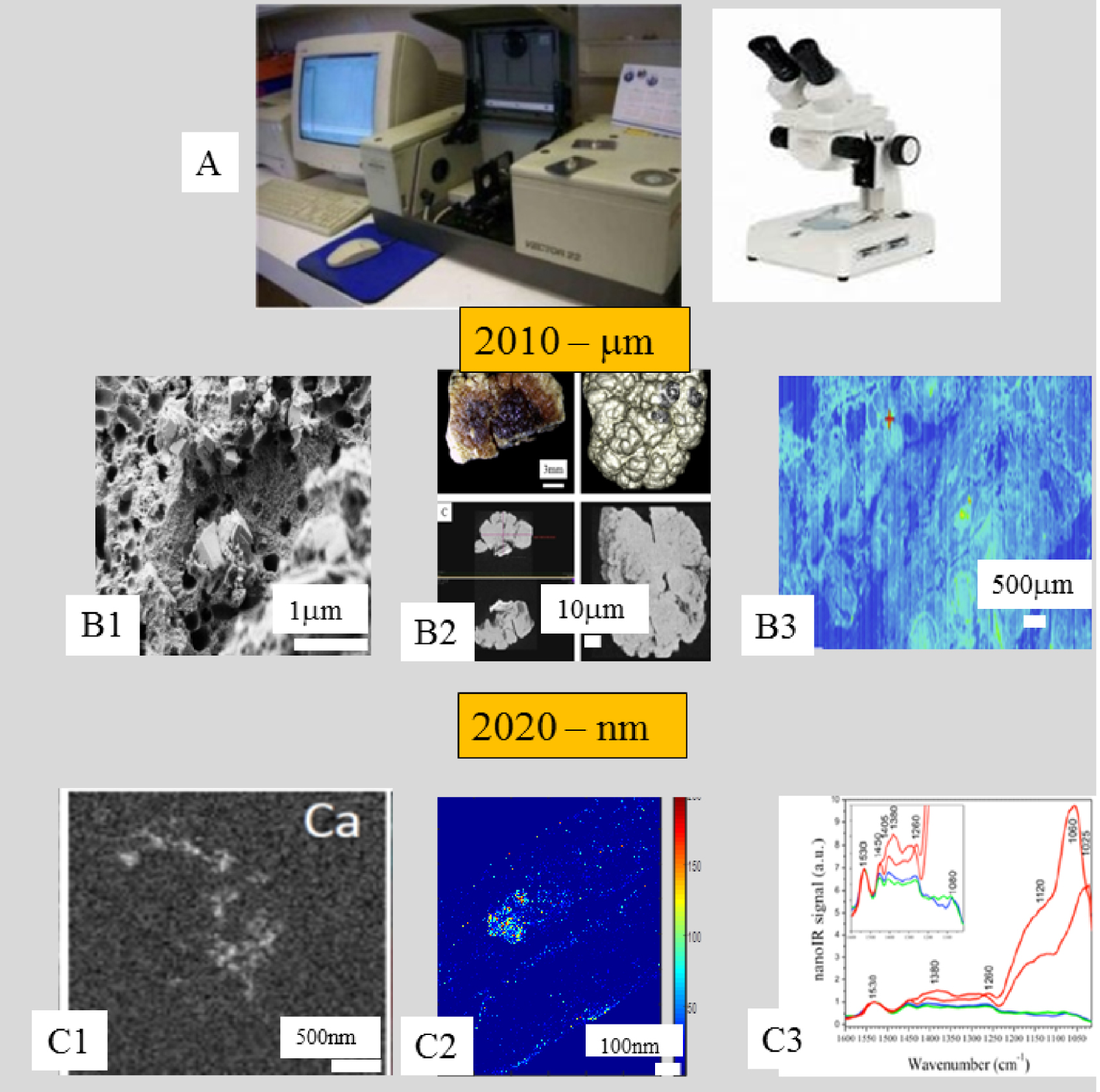
Letter of J. Friedel regarding the interaction between NO and a nanometer scale metallic cluster.
Some fundamental aspects of the adsorption process.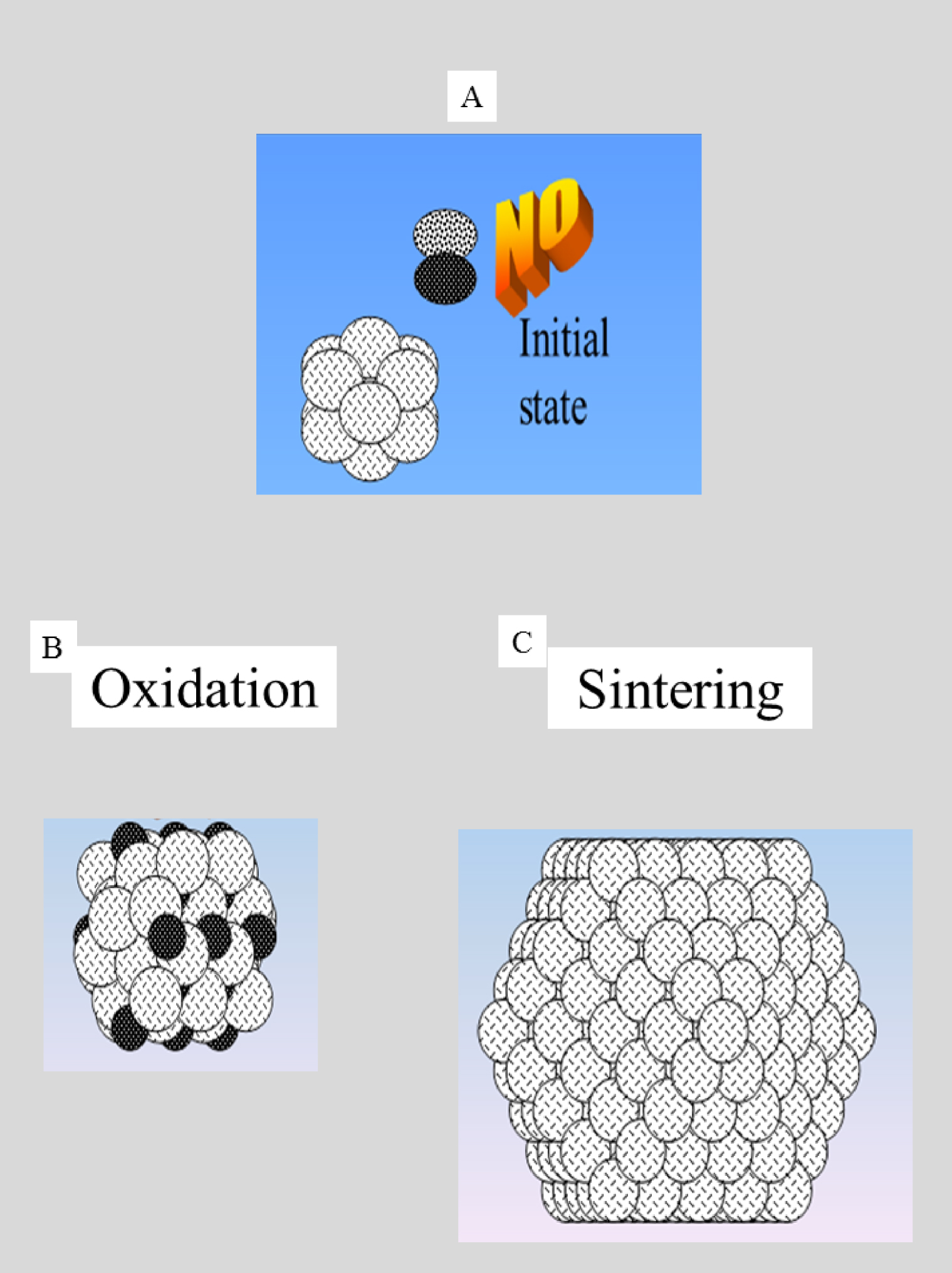
From there, we proceed to micrometer scale characterization, including determination of trace elements in order to assess their role in the pathogenic process [134, 135]. Currently we also use characterization techniques to describe the pathological calcifications at the nanometer scale. We are able to assess not only the morphology but also the chemistry of pathological calcifications either by determining the elements which constitute the abnormal deposit [141, 142] or by an identification of specific chemical compounds at the nanometer scale [171, 172]. To achieve this, we have combined atomic force microscopy and IR lasers (AFMIR) [173, 174], TEM integrated with electron energy loss spectromicroscopy [175], and synchrotron radiation-induced nano X-ray fluorescence [176].
We have already investigated biological samples from different organs and parts of the human body, namely kidney [177, 178, 179, 180, 181, 182, 183], prostate [184, 185], breast [13, 103], thyroid [104, 105, 106], cartilage [186, 187, 188, 189, 190, 191, 192, 193], bone [194, 195, 196, 197], tooth [198], pancreas [199], skin and hairs [53, 54, 55, 147, 165, 168, 200, 201, 202, 203] and cardiovascular system [140, 204, 205]. We have also investigated cells [206], mice [207, 208, 209, 210], and medical devices [211, 212], as well as chemical compounds identified in kidney stones synthesised using microfluidics [213]. Investigations of such diverse biological samples requires collaboration between physico-chemists and the medical community and leads smoothly to the establishment of efficient networks. Regarding the analysis procedure, it is worth emphasizing that all these data are analysed taking into account the soft chemistry concepts introduced by Professor J. Livage (Collège de France), as well as those rooted in surface science (Figure 4).
Finally, we have recently complemented our research via theoretical Density Functional Theory (DFT) approaches [214, 215, 216, 217], an efficient approach to predict and elucidate the crystallographic structure of compounds such as whitlockite [215] or calcium oxalate polyhydrates [216]. Particular effort has recently been devoted to modelling the interaction between proteins and calcification processes. For this purpose, we simplify interactions by modelling calcification components as nanometer scale metallic clusters and the proteins as simple diatomic molecules [218, 219]. Even though this is a drastic simplification, the parameters which determine the differences between molecular, and dissociative, adsorption, such as temperature, the geometrical and the electronic nature of the surface, and the adsorption site are not well understood [220, 221]. Figure 6 shows a letter from Professor J. Friedel supporting this hypothesis based on which such investigations have to be performed. Although simplified, much can be learnt from this system.
Figure 7 shows the initial state (nanometer scale metallic particle, and a NO molecule), and subsequent possibilities for mutual interaction [222, 223, 224].
Dissociative adsorption of NO on the metallic cluster leading to the formation of an oxide and the desorption of N2 [225] is the first possibility. The second is physisorption of the NO on the metallic cluster, which can induce a significant growth of the nanometer scale cluster [226, 227]. What can we learn from these alternative possibilities? In the first case, we have a significant modification of the chemistry of the nanometer scale cluster (from metallic to an oxide) and a significant modification of the adsorbed molecule (dissociation process). Returning to our model in which the metallic cluster represents the calcification and the NO molecule the protein, it is clear that the final character of the calcifications, and by analogy with NO, the protein, can differ considerably from the initial. Such a fundamental approach is a necessary step in understanding precisely how molecules interact with nanocrystals and possibly modify their morphology [228, 229, 230, 231, 232].
As a preliminary conclusion, the characterization of pathological calcifications beyond observations initiated from routine clinical observations [111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122] and performed at the hospital (optical microscopy and μFTIR spectroscopy) has led to many significant breakthroughs based on more cutting edge physicochemical characterization techniques. A complete set of results provides more precise information about the relationship between calcification and disease, and the development of new diagnostic tools. All these data have been published in major medical and chemistry journals such as New England Journal of Medicine [35, 36], Chemical Review [3], ACS Nano [142], Arthritis Rheumatology [186, 187], J. Am. Soc. Nephrol. [62, 207], Kidney International [233, 234] and JAMA [200].
4. Diversity in the very first pathogenic stages
Recently, we have overviewed the various different mechanisms described for the nucleation of pathological calcifications [10]. Nephrolithiasis is generally the result of crystal formation, aggregation and retention in the kidney and is thus related to homogeneous nucleation (Figure 8) in supersaturated solutions [235, 236, 237, 238]. Note that Randall’s plaque [239, 240] plays a key role in the pathogenesis of kidney stones [241, 242, 243, 244]. Kidney stones may also be initiated from tubular plugs which are a frequent finding in human distal tubules [244, 245].
As illustrated in several publications, the nature of the deposit may differ according to the specific area of the organ where it was observed, underlining the close relationship to the local tissue physiology [3, 123]. In other pathological conditions like metabolic syndrome and/or diabetes mellitus, multiple supersaturations of both calcium oxalate and uric acid may favour crystallization of one species (for example uric acid) and only secondarily of the second (for example calcium oxalate), explaining why patients suffering these diseases may form mixed stones containing alternating layers of these two compounds. In other cases, the two crystalline species are closely mixed. Notably, uric acid stones also contain calcium oxalate in more than 63% of cases. These two chemical species can crystallize in urine and form aggregates by heterogeneous nucleation or by epitaxial processes [10]. About one third of urine samples containing uric acid crystals also contain those of calcium oxalate.
(a) Homogeneous nucleation. When the patient has a chronic high number of crystallites in urine (a1), a kidney stone is generated (a2). In the case of heterogeneous nucleation, part of the DNA may serve as a nucleus (b1) alizarine staining to reveal the calcification and (b2) immunostaining to visualise DNA). In the case of a Randall’s plaque, crystallites generated through homogeneous nucleation are trapped by a calcification present on the tissue (white spots on c1) and a kidney stone with a RP is generated (white spots on c2). Finally, the presence of vesicles containing calcium salts (phosphate and/or carbonate) has been noted in the kidney (d).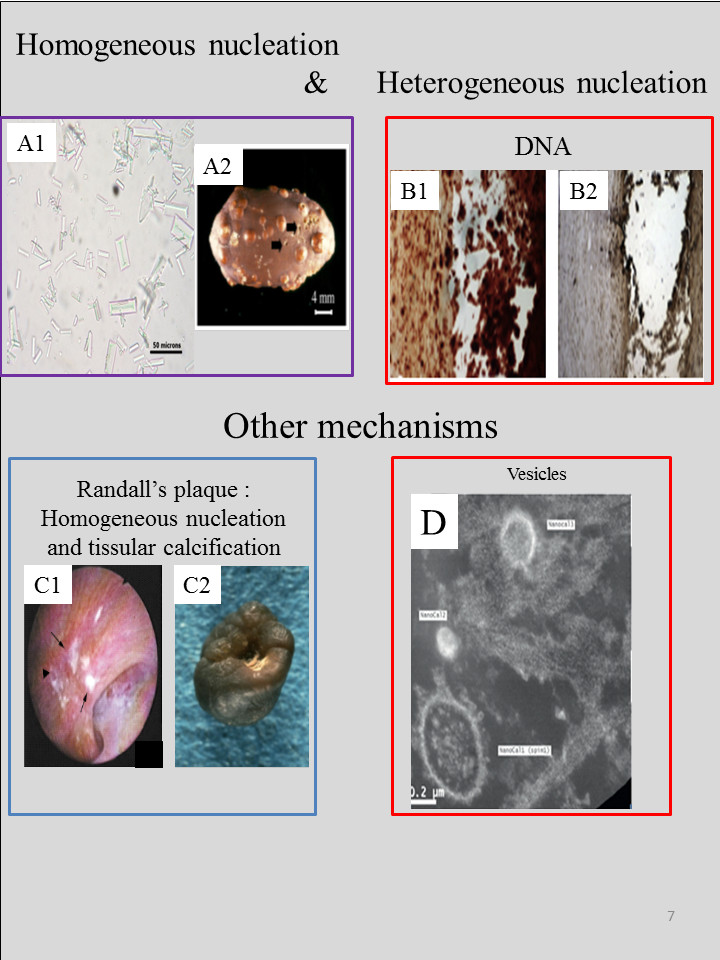
Heterogeneous nucleation is another mechanism involved in the formation of other pathological calcifications. DNA [246] and elastin [247] have been identified as organic scaffolds. The role of other proteins may be more complex. For example, Cerini et al. [248] have studied the promotion of calcium oxalate crystallization by albumin, with specific favouring of the dihydrate form. The authors conclude that due to rapid nucleation of small crystals, saturation levels fall, and thus albumin might be protective.
Thanks to nanometer scale structural descriptions, another mechanism, via extracellular matrix vesicles (MV) containing a high level of alkaline phosphatase, has been identified in the induction of de novo mineral deposition in cartilage, bone, and dentin [249]. The presence of MV in kidney [141, 142, 250] as well as vascular smooth muscle [251] has also been reported.
5. Calcifications versus tissues—How calcifications may alter tissues?
In the case of Randall’s plaque, we know that ectopic calcifications made of calcium phosphate apatite may pass through the tissue and appear at the surface of the papillary epithelium where they play a crucial role in the pathogenesis of kidney stones [239, 240, 241, 242, 243, 244]. In fact, such major tissue alteration occurs in other pathological processes. In the case of epidermal necrolysis, i.e., Stevens Johnson syndrome and toxic epidermal necrolysis, atypical healing retardation with calcinosis cutis, may be explained by the presence of calcification. Lastly, a simple change in wall composition (medial calcium overload of elastic fibers) can decrease aortic elasticity. Elastocalcinosis can induce destruction of elastic fibers, leading to arterial stiffness, which has been associated with the development of left ventricular hypertrophy in a normotensive model [252].
Similar pathological processes may occur in other parts of the human body. For example, in breast cancer, Ductal carcinoma in situ (DCIS) is considered as a non-invasive or pre-invasive breast cancer and is associated with cast-type calcifications. It is possible that these pathological calcifications may break the milk ducts leading to cancer cells spreading into surrounding breast tissue.
6. Presence of pathological calcification and loss of the renal function
An interesting clinical and physicochemical question is raised by the relationship between the presence of pathological kidney calcification and loss of renal function.
Are pathological calcifications responsible for renal dysfunction?
We can open the discussion with the case of kidney stones. The obstruction of one or both ureters prevents urine passing into the bladder and out of the body. If not treated rapidly, kidney damage may ensue, in severe cases leading to kidney failure, sepsis (life-threatening infection), or ultimately death. Such obstructions don’t depend on the chemical phase of the stone.
Intratubular crystalline precipitation can also lead to acute renal insufficiency. For example, resultant blockage of urine flow can also be associated with the presence of plugs composed of apatite or other crystalline phases. These cases involve the collecting duct(s). Some genetic diseases such as APRT deficiency, a rare autosomal recessive disorder, may cause 2,8-dihydroxyadenine stones, and, secondarily, renal failure due to intratubular crystalline precipitation. Repeated obstruction of tubules and interstitial deposits by 2,8-dihydroxyadenine crystals may result in irreversible loss of kidney function [34, 253, 254] and sometimes end-stage renal failure [255, 256, 257, 258]. Finally, several medications, notably acyclovir, sulfonamides, methotrexate, indinavir, and triamterene, are associated with the production of crystals that are insoluble in human urine [61, 62, 63, 64, 259].
This all raises a major question: Is it simply the obstruction which leads to renal insufficiency, or is it in fact tissue inflammation induced by the presence of crystals? Recent studies suggest the likelihood that inflammatory processes act together with obstruction to promote renal damage [86], a process which has been discussed in detail in 2,8-dihydroxyadenine nephropathy [86], and development of Randall’s plaques [260].
Besides this, the presence of only very few crystallites in a biopsy may reveal a pathological condition. In a kidney biopsy in which only a single crystallite of calcium oxalate was observed, UV–visible spectroscopy experiments clearly showed a significant oxalate signal in numerous surrounding cells, suggesting that the presence of even one crystal is an indicator of local supersaturation. Thus it seems that this supersaturation leading to pathogenic crystals also significantly affects neighbouring cells.
7. A specific example: the effect of plant extracts on kidney stones
We should highlight the effects of plants in our urolithiasis research [261]. For example, we have used FE-SEM to assess the ability of plant extracts, namely Arenaria ammophila (leaves and stems), Parietaria officinalis (leaves, and flowers, studied separately), Paronychia argentea (flowers), to dissolve cystine stones in vitro. All these plants are widely claimed to treat or prevent urolithiasis [262] in traditional medicine.
Recently, we have also studied the effect of green tea infusions in hypercalciuric renal stone patients [263]. It is well known that excessive consumption of plants such as rhubarb containing high levels of oxalate may lead to significant quantities of calcium oxalate monohydrate in kidney tissues leading to a loss of kidney function [264, 265]. By contrast, in the case of tea, an oxalate-rich plant, tea extracts, particularly of green tea, may have beneficial clinical effects due to the presence of antioxidant polyphenol compounds such as catechins. In our study, the data showed no evidence for increased stone risk factors or oxalate-dependent stones in daily green tea drinkers [266]. Also, it is worth emphasizing that other studies have been devoted to tea due to the presence of aluminium and other toxic elements in the leaves [267].
Finally it is noteworthy that absorption of large quantities of caffeine may lead, combined with other factors, to the formation of kidney stones; in a recent publication, 1-methyluric acid nephropathy has been identified in three kidney biopsies [268].
8. Beyond pathological calcifications
Note that our research is not limited to pathological calcifications. We also participate in other research fields (Figure 9), namely the nephrotoxicity of Pt anticancer drugs [269, 270], the vectorisation of drugs through “quantum rattle-gold quantum dots” (QR-AuQDs) [271], Wilson’s disease (WD) which is a result of copper accumulation [272], urothelial carcinoma grades [273], and TiO2 particles at hair surfaces [168]. In this research, we take advantage of our previous investigations using μX-ray fluorescence on trace elements in kidney stones and in tissues.
(a1) TEM image of the Au25(4ATP)18 cluster: Spatial repartition of different elements, namely Zn (a2) and Au (a3), obtained using the intensity of the corresponding X-ray fluorescence emission lines (QR-AuQDs exposed rat kidney embedded in paraffin) as contrast image (map: 13.7 mm × 19.3 mm, 30 μm resolution, 20 ms acquisition time). (b1) and (b2): Spatial repartition of Zn and Pt in a kidney biopsy collected on a patient treated with Pt anticancer drugs. (c1 to c4): Tissue sections of 5 μm thickness were stained with HES. Portal tract and centrilobular vein are present on the section of normal liver (patient #3) (upper image). WD liver (patient #46) exhibits nodules surrounded by fibrosis (lower image). Synchrotron radiation XRF spectra were acquired on the area delimited by the black squares. Representative XRF spectra acquired on tissue sections from normal liver and WD showing peaks characteristic of the composite elements.
In the case of Pt anticancer drug nephrotoxicity [269, 270], we have investigated mice as well as patients, the ultimate goal being to prove that such X-ray fluorescence measurements can find a place in clinical practice. Indeed, as we can see in Figures 8 b1 and 8 b2, it is possible to confirm the presence of platinum in patient kidney biopsy. Such data can form part of the patient clinical file and help the clinician to assess the loss of kidney function.
The first example concerns nanometer-scale systems developed recently for the treatment of various severe pathologies [274, 275]. The characterization of such nanomaterials, which can contribute to both diagnosis and therapy, can be easily performed using synchrotron radiation-specific techniques [276, 277, 278, 279, 280, 281, 282, 283, 284], in order to determine their size [276, 277, 278, 279], their morphology [280, 281], the modification of their electronic state during the growth process [282, 283], or the adsorption process of molecules at their surface [284, 285]. Here, using a completely new technology, the “flyscan” mode, it was possible to assess the presence of QR-AuQDs in various rat organs [271]. Such observations constitute a significant breakthrough opportunity to test new nanomaterials with potential medical uses.
The last example focuses on Wilson’s disease, also known as hepatolenticular degeneration, which is a severe disorder of copper homeostasis [272]. Several excellent physicochemistry based studies have been performed on liver [286, 287]. As shown in Figure 8c, X-ray fluorescence is a more efficient technique than the classical staining currently used in the clinic to confirm the presence of copper.
9. Building a national and international network
The different selected examples discussed in this contribution show the complexity of pathological calcification research. The dynamism of our research is proven by some standard publication indicators, such as 135 results on Web of Science for 2119 citations, or 71 results on PubMed.
At the national level, our research involves collaborations among several hospitals, namely Tenon, Bichat, St Louis, Lariboisière, Rothschild, Ambroise Paré in the Paris area and other hospitals in various French regions (CHU of Lille, Toulouse, Nantes, CH of Pontoise…).
At the international level, numerous strong collaborations with research groups in different countries have been established and resulted in several publications. Among them we can cite Dr. A. Pozdzik (Department of Nephrology, Dialysis and Renal Transplantation, Brugmann Hospital, Université Libre de Bruxelles, Brussels, Belgium), Professor F. Tielens (Free University of Brussels, Belgium) , Professor J. C. Williams (Department of Anatomy and Cell Biology, Indiana University School of Medicine, Indianapolis, Indiana, USA) [287, 288], Professor M. Duer and Dr. D. Reid (Department of Chemistry, University of Cambridge, Great Britain), Dr. F. Preitner (Mouse Metabolic Facility of the Cardiomet Center, University Hospital, Lausanne, Switzerland), Dr. J. Bertazzo (Department of Materials, South Kensington Campus, Imperial College, London, Great Britain), and Dr. X. Keller [289] (Zurich hospital, Switzerland).
10. Knowledge transfer
Our collaborations between physicists and physicians provides the opportunity for young doctors from different medical specialties (cardiology, dermatology, nephrology, otorhinolaryngology, rheumatology, urology) to receive training and insights in physicochemical characterization techniques and possibilities.
11. Perspectives on the synchrotron soleil upgrade (In collaboration with Dr. E. Boudelique, Dr. H. Colboc, Dr. E. Esteve)
As previously shown [290, 291, 292], synchrotron radiation plays a pivotal role in our research. Experiments have been performed on seven different beamlines at soleil (SMIS, AILES, DISCO, CRISTAL, PROXIMA, NANOSCOPIUM, DIFFABS), one on ESRF, and one on another large instrument, the LLB (G4.1), where we have used neutrons as a probe, in very diverse research related to urology, nephrology, rheumatology, and dermatology.
In this research, the role of the beamline scientists is clearly of major significance, and numerous fruitful discussions regarding the information yielded by each technique as well as sample preparation protocols have taken place. Thanks to the SMIS beamline, the first set of μFTIR experiments on 15 kidney biopsies served to initiate a research project which was accepted and funded by the CORDDIM, allowing a platform to be created consisting of two μFTIR imagers, on which more than 1600 human kidney biopsies have been performed. The technology allowing this transfer is of prime importance for the clinician.
Following various discussions around the upgrade of the synchrotron Soleil, we have defined some priorities for analysis of tissue at a very small scale. It is of primary importance to be able to precisely know the position of the micro or nano probe on the sample. For exemple, in the case of kidney, the nephron is constituted of different elements, namely the glomerulus, the proximal tubule, the loop of Henle, and the distal tubule, in each of which the biochemical environment (pH, calcium concentration, etc…) can be very different. Calcifications may also take place in the interstitium in the cortex and the medulla, and also in the papilla where we can find Randall’s plaques. A comparison between the chemical composition of pathological calcifications and the normal physiological environment is clearly a key to understand the disease. In a perfect world, the kidney would be placed under the synchrotron beam in such a way that the clinician would be able to position the nanometer probe on a specific organ structure such as a glomerulus, or a proximal or distal tubule. We thus would like to have a functional optical device comparable to those in the hospital (magnification ×100), and be able to know the exact position of the beam on the sample via an optical laser. Also, it will be advantageous to automate rapid sample mounting and demounting, as is already done on various beamlines [293].
In terms of the acquisition procedure, it is of primary importance to define experimental parameters such the size of the map directly onto a touch-sensitive screen without prior knowledge of the software code. Moreover, it must be possible to perform experiments remotely [294].
It is very convenient for the clinician to carry out different experiments on the same sample. For example, on a beamline such Diffabs, it is possible to collect X-ray diffraction as well as X-ray absorption data on the same sample. In the case of cartilage this experimental configuration generates very valuable information from which trace elements and chemical composition of pathological calcifications can be deduced. Also, it would be advantageous if the sample support used for synchrotron experiments is compatible with other techniques such FE-SEM or μFTIR spectroscopy. This will require a significant effort to achieve, particularly for studies at the nanometer scale where new physical challenges will have to be solved.
Nanometer scale measurements may yield significant information regarding interaction between mineral and proteins or between mineral and cells. The first experiments performed on nanoscopium opens a major perspective on the 3D distribution of mineral as well as trace elements such Zn.
12. Conclusion
The quest for characterization techniques to generate significant information on microcrystalline pathologies for the clinician is constantly active. In Figure 10, we present a short list of selected results. The latest efforts towards this goal are related to the fact that these biological entities contain organic as well as inorganic compounds.
(A) SEM as a diagnostic tool for primary hyperoxaluria. (B) μFTIR spectroscopy on kidney tissue as a diagnostic tool, in a case of APRT deficiency. (C) Neutron scattering to assess a drug effect for the treatment of cystinuria. (D) NanoUV spectroscopy to assess the presence of oxalate in kidney tissue.(E) Micro X-ray fluorescence performed at ESRF indicating the presence of Al in kidney stone. (F) SEM illustrating the process of kidney stone growth on RP. (G) SEM as a diagnostic tool for urinary infection in the absence of clinical symptoms (H) Neutron scattering to define UA crystal size modifications which occur between metabolic syndrome and diabetes (I) NanoIR indicatinging the presence of cysteine in intracellular crystals. (J) X-ray fluorescence showing an abnormal quantity of Zn in RP, indicating an inflammatory process. (K) Xanes spectra collected at Ca K edge of RP (L) NanoX-ray fluorescence performed on Synchrotron Soleil indicating the presence of Ti on hair.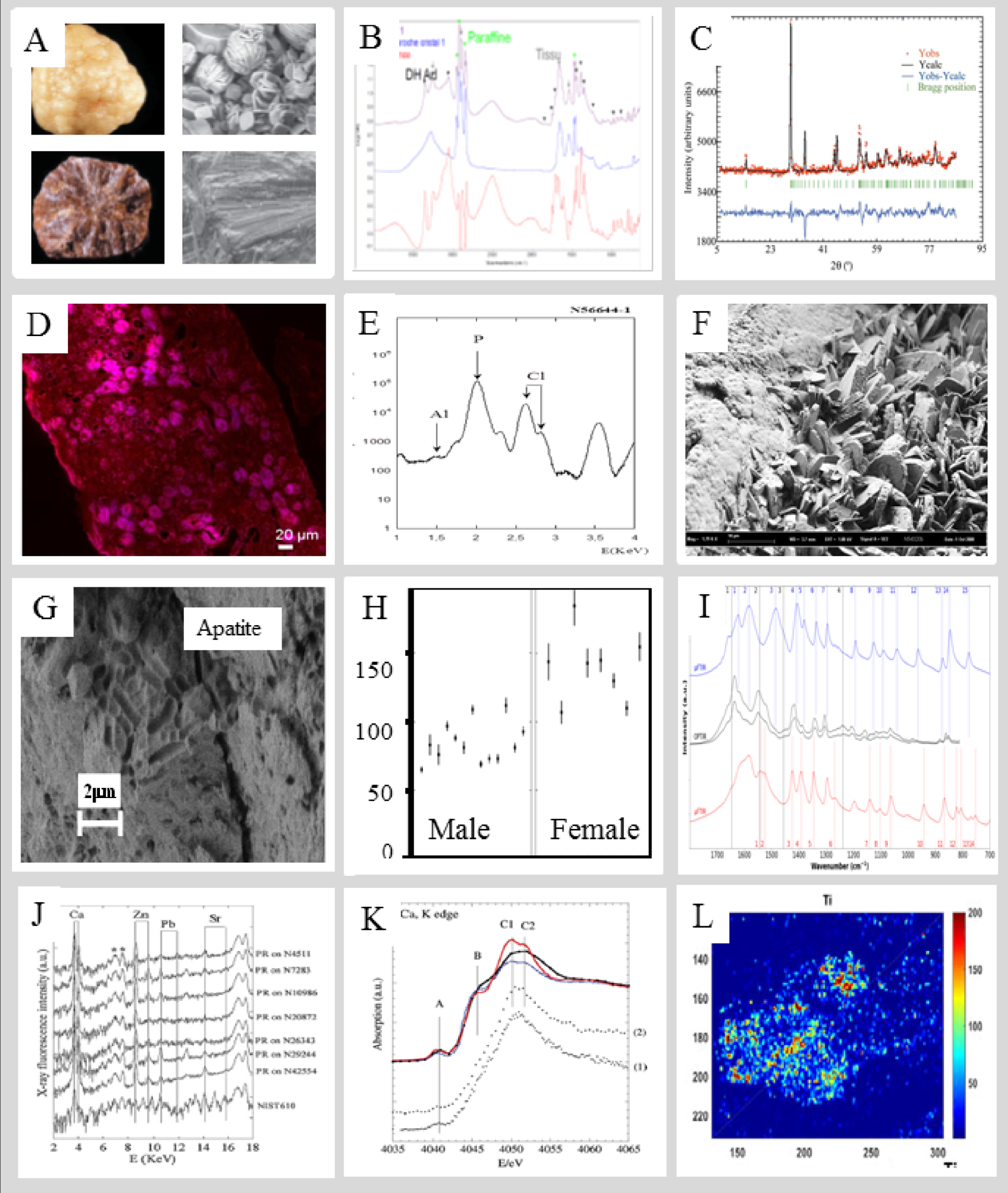
This prompted us to perform experiments on human biopsies using non-linear optical spectroscopy, namely Second Harmonic Generation (SHG), as well as fluorescence, in order to assess its potential on ectopic calcifications. Non-linear optical spectroscopy is a non-invasive technique which enables investigations of different biological tissues based on SHG spectroscopy [295, 296, 297, 298]. Among the different indispensable laboratory techniques allowing characterization of nanometer scale materials [299] are NanoIR i.e. AFM-IR [171, 172, 173, 174] as well as PTO-IR (Optical PhotoThermal IR) [300, 301], which should lead to major breakthroughs in the coming years.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgments
It is a great honour to thank all the people who have played a significant role in building this interface between physics, chemistry and medicine: Dr. X. Carpentier (Nice Hospital), Dr. Ch. Chappard (Lariboisière Hospital), Professor P. Conort (La Pitié-Salpetrière Hospital), Dr. P. Dorfmüller (La Pitié-Salpetrière Hospital), Dr. E. Estève (Tenon Hospital), Professor D. Hannouche (Lariboisière Hospital), Professor P. Jungers (Necker Hospital), Professor B. Knebelman (Necker Hospital), Dr. E. A. Korng (Lariboisière Hospital), Professor F. Liote (Lariboisière Hospital), Dr. M. Livrozet (Tenon Hospital), Professor M. Mathonnet (Limoges Hospital), Professor P. Méria (Saint-Louis Hospital), Dr. C. Nguyen (Lariboisière Hospital), Dr. J. Rode (Tenon Hospital), Professor P. Ronco (Tenon Hospital), Dr. I. Tostivint (La Pitié-Salpetrière Hospital), Professor O. Traxer (Tenon Hospital) and Professor J. C. Williams (Department of Anatomy and Cell Biology, Indiana University School of Medicine, Indianapolis, Indiana, USA) for providing samples and useful discussions.
Also, regarding the physicochemistry, this research could not have been performed without the scientific advice of Dr. P.-A. Albouy (LPS), Dr. G. André (LLB), Dr. A. Bianchi (INSERM-U7561), Dr. P. Chevallier (LURE), Dr. A. Cousson (LLB), Dr. P. Dumas (SOLEIL Synchrotron), Professor M. Duer (Department of Chemistry, University of Cambridge, United kingdom), Dr. E. El Kaim (SOLEIL Synchrotron), Dr. B. Fayard (LPS-ESRF), Dr. E. Foy (Laboratoire Pierre-Süe), Dr. J.-L. Hazemann (ESRF), Dr. L. Hennet (CEMHTI), Dr. F. Jamme (SOLEIL Synchrotron), Dr. A. Lebail (Laboratoire des Fluorures), Dr. F. Lenaour (Hôpital Paul Brousse), Dr. O. Mathon (ESRF), Dr. K. Medjoubi (SOLEIL Synchrotron), Dr. G. Matzen (CEMHTI), Dr. C. Mocuta (SOLEIL Synchrotron), Dr. R. Papoular (CEA), Dr. P. Reboul, (UMR 7561), Dr. M. Réfrégiers (SOLEIL Synchrotron), Dr. S. Reguer (SOLEIL Synchrotron), Dr. D. Reid (Department of Chemistry, University of Cambridge), Dr. S. Rouzière (LPS), Dr. S. Kaščáková (Hôpital Paul Brousse), Dr. J.-P. Samama (SOLEIL Synchrotron), Dr. C. Sandt (SOLEIL Synchrotron), Dr. M. C. Schanne-Klain (LOB, Polytechnique), Dr. D. Reid (Department of chemistry, Cambridge University, United kingdom), Dr. A. Somogy (SOLEIL Synchrotron), Dr. D. Thiaudière (SOLEIL Synchrotron), Dr. E. Véron (CEMHTI) and Dr. R. Weil (LPS).
This work was supported by the Physics and Chemistry Institutes of CNRS and by contracts ANR-09-BLAN-0120-02, ANR-12-BS08-0022, ANR13JSV10010-01, convergence UPMC CVG1205, Labex Matisse, Labex Michem and CORDDIM-2013-COD130042. The authors are grateful to the SOLEIL Synchrotron Facility and the Leon Brillouin laboratory for beam time allocation.




 CC-BY 4.0
CC-BY 4.0