1. Introduction
Epidermal necrolysis (EN) (i.e., Stevens Johnson syndrome and toxic epidermal necrolysis (TEN) according to the extent of detached body surface area) is a rare life-threatening condition, usually drug-induced, characterised by a diffuse epidermal and mucosal detachment. Skin histology reveals pan epidermal necrolysis. Mucocutaneous healing usually lasts 2 to 3 weeks [1]. Bacterial or fungal infections, with skin as a portal of entry, are a main complication of the disease, especially in TEN [2, 3].
As recalled by Le and Bedocs [4], calcinosis cutis is classified into five main types: dystrophic, metastatic, idiopathic, iatrogenic, and calciphylaxis. Dystrophic calcification is the most common cause and occurs preferentially with tissue damage. Little is known regarding the chemical composition of calcium salts associated to calcinosis cutis which are deposited in the skin and subcutaneous tissue.
From a chemical point of view, the chemical diversity of abnormal deposit in skin [5] with an exogenous origin has been investigated in several papers dedicated for example to tattoo [6, 7, 8, 9] or to sarcoidosis [10, 11]. The chemical diversity of abnormal deposits in skin with an endogenous origin is less documented but has been underlined. In our case, for calcification of endogenous origin, we give evidence through physicochemical characterization techniques of the presence of calcium carbonate in the case of sarcoidosis [12, 13] or of calcium phosphate apatite in the case of calcific uremic arteriolopathy [14].
Such chemical diversity of pathological calcifications [15, 16, 17, 18, 19, 20, 21] calls for a characterization through physicochemical techniques such vibrational spectroscopies namely Fourier Transform Infra-Red (FTIR) or Raman spectroscopies [18, 22, 23, 24, 25, 26] which are able to describe precisely their chemistry. Imaging through field-emission scanning electron microscopy (FE-SEM) coupled with an energy-dispersive X-ray (EDX) spectrometer have also to be performed to describe and localize precisely at the submicrometre scale such abnormal deposits [18, 27, 28, 29].
Here we describe three patients who presented atypical healing retardation due to calcinosis cutis, a finding not previously described in EN. Part of the results presented in this investigation has been already published in a letter [30]. Here, a completely new set of unpublished data encompassing FE-SEM observations, EDX and μFTIR measurements is presented in order to discuss more precisely the pathogenesis process related to calcinosis cutis.
2. Methods
The main characteristics of the three patients, admitted to the intensive care unit of our SJS/TEN reference centre between October, 2017 and February 2021, were retrospectively obtained from medical charts.
To investigate the origin and the composition of the epidermal calcifications, we conducted several experiments. For the hypothesis of calcium deposition induced by a trans-differentiation of fibroblasts or keratinocytes to an osteochondrogenic phenotype, immunohistochemistry was used to investigate classical osteogenic markers. Sections of paraffin-embedded skin biopsy, 4 μm thick, were dewaxed, heated in citric acid solution (Target Retrieval Solution pH6, Dako), then incubated with antibodies. After blockade of endogenous peroxidase (peroxidase blocking solution, Dako), sections were immunostained with antibodies specific for the following osteogenic markers: bone morphogenic protein 2 (BMP-2, polyclonal, 1:300; Abcam), runt-related transcription factor 2 (Runx2, polyclonal, 1:200; GeneTex), alkaline phosphatase (monoclonal, 1:200; Abcam) and sclerostin (polyclonal, 1:100; Santa Cruz Biotechnology). Immunostaining was revealed by specific Histofin (Nichirei Biosciences) and AEC (k34769, Dako) staining.
Additional 4 μm thick sections of paraffin-embedded skin biopsies were deposited on low-e microscope slides (MirrIR, Kevley Technologies, Tienta Sciences, Indianapolis, IN, USA) for μFTIR spectroscopy, FE-SEM observations and EDX measurements.
μFTIR spectra have been collected by using a Spectrum Spotlight 400 imaging system (Perkin-Elmer Life Sciences), with 6.25 μm spatial resolution and 8 cm−1 spectral resolution [22, 23, 30, 31, 32]. The concise description of the topology at the submicrometer scale of the sample is obtained using a FE-SEM (Zeiss SUPRA55-VP) microscope. High-resolution images were obtained with an Everhart-Thornley secondary electron detector. Measurements were taken at low voltage (less than 1 kV generally), without the usual carbon-coating of the sample surface. Finally, EDX spectroscopy was also used to identify the different elements present in the sample. Special attention has been taken to calcium and phosphor in tissue [18, 27, 28, 29].
3. Results and discussion
3.1. Clinical data
Regarding epidemiology, calcinosis cutis commonly occurs in patients with systemic sclerosis, especially the limited form (CREST syndrome). Twenty-five to fourty percent of patients with limited systemic sclerosis will develop calcinosis cutis ten years after the onset of disease. Calcinosis cutis is seen in 30% of adults and up to 70% of children and adolescents with dermatomyositis. Patients with systemic lupus erythematosus can present with periarticular calcification in 33% of cases and soft tissue calcification in 17%.
Here we report three patients with severe TEN in whom secondary epidermal detachment with calcinosis cutis developed that was not previously described in this condition. This event was observed in the three cases a few days after caspofungin administration. One patient died and the two other patients presented an unusual, prolonged cutaneous healing. Clinical characteristics and histological results for the three patients are presented in Table 1.
Clinical and demographic data of three patients admitted to the intensive care unit for toxic epidermal necrolysis
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age | 57 | 49 | 59 |
| Sex | Female | Male | Male |
| Drug culprit of the TEN | Pantoprazole | Ibuprofen | Allopurinol |
| SCORTENa at admission | 2 | 4 | 3 |
| Maximal body surface area involved | 100% | 100% | 80% |
| Cutaneous biopsy at admission | Epidermal necrolysis | Epidermal necrolysis | Epidermal necrolysis |
| Time between caspofungin administration and abnormal epidermal detachmentb | 8 days | 1 day | 3 days |
| Cutaneous biopsy at epidermal detachment | Necrosis of the corneal layer associated with calcium deposition in the superficial dermis and epidermis | Necrosis of the corneal layer associated with calcium deposition in the superficial dermis | Necrosis of the corneal layer associated with calcium deposition in the superficial dermis |
| Type of delayed cutaneous healing | Relapse of fibrinous erosions 17 days after complete healing | Extension of fibrinous erosions 3 days after the beginning of healing | Relapse of cutaneous erosions 22 days after complete healing |
| Topography of detachment | Anterior trunk and limbs | Limbs, trunk | Upper trunk, shoulders, back |
| Time to complete healing | 166 days | Not available (died at day 33) | Not obtained at day 156 |
a SCORTEN is a specific severity-of-illness score for TEN, ranging from O to ⩾5. A high SCORTEN is associated to a higher mortality rate.
b As defined by the day it was evidenced by the dermatologist.
Patient 1 was a 57 year-old woman, with TEN induced by pantoprazole. At day 6 after admission in our dermatology department, she was referred to ICU for a first septic shock to P. aeruginosa and P. mirabilis. On day 12 after admission, cutaneous healing was almost complete (Figure 1A). On day 18, she presented septic shock with Candida parapsilosis bloodstream infection and received caspofungin for 14 days. On day 29, dermatological examination revealed a diffuse epidermal detachment associated with atone fibrinous plaques (Figure 1B). Thereafter, the evolution was slowly favourable and the patient was discharged from the ICU. Complete healing occurred 166 days after the second epidermal detachment.
Clinical presentation of patient 1, showing delayed healing with prolonged erosions covered with yellowish crusts.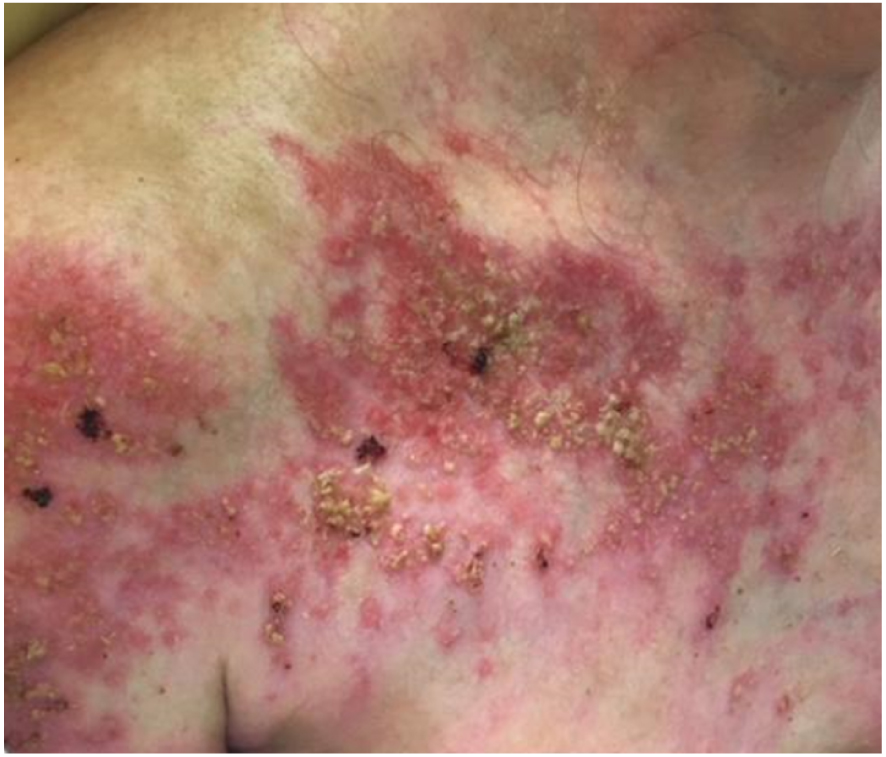
Patient 2 was a 49 year-old man TEN with ibuprofen suspected. He was transferred to ICU because of respiratory distress. At day 3 after admission in ICU, he showed places of beginning of healing. On day 5, he presented non-documented septic shock and received vancomycin, piperacillin-tazobactam and caspofungin. On day 6, diffuse epidermal detachment was noted, together with extended fibrinous areas. He died on day 33 of multiple organ failure, without healing of fibrinous plaques.
Patient 3 was a 59 year-old male with TEN induced by allopurinol. He was transferred in the ICU and was rapidly treated with piperacilline-tazobactam for Klebsiella aerogenes pneumonia. On day 12 after admission, cutaneous healing was almost complete. The same day, caspofungin was administered for Candida lusitaniae catheter infection, with fluconazole relay 2 days later. On day 26, the patient presented a septic shock to Candida parapsilosis. Caspofungin was reintroduced for 2 days, followed by fluconazole for 12 more days. On day 34, dermatological examination revealed a diffuse epidermal detachment with extended atone fibrinous areas. On day 156, complete mucocutaneous healing still had not occurred.
In the three cases, skin biopsy performed at diagnosis revealed epidermal necrosis typical of EN [33]. In light of healing retardation, a new biopsy revealed diffuse necrosis of the corneal layer associated with calcium deposition in the superficial dermis and in the epidermis, which was confirmed by Von Kossa staining (Figure 2A). Median ionized calcemia was 1.19 (0.97–1.25) mmol/L (normally 1.15–1.33). No patient had received calcium supplementation. Median phosphoremia was 0.89 mmol/L (normally 0.61–1.11 mmol/L). Phosphate supplementation was administered in routine care [1]. The three patients received caspofungin for fungal sepsis, a rare infectious event in EN.
Skin biopsy sections. Diffuse necrosis of the corneal layer associated with calcium deposition in the superficial dermis. A, Von Kossa staining (×100). B and C, FE-SEM observations at low magnification. Red arrows indicate the voluminous calcifications.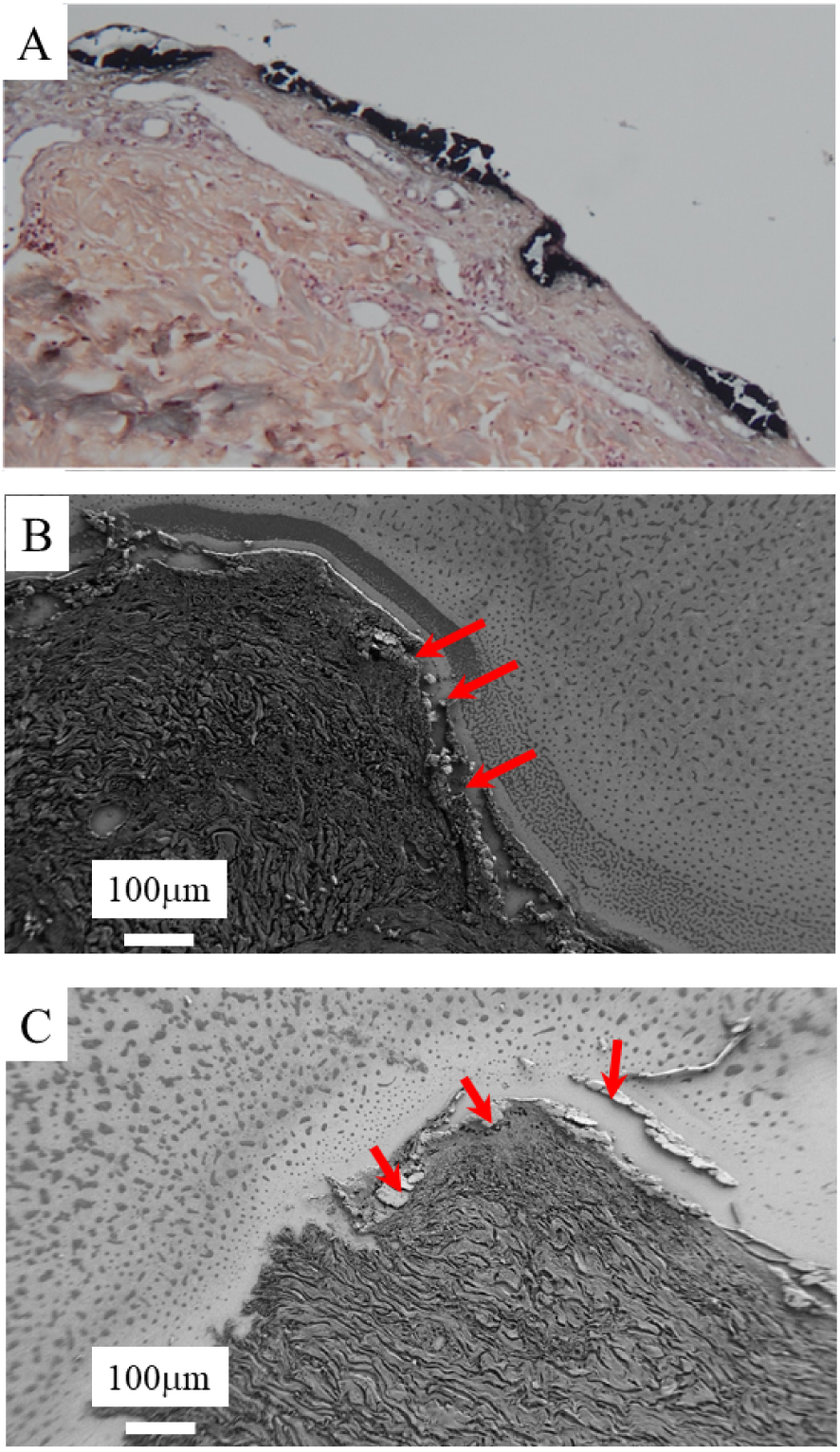
On retrospectively reviewing all patients (n = 115) in our centre between January, 2015 and December, 2020, four other patients with TEN had also received caspofungin: three had died in the following days without any dermatological examination. In the fourth case, new fibrinous plaques were described in the medical chart 4 days after caspofungin administration (no imaging or histology performed).
Caspofungin is an inhibitor of the 𝛽-D glucan synthesis of fungal pathogens [34]. Nonetheless, caspofungin is also a proven agonist of ryanodine receptor (RyR), found on cardiomyocytes and on pulmonary epithelial cells, inducing intracellular calcium influx from endoplasmic reticulum to cytosol [35, 36]. Although the effect of caspofungin on keratinocytes has not been studied, Denda et al. [37] reported that RyR is strongly expressed in keratinocytes and that the application of another RyR agonist induced intracellular calcium flux, leading to a precocious keratinocyte differentiation in an in vitro model and a delayed cutaneous healing in a mouse model. All osteogenic markers were negative on keratinocytes as on fibroblasts, excluding a trans-differentiation to an osteochondrogenic phenotype.
3.2. The three different areas as defined by SEM observations
FE-SEM revealed that these deposits consisted of voluminous plaques (Figures 2B and C). FE-SEM observations at higher magnification (Figure 3) underline the presence of the voluminous plaques (area 1 in Figure 3). Just below, we can see spherical entities within the papillary dermis (area 2 in Figure 3). Finally, an area which seems to be free of calcification can be defined (area 3 in Figure 3).
Skin biopsy sections as seen with a FE-SEM at higher magnification. A and B voluminous calcification (inside the blue rectangle) and spherical calcifications within the papillary dermis.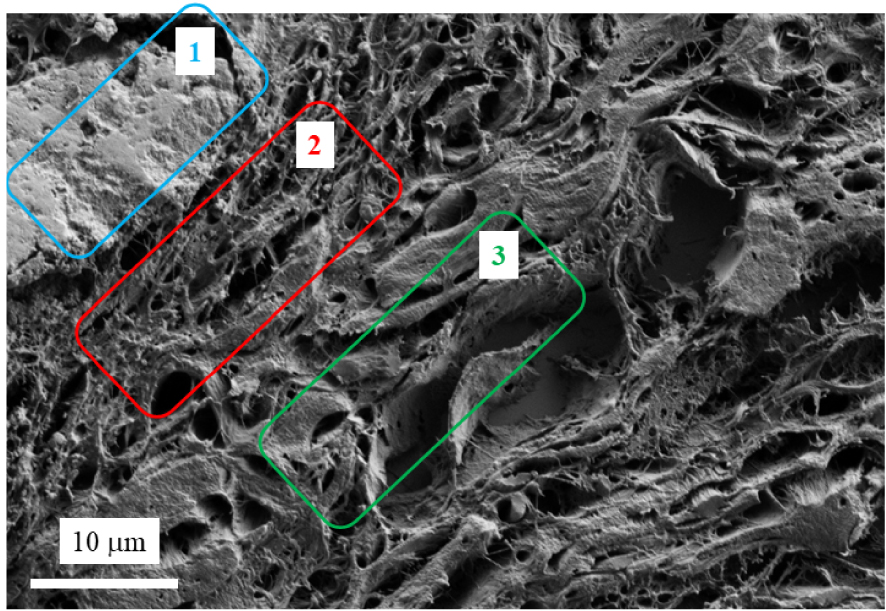
In order to gather information regarding the chemistry of the abnormal deposits, we have performed EDX and μFTIR. Figure 4 shows three EDX spectra. In the first one corresponding to the support, different elements are identified, namely O (K𝛼1 = 0.524 KeV), Zn (K𝛼1 = 8.638 KeV, KL1 = 1.011 KeV), Si (K𝛼1 = 1.740 KeV), P (K𝛼1 = 2.013 KeV), Ag (L𝛼1 = 2.985 KeV, L𝛽1 = 3.150 KeV), K (K𝛼1 = 3.310 KeV) and Ca (K𝛼1 = 3.690 KeV, K𝛽1 = 4.010 KeV) [38, 39]. In EDX spectra (red line in Figure 4) related to the large calcification (area 1 in Figure 3), contribution coming from some elements present in the support namely Si, Ag, K and Zn are no more visible. Instead, contributions of Ca and P are associated with a strong amplitude. Note that the X-ray fluorescence peak at 1.0 KeV corresponds to a sum peak (star in Figure 4) due to the coincidence of two O K𝛼1 photons. Finally, in EDX spectra (blue line in Figure 4) related to the spherical calcifications (area 2 in Figure 3), contributions of Ca and P related to the calcifications and of other elements present in the support are measured.
EDX spectra of the support (black line), calcification 1 (red line) and spherical entities which belong to calcification 2 (blue line). We can see the different contributions coming from the support namely O (K𝛼1 = 0.524 KeV), Zn (K𝛼1 = 8.638 KeV, KL1 = 1.011 KeV), Si (K𝛼1 = 1.740 KeV), P (K𝛼1 = 2.013 KeV), Ag (L𝛼1 = 2.985 KeV, L𝛽1 = 3.150 KeV), K (K𝛼1 = 3.310 KeV) and Ca (K𝛼1 = 3.690 KeV, K𝛽1 = 4.010 KeV).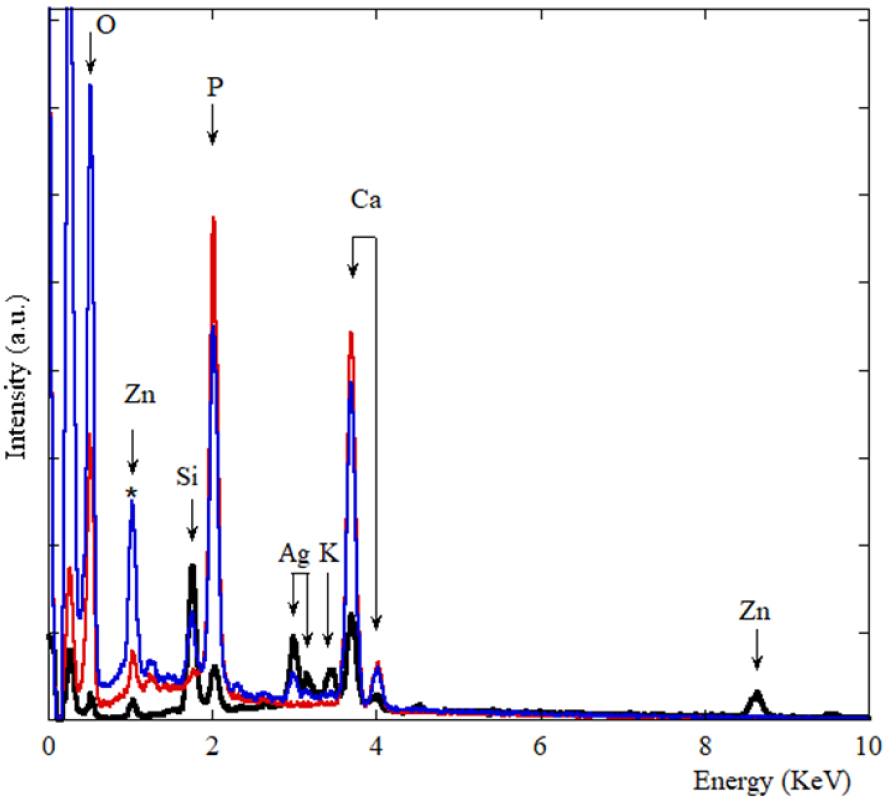
On Figure 5, μFTIR spectra corresponding to large calcifications (red line in Figure 5) and tissue (black line in Figure 5) are visualized. μFTIR spectroscopy revealed that calcifications consisted of amorphous calcium phosphate and calcium-phosphate apatite which is consistent with the composition of most dystrophic skin calcifications [40, 41, 42, 43].
μFourier transform infra-red spectroscopy of calcium deposition. The presence of amorphous calcium phosphate and calcium phosphate apatite spectrum with characteristic peaks (1060 cm−1 and 1030 cm−1) in a protein matrix (skin tissue) is detected.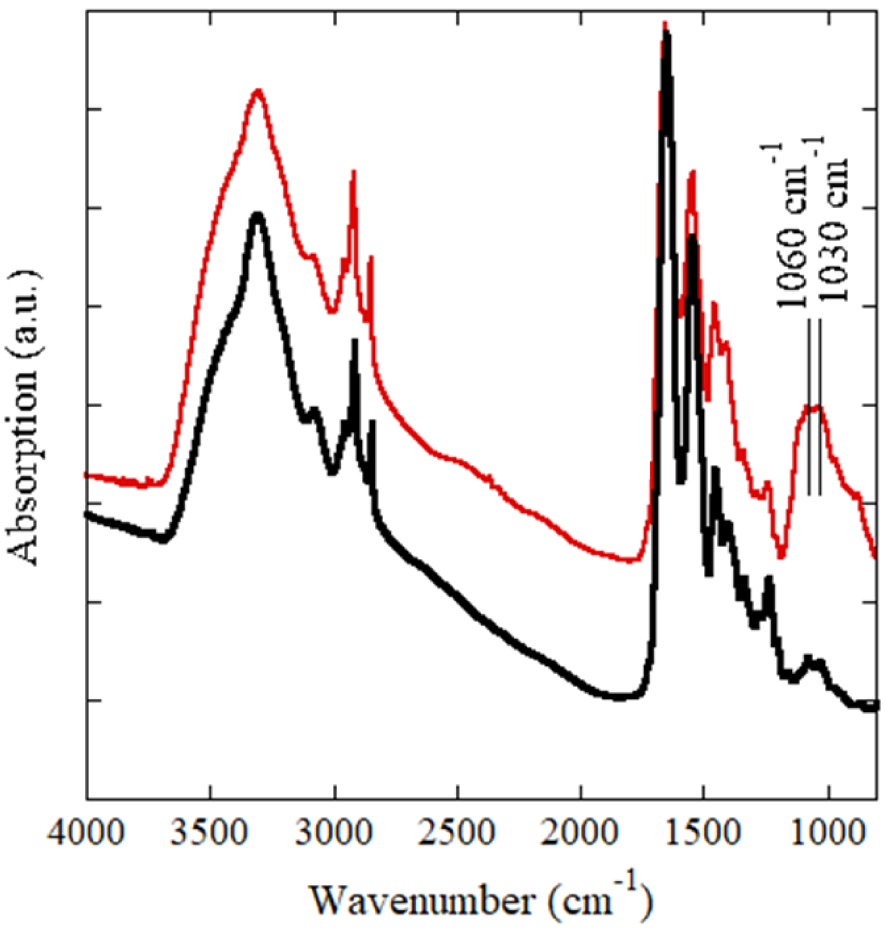
3.3. The pathogenesis of large calcifications
On Figure 6, we can see that “large” submillimeter scale calcifications are the result of an agglomeration of micrometer scale spherical entities. Such fusion process of spherical entities leading to “large” submillimeter scale calcifications has been also observed in other organs such breast [44].
Area 1 is made of “large” calcifications (area 1 in Figure 3) which are the result of an agglomeration of small spherical entities (area 2 in Figure 3).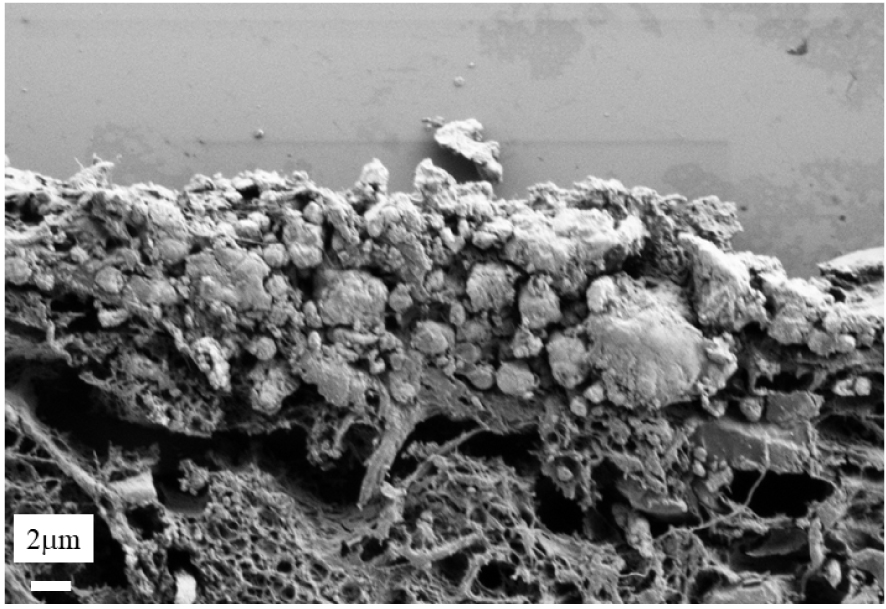
3.4. The pathogenesis of micrometer spherical entities
It is worth to underline that the internal structure of spherical entities may display different configuration as we have shown previously in the case of an investigation dedicated to breast calcifications (Figure 7) [44].
Spherical entities may display different internal structures: (A) Radial structures; (B): Concentric layers; (C): Both radial and concentric structure (A to C images are breast calcifications). In the case of calcinosis cutis (D), microspherical entities (blue arrows) result of an agglomeration of nano-spherules (red arrows).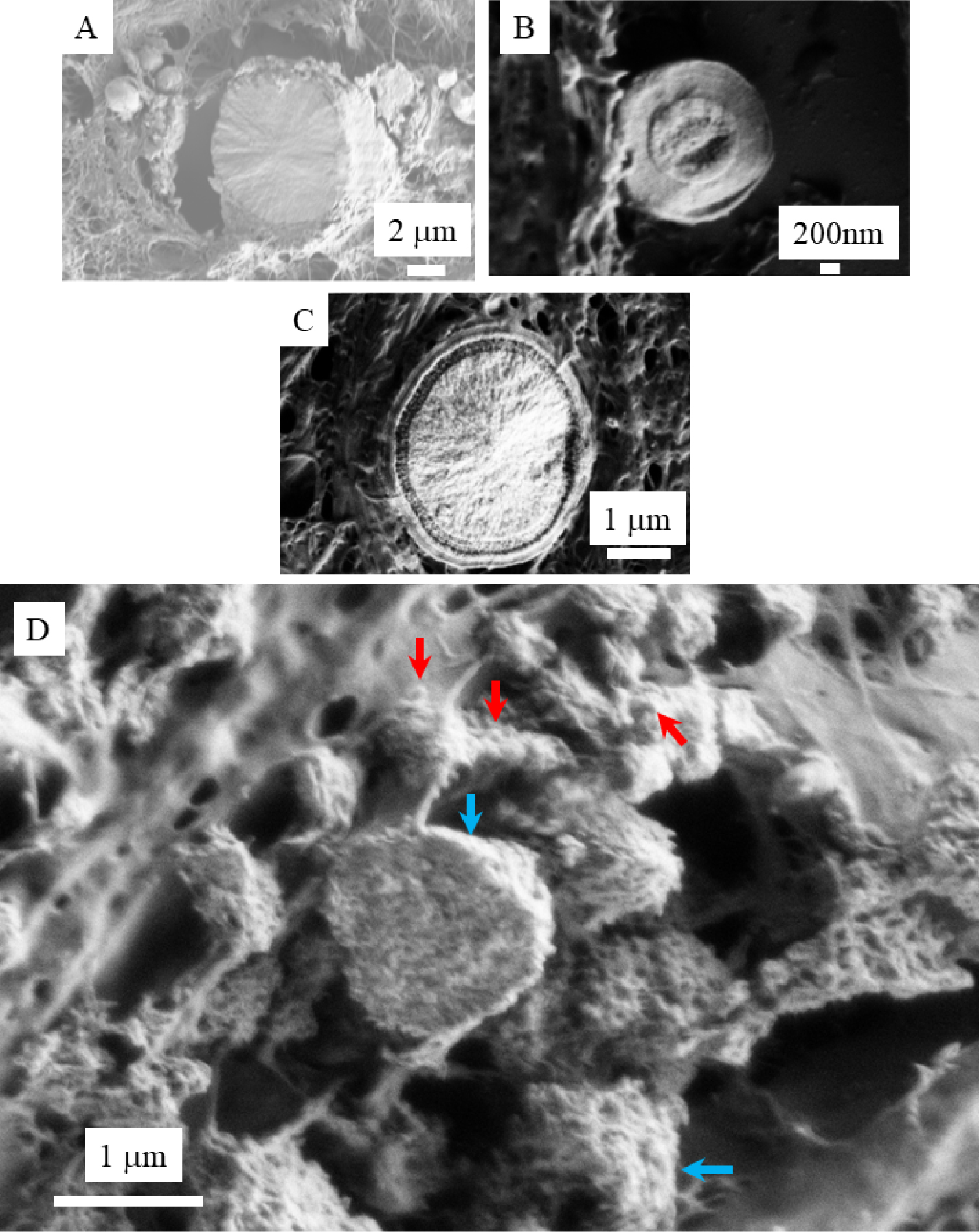
3.5. The very first steps of the pathogenesis of micrometer spherical entities
Finally, we have tried to localize precisely the very first steps of the calcification process. In Figure 8, we can see a set of nanometre scale spherical entities without micrometre spherical ones. It seems that the organisation of the skin is altered by the presence of the nanospherical calcifications. Intermingled tiny fibres are clearly visible around these calcifications and might be reticulin. Note that ectopic presence of reticulin has been found in pseudoxanthoma elasticum, a genetic calcifying skin disorders, suggesting the existence of a tropism of the calcification for such kind of fibres [45].
(A, B) Spherical entities as seen at two magnifications; (C) black line corresponds to the EDX spectra of the support and blue line corresponds to spherical entities visible in (A).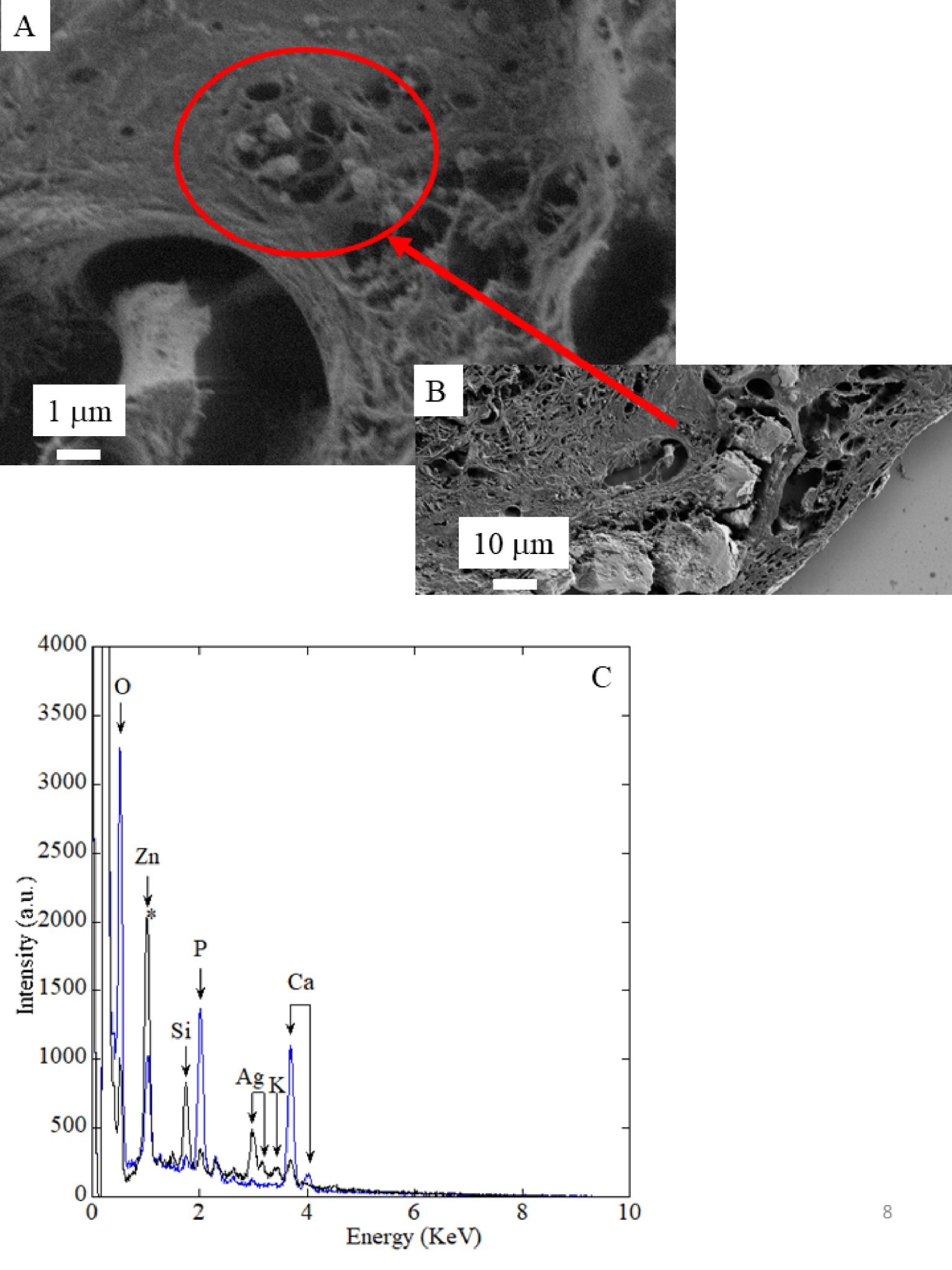
3.6. Physiological and biochemical considerations
The presence of spherical calcified entities within the upper but not deeper dermis suggests that these calcifications formed in the upper part of the skin tissue, contiguous to the epidermal necrolysis. Such co-location suggests a link between inflammation induced by the EN and calcifications, as in other dystrophic calcified skin diseases such as dermatomyositis [46, 47].
Regarding the pathogenesis of these calcifications, caspofungin administration might have disrupted the extracellular/intracellular gradient of calcium in keratinocytes, causing an extracellular calcium deposit, associated with secondary epithelial detachment in these patients with previous skin fragility (ongoing TEN healing) and prolonged inflammation. Because of the negativity of osteogenic markers in all three patients, the hypothetical mechanism of trans-differentiation of fibroblasts or keratinocytes to an osteochondrogenic phenotype, as suspected with vascular smooth muscle cells of dermal arterioles in calciphylaxis, seems unlikely [48]. We acknowledge some limitations of the study. The first is the retrospective design associated with a limited number of patients and the absence of a control arm. Moreover, we did not use an in vitro model of the possible pathogenesis link between calcification and caspofungin. Nonetheless, the occurrence of the same situation in three similar patients after caspofungin administration associated with a possible pathogenesis hypothesis raises the possible causality of caspofungin. Liposomal amphotericin B or fluconazole might therefore be preferred for patients with TEN in case of invasive fungal infection.
4. Conclusion
We have investigated a set of biopsies corresponding to patients who presented atypical healing retardation due to calcinosis cutis. Through SEM observations at the nanometer scale, we describe different areas: voluminous calcifications present at the surface of the skin tissue, submicrometer spherical entities within the papillary dermis and then normally structured dermal fibers. FE-SEM observations show clearly that “large” calcification are the result of an agglomeration of micrometer spherical entities. Moreover, micrometer scale spherical entities are the results of an agglomeration of nanometer scale spherical entities. Finally, the last set of data seems to show that the starting point of the calcifications process is “distant” from the epidermis in part of the dermis which appears undamaged.
From a medical point of view, our cases alert to the administration of caspofungin in patients presenting diffuse epidermal detachment and pave the way for further studies regarding dermatological adverse events induced by caspofungin, especially on mucocutaneous healing.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgment
The patients in this manuscript have given written informed consent to publication of their case details.




 CC-BY 4.0
CC-BY 4.0