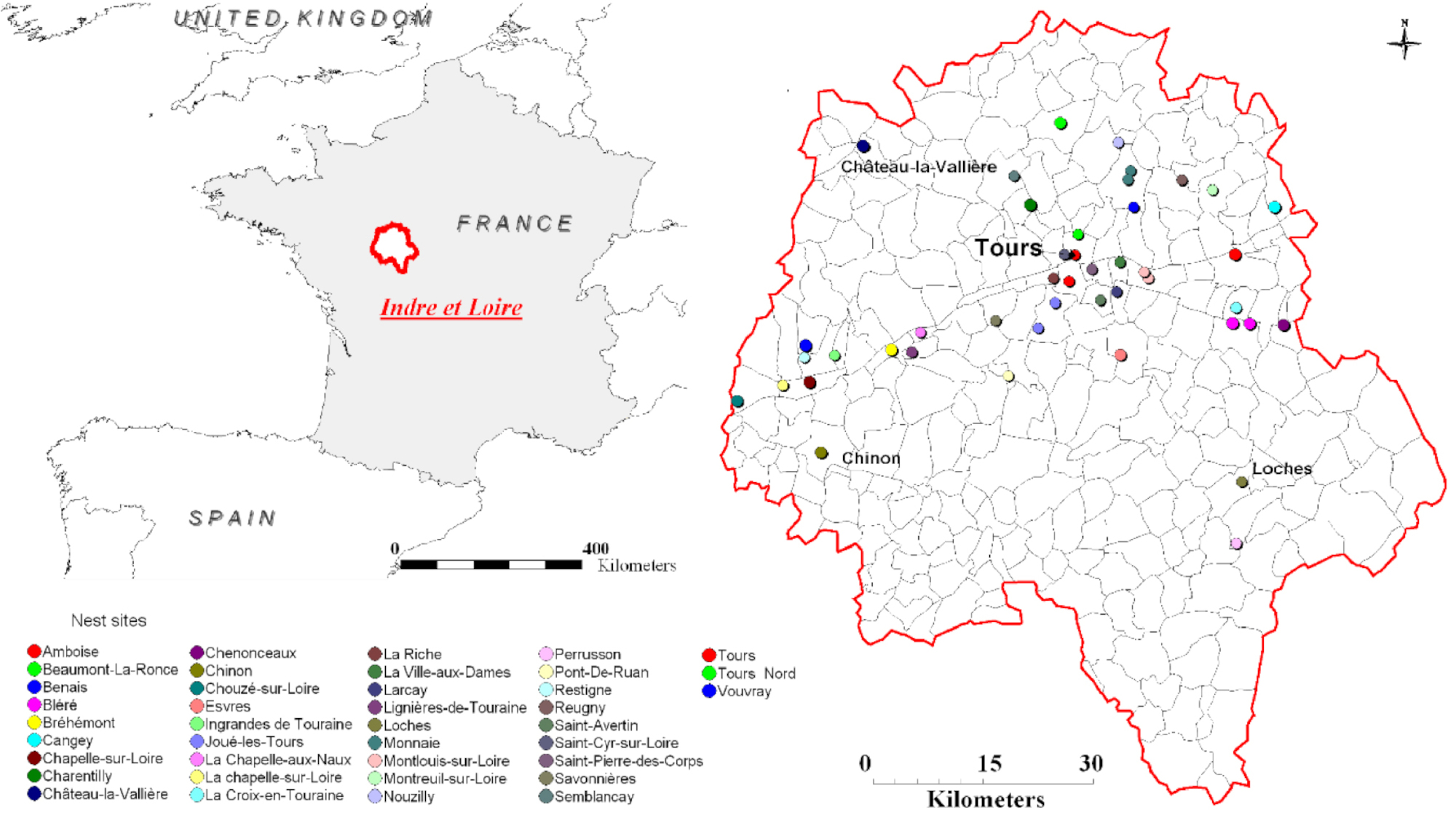
1. Introduction
Invasive species are increasingly affecting the Earth’s ecosystems [1]. They are permanently modifying the distribution and diversity of native species worldwide [2] at a variety of biological scales and with differing degrees of severity [3]. In many different habitats, social insects are highly successful invaders because of their excellent ability to disperse, compete for resources, mount effective defenses, reproduce at high rates, and exploit a broad range of habitats and diets [4]. Native to China, the invasive yellow-legged hornet, Vespa velutina nigrithorax [5], was accidentally introduced into southwestern France around 2004 [6]. It is a hardy, adaptable species whose flexible dietary requirements have allowed it to invade and establish itself within a variety of habitats [7]. Vespa v. nigrithorax has successfully expanded its range and is now found throughout most of France [8] as well as in several other European countries [9, 10, 11, 12, 13]. Ecosystems invaded by this hornet are impacted in various ways since V. v. nigrithorax preys upon several insect and arthropod taxa [14], including the domestic honey bee, Apis mellifera [15]. Finally, V. v. nigrithorax presents human health risks as hornets can aggressively spray liquid in people’s eyes [16]; also, their stings are painful and sometimes even fatal [17, 18]. Consequently, medical practitioners and researchers are increasingly seeking information on V. v. nigrithorax venom for diagnostic and treatment purposes [17, 19]. The hornet’s introduction into Europe led to calls for control efforts, which have largely focused on passively trapping adults using homemade or commercial poison baits [20]. Unfortunately, this approach has done little to limit V. v. nigrithorax population sizes [20, 21]. Furthermore, because they lack specificity, these traps can have significant ecological impacts on a wide range of non-target species [22, 23]. As a result, work is currently underway [20] to develop new species-specific systems that are based on pheromones [24, 25, 26, 27].
Chemical signals are an essential component of animal communication systems [28, 29] and mediate a variety of behaviors, including social organization. In insects, long-distance signals play an important role in intra- and interspecific communication [29] and elicit species-specific responses. Such communication provides fitness benefits by enhancing colony cohesion, defense, and alarm systems. There is a long history of research on chemical signals (e.g., pheromones) in social insects (e.g., ants, bees, hornets, and termites); these signals often trigger different behaviors (recruitment, dispersal, aggressiveness, or aggregation) that can affect colony survival [30, 31]. Thus, a promising pest control approach is to develop species-specific attractants (e.g., trap bait) or repellents that target specific life stages. Alarm pheromones are of key importance because they are deployed when colonies are threatened: they prompt nestmate recruitment and defense behaviors [32, 33]. They can also be used by workers to signal danger in areas farther away from the nest [34]. The most effectively disseminated signals are made up of highly volatile compounds that act quickly and then dissipate, limiting further responses. Most alarm pheromones in social insects are multicompound blends [24, 35, 36] that are generally involved in both offensive and defensive reactions by workers. The venom gland tends to be the primary source of alarm pheromones [33, 37], although interspecific variation exists. Thus, during aggressive interactions, alarm signals are released at the same time as venom [38]. Such is the case in several wasp and hornet species, including Polistes dominulus [39], Vespula squamosa [40], Vespa crabro [41], Vespa mandarinia [37], and Vespa simillima xanthoptera [37].
The first study conducted on alarm pheromones in a subspecies of the yellow-legged hornet characterized the volatile compounds found in worker venom glands in a native V. velutina auraria population in China [42]. A second study [43] did the same in an invasive V. v. nigrithorax population in France. In both cases, researchers identified some venom gland compounds that elicited hornet attacks when colonies were threatened. When the alarm pheromones were released, they attracted other workers and encouraged stinging behavior. However, compound abundance differed between the two subspecies. Next, researchers explored the chemical signals associated with caste and species’ reproductive status using four colonies in an invasive population in Italy; the study looked at compounds in volatile venom gland mixtures in gynes and workers [27]. Twelve major compounds were observed, which contrasted with the 16 and 17 compounds observed previously (Refs. [42, 43] respectively). Five of these 12 compounds had been seen in the first two studies. However, others had never been reported before, including 4 unknown acetates of aliphatic alcohols and citronellyl acetate, which had the second highest abundance [27]. Finally, work on an invasive population in Spain observed 8 compounds [26], collected in vivo by SPME from seven hornets, including 3 previously seen ketones [27, 42, 43]. However, in this most recent study, individuals displayed marked diversity in their chemical profiles since 2 of the 8 compounds were found in only one hornet and 3 others in fewer than 3 hornets [26]; only one compound occurred across all the profiles. There is clearly a need for further research given this range of results. First, a better understanding of the chemical composition of V. v. nigrithorax’s alarm pheromone is needed. Second, since gynes, foundresses, and queens alike use alarm pheromones, we also must clarify associations between hornet caste and alarm pheromone composition. However, in the latter three cases, the volatile compounds involved remain poorly characterized. Here, we hypothesized that females from different castes and/or with different tasks might differ in their venom gland profiles.
This study thus had two goals. First, we assessed differences in venom gland profiles among the four types of females: queens, foundresses, pre-wintering gynes, and workers. Second, we experimentally explored venom gland profiles of V. velutina nigrithorax workers and pre-wintering gynes via an in vivo approach (collection of volatiles, solid-phase microextraction (SPME)) and an in vitro approach (liquid extraction of compounds from the venom gland).
2. Materials and methods
2.1. Study sites and hornet sampling
Hornet colonies were collected at 44 different sites in the administrative department of Indre-et-Loire, which is part of the Centre-Val-de-Loire region of France (Figure 1). The yellow-legged hornet is classified as an invasive species, and all research was carried out in compliance with relevant national guidelines. To determine hornet caste (worker versus reproductive), mass and wing spacing were measured [44]. The reproductives were either pre-wintering gynes (females without nest), foundresses (mated females with small nest and some eggs), or queens (mated females with workers). To characterize the venom gland’s compounds, hornets of each caste were stored at −80 °C until they could be dissected. For the second experiment, individuals from a given colony were placed in a mesh breeder box and fed an ad libitum mixture of honey and water, which was replenished daily. The hornets underwent SPME within three days of collection.
2.2. Analysis of volatile compounds
To characterize the venom gland’s compounds, hornets (n = 75 workers, 40 pre-wintering gynes, 30 foundresses, and 30 queens) were dissected under a binocular microscope. Within each group, a set of 5 undamaged venom sacs (n = 15, 8, 6, and 6 replicates for the workers, pre-wintering gynes, foundresses, and queens, respectively) was placed in a vial containing heptane (1 mL). The vials were then stored at −20 °C. Glands were perforated right before performing the gas chromatography-mass spectrometry (GC-MS) analysis; the vials were vortexed at 300 rpm for 1 min. Gland extract (2 μL) from each vial were analyzed using a gas chromatograph (Agilent Technologies 7890B) coupled to a mass spectrometer (Agilent Technologies 7000C GC/MS Triple Quad, Les Ulis, France) equipped with an HP-5 capillary column (Agilent Technology, USA; 30 m × 250 μm × 0.25 μm) and using a Gerstel MPS autosampler (electron impact at 70 eV). Helium was the carrier gas (flow rate = 2.3 mL/min). Injectors were used in splitless mode (splitless time = 2 min) at a constant temperature of 250 °C. The oven temperature was set to ramp up from 50 °C to 200 °C at a rate of 8 °C/min and then from 200 °C to 315 °C at a rate of 5 °C/min (held constant for 5 min). Masses were scanned between 15 and 550 amu at 0.1 scan/s. Immediately before the analysis, 10 μL of internal standard (n-eicosane at 10−3 g/mL diluted in heptane) was added to each sample.
To identify and quantify the compounds released by workers or pre-wintering gynes in response to stress, two experimental stimuli were assessed. The experiments were conducted with sets of four live co-workers or four live pre-wintering gynes. The hornets were cold anesthetized (−18 °C for 10 min) and were subsequently placed in a clean 350 mL glass vial at room temperature (19–21 °C for 10 min). The hornets were still able to move but not to fly. Each hornet was exposed once to either a stressed hornet group (SH, replicates: pre-wintering gynes n = 4; workers n = 8) or a wounded hornet group (WH, replicates: pre-wintering gynes n = 4; workers n = 8). The SH treatment consisted of gently shaking the hornet group inside the glass vial. The WH treatment consisted of applying pressure to a hornet’s thorax using metal forceps; wounded hornets were then resealed before closing the glass vial. We assumed that the WH treatment induced a higher stress level than the SH treatment. To extract the resulting volatile compounds, a 40-min procedure was carried out using red solid-phase microextraction (SPME) fibers coated with polydimethylsiloxane (PDMS, 100 μm; Supelco). Immediately after the treatments, the fibers were placed within a metal wire to prevent any physical contact with the hornets. First, for each caste, two fibers were desorbed, and the resulting compounds were identified using the temperature program and GC-MS procedure described above. Second, individual fibers representing different castes and treatment groups were desorbed (WHworkers n = 8; SHworkers n = 8; WHgynes n = 4; SHgynes n = 4) using a GC system (CPG Agilent Technologies 7820A) equipped with a flame-ionization detector (FID) and a capillary column (HP-5 Agilent Technology, Santa Clara, USA; 30 m × 0.32 mm × 0.25 μm); helium served as the carrier gas (1.7 ml/min). The temperature program was the same as above. The relative area of each compound was calculated based on the proportions represented by each peak within the chromatogram using ChemStation (v. 04.02, Agilent).
2.3. Compound identification
The data were analyzed using MassHunter (v. B.07.00; Agilent) and AMDIS (v. 2.0g; NIST, 2011). Compounds were identified based on their mass spectra, which were interpreted via fragmentation analyses [45]. The results were then compared to published spectra and/or confirmed using commercial standards (Table 1) purchased from different suppliers (Sigma-Aldrich, Saint-Louis, Missouri, USA; GreenPharma, Orléans, France; and ChemSpace, Monmouth Junction, New Jersey, USA). The molecules 4,8-dimethyl-7-nonen-2-one and 4,8-dimethyl-1,7-nonadiene were synthesized by Synthenova (Hérouville-Saint-Clair, France). Kovats retention index (KRI) values were calculated based on the retention times of C8–C20 n-alkane standards (Fluka, 94234) that were analyzed under the same set of GC and GC-MS conditions.
Compounds found in venom glands of V. velutina subspecies
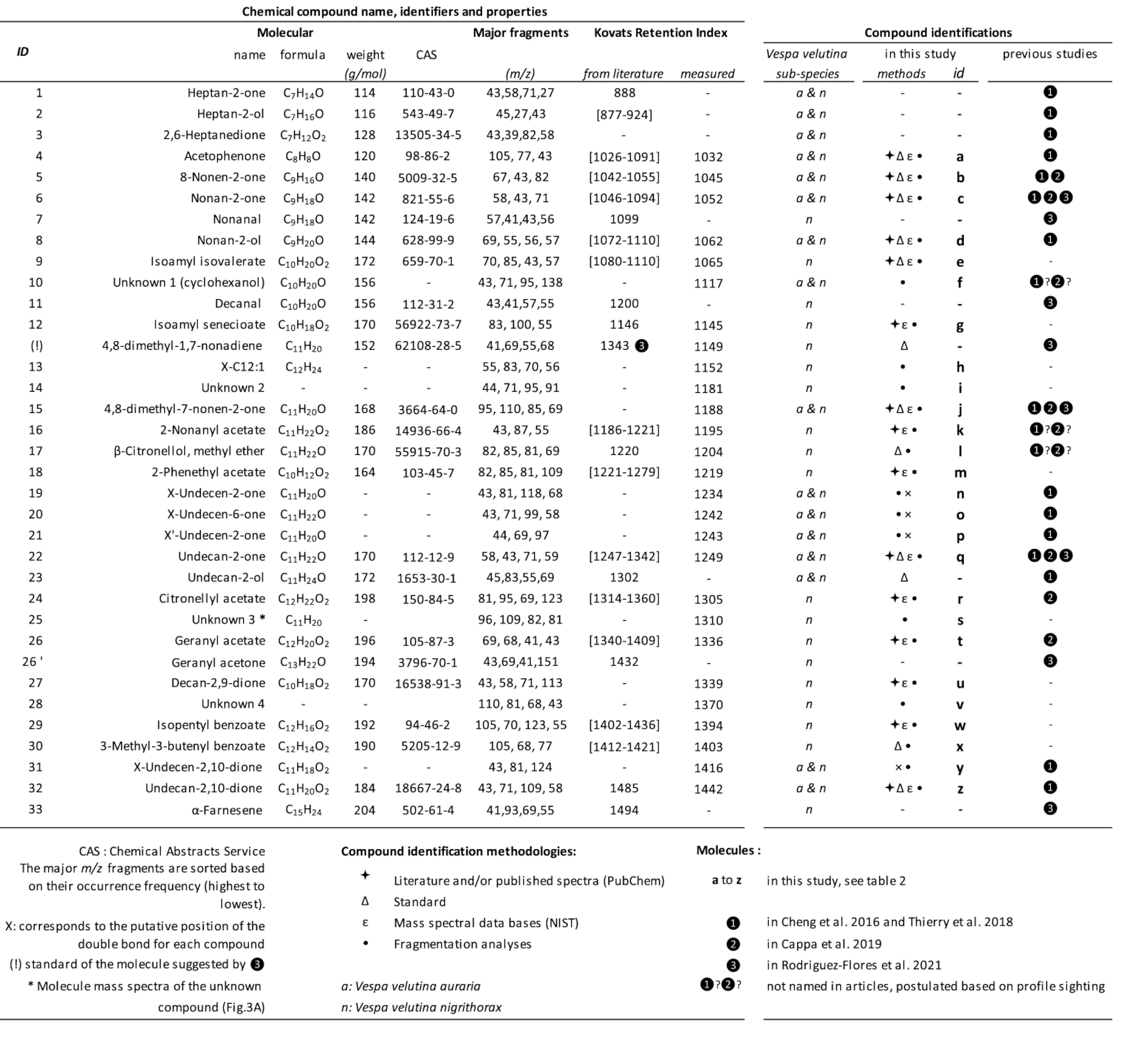 |
 Peaks present in both V. v. auraria [42] and V. v. nigrithorax [43]. Peaks present in V. v. nigrithorax populations in
Peaks present in both V. v. auraria [42] and V. v. nigrithorax [43]. Peaks present in V. v. nigrithorax populations in  Italy [27] and
Italy [27] and  Spain [26]. All the compounds observed in this study (a to z) underwent MS analysis (•) and were identified by comparing their mass spectra with those in the NIST 2017 database (𝜀) and/or in the literature (
Spain [26]. All the compounds observed in this study (a to z) underwent MS analysis (•) and were identified by comparing their mass spectra with those in the NIST 2017 database (𝜀) and/or in the literature ( ); final validation was performed using available standards (𝛥). The major m∕z fragments are sorted based on their occurrence frequency (highest to lowest). The Kovats retention index values were calculated and obtained from PubChem and the literature. Chemical Abstracts Service (CAS) of the American Chemical Society. (!) compound (Figure 3B) identified by Rodríguez-Flores et al. [26] on the basis of the HS-SPME chromatogram and mentioned as eluting after undecan-2-one. However, its MS spectra, retention time, and KRI value differed from those of the 4,8-dimethyl-1,7-nonadiene standard. ∗ Mass spectra of unknown compound s (Figure 3A) isolated from the venom gland (Figure 3). n: Vespa velutina nigrithorax. a: Vespa velutina auraria.
); final validation was performed using available standards (𝛥). The major m∕z fragments are sorted based on their occurrence frequency (highest to lowest). The Kovats retention index values were calculated and obtained from PubChem and the literature. Chemical Abstracts Service (CAS) of the American Chemical Society. (!) compound (Figure 3B) identified by Rodríguez-Flores et al. [26] on the basis of the HS-SPME chromatogram and mentioned as eluting after undecan-2-one. However, its MS spectra, retention time, and KRI value differed from those of the 4,8-dimethyl-1,7-nonadiene standard. ∗ Mass spectra of unknown compound s (Figure 3A) isolated from the venom gland (Figure 3). n: Vespa velutina nigrithorax. a: Vespa velutina auraria.
2.4. Statistical analyses
Venom gland profile composition was analyzed using multivariate principal component analysis (PCA) performed in Statistica (v. 10; Statsoft). The analysis used the percentage representation of identified compounds that occurred above a minimum threshold of 0.5%. The worker and gyne profiles obtained in the stress experiment were explored using another PCA, which employed the percentage representation of all the compounds collected via PDMS-SPME. To assess profile-based grouping patterns, we conducted a K-means cluster analysis [46]. Because the number of groups is unknown ahead of time, the choice of initial group number is somewhat arbitrary. To optimize this number, we used the Caliñski-Harabasz index [47].
3. Results
3.1. Identification of venom gland compounds
The venom gland profiles contained 38 compounds with chain lengths ranging from 8 to 12 carbons (Figure 2, Tables 1 and 2). Of these, 26 occurred at levels higher than the 0.5% threshold (Table 2). This group included 11 ketones, 8 esters, 4 alcohols, and 3 unknown molecules. The MS spectrum were investigated, and the identities of the compounds were confirmed using chemical standards (Table 1). The unknown compounds were subject to MS interpretation. The third unknown compound (s, Table 2, Figure 3A) was thought to be 4,8-dimethyl-1,7-nonadiene (Figure 3B, Table 1). Indeed, this compound was identified [26] based on the HS-SPME chromatogram. However, its MS spectra, retention time, and KRI value differed from those of the 4,8-dimethyl-1,7-nonadiene standard (Figure 3). The MS spectra for compound s (Figure 3A) and citronellyl acetate (compound r) shared some similarities. However, they differed in the presence of a fragment at m∕z 152 fragment and a trace of m∕z 170. Furthermore, compared to the citronellyl acetate, compound s had higher peaks at m∕z 137 (instead of at m∕z 138), m∕z 96, and m∕z 109. Unfortunately, spectra for the citronellyl family remain quite rare, limiting more detailed comparisons. Additionally, we found no other commercial compounds with more similar spectra, thus we were not able to confirm our hypothesis.
Chemical profiles of hornet workers, gynes, foundresses, and queens (unit of analysis = pools of five venom glands). The letters refer to the different compounds observed (Table 2).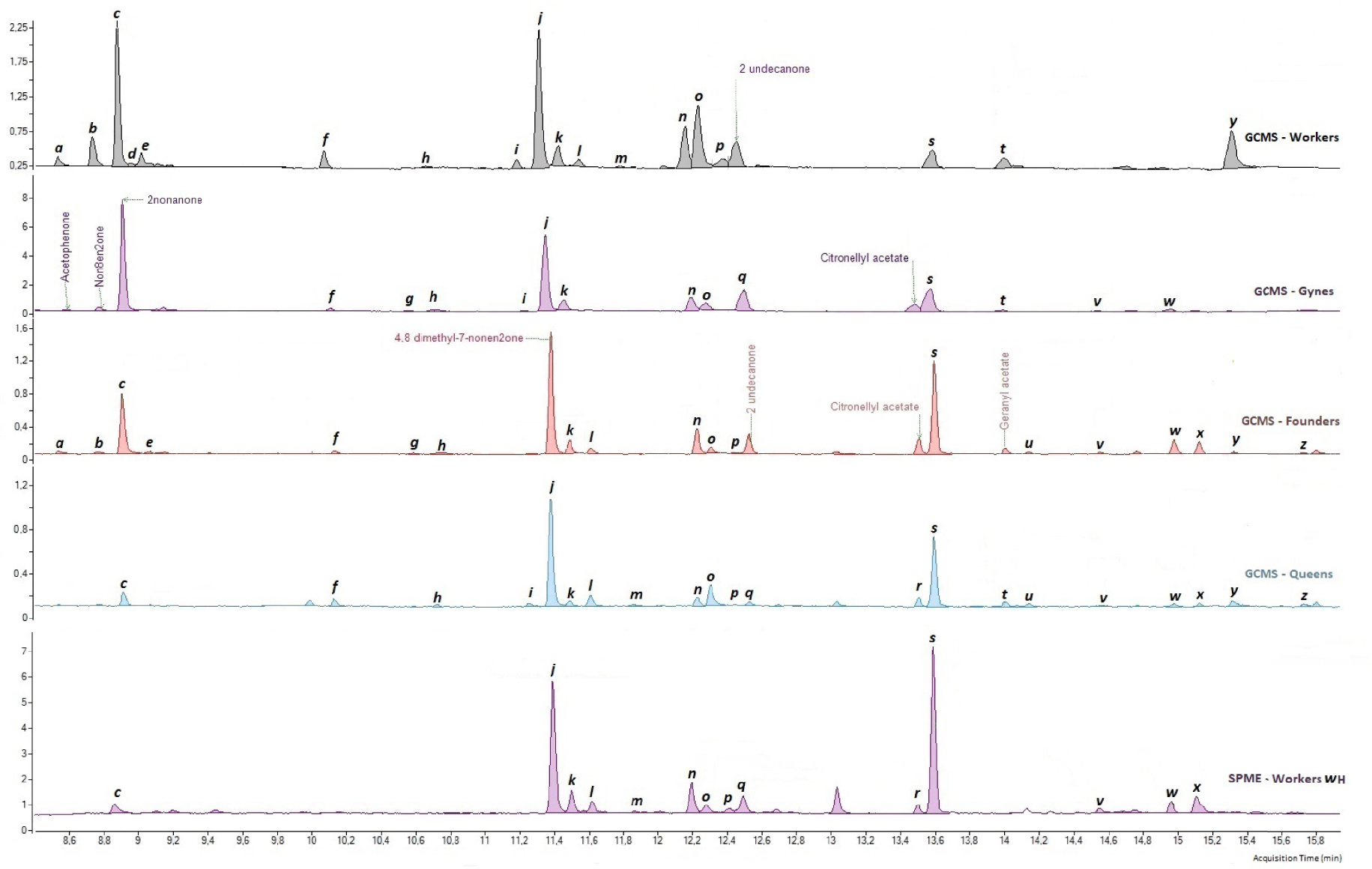
(A) Mass spectra for unknown compound s (Kovats retention index = 1310), isolated from the venom gland of V. v. nigrithorax. (B) Mass spectra for the 4,8-dimethyl-1,7-nonadiene standard (Kovats retention index = 1149). (C) Combined GC-MS profiles for C10-C18 and 4,8-dimethyl-1,7-nonadiene. The blue arrow represents the molecule s location (RT), in the gland profile.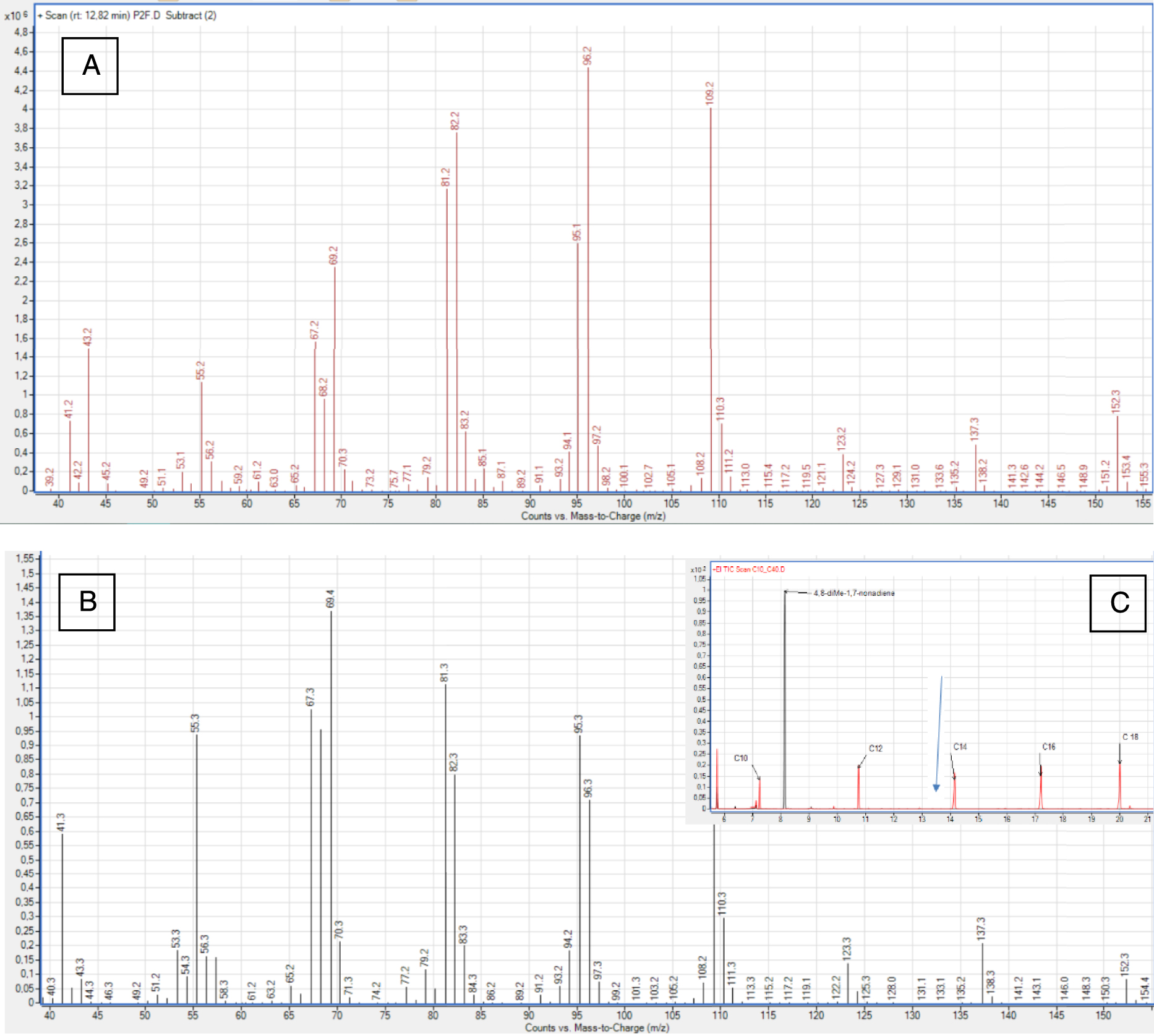
Chemical composition of the venom glands of V. v. nigrithorax queens, foundresses, workers, and pre-wintering gynes (percentages ± standard error), which were obtained either via solvent- or fiber-based extraction
| Name | VENOM GLAND’s profile | SPME analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SH | WH | SH | WH | ||||||
| Queens | Foundresses | Workers | Prewintering gynes | Workers | Prewintering gynes | ||||
| Acetophenone | a | 1.7 ±1 | 0.4 ±0.3 | 0.2 ±0.2 | 8 ×10−3 ±1 ×10−3 | - | - | - | - |
| 8-Nonen-2-one | b | 0.4 ±0.3 | 0.8 ±0.2 | 3.7 ±0.9 | 3.2 ±0.5 | 1.1 ±0.9 | 1.4 ±0.4 | 3.1 ±2.5 | 1.1 ±1.05 |
| Nonan-2-one | c | 3.4 ±0.9 | 16.4 ±2.8 | 18.2 ±3.2 | 14.2 ±3.7 | 7.7 ±3.8 | 10.9 ±3.8 | 3.3 ±2.2 | 6.3 ±2.6 |
| Nonan-2-ol | d | 0.3 ±0.3 | 0.4 ±0.2 | 1.6 ±0.8 | 0.8 ±0.9 | 1.2 ±0.7 | 1.5 ±0.5 | 1.8 ±1.1 | 0.6 ±0.3 |
| Isoamyl isovalerate | e | 0.1 ±0.1 | 0.8 ±0.2 | 2.0 ±0.9 | 2.5 ±0.8 | 1.2 ±1.3 | 0.8 ±0.5 | 0.4 ±0.5 | 1.2 ±0.9 |
| Unknown 1 (cyclohexanol) | f | 3 ±0.4 | 0.7 ±0.1 | 1.3 ±0.9 | 0.8 ±0.7 | - | - | - | - |
| Isoamyl senecioate | g | 0.6 ±0.5 | 0.2 ±0.1 | 0 ±0 | 0.1 ±0.1 | - | - | - | - |
| X-C12:1 | h | 0.4 ±0.4 | 0.4 ±0.1 | 0.7 ±0.9 | 0.8 ±1.1 | - | - | - | - |
| Unknown 2 | i | 1.4 ±0.3 | 0.2 ±0.1 | 0.8 ±0.6 | 0.5 ±0.5 | - | - | - | - |
| 4,8-dimethyl-7-nonen-2-one | j | 28.3 ±5.7 | 21.8 ±2.9 | 23.3 ±5.3 | 24.6 ±3.1 | 34.4 ±4.8 | 38.2 ±3.1 | 37.7 ±7.6 | 32.5 ±5.5 |
| 2-Nonanyl acetate | k | 1 ±0.6 | 3.7 ±0.5 | 3.4 ±1.1 | 4 ±0.8 | 5.6 ±1.4 | 6.7 ±1.6 | 6.7 ±1.2 | 5.2 ±1.1 |
| β-Citronellol, methyl ether | l | 4.5 ±2.1 | 1.0 ±0.4 | 1.2 ±0.8 | 1.7 ±0.8 | 4.1 ±0.6 | 3.5 ±1.1 | 5.7 ±2.1 | 3.8 ±0.8 |
| 2-Phenethyl acetate | m | 0 ±0 | 0.4 ±0.2 | 0 ±0 | 0.2 ±0.3 | - | - | - | - |
| X-Undecen-2-one | n | 2.6 ±0.2 | 5.5 ±0.4 | 9.3 ±2.9 | 5.8 ±1.4 | 7.1 ±2.4 | 7.5 ±2.8 | 4.9 ±1.2 | 5.7 ±2.4 |
| X′-Undecen-6-one | o | 6.7 ±1.8 | 2.3 ±0.7 | 8.5 ±2 | 11.5 ±2.0 | 1.3 ±0.8 | 0.9 ±1.3 | 1.5 ±1.3 | 3.5 ±0.7 |
| X′′-Undecen-2-one | p | 0.2 ±0.3 | 0.2 ±0.1 | 1.2 ±0.9 | 1.5 ±0.5 | 1.5 ±0.5 | 1.3 ±0.3 | 0.3 ±0.2 | 1.5 ±0.4 |
| Undecen-2-one | q | 2.4 ±1 | 6.1 ±1 | 7.7 ±1.6 | 7.7 ±2.0 | 6.9 ±2.9 | 8.1 ±3.1 | 2.7 ±0.4 | 5 ±1.4 |
| Citronellyl acetate | r | 1.6 ±0.8 | 6.9 ±2 | 0.5 ±0.5 | 0.7 ±0.6 | 5.8 ±3.8 | 1.8 ±1.1 | 7.9 ±3.3 | 11.3 ±4.9 |
| Unknown 3 | s | 23 ±5 | 15.8 ±2.2 | 5.2 ±1.6 | 7.2 ±2.6 | 21.9 ±6.1 | 17.3 ±5.6 | 23.7 ±10 | 22.4 ±5.9 |
| Geranyl acetate | t | 1.2 ±0.5 | 1.7 ±0.4 | 0.1 ±0.1 | 0.1 ±0.2 | - | - | - | - |
| Decan-2,9-dione | u | 0.2 ±0.2 | 0.1 ±0.2 | 0.8 ±0.6 | 0.6 ±0.4 | - | - | - | - |
| Unknown 4 | v | 0.2 ±0.2 | 0.5 ±0.1 | 1.2 ±0.7 | 1 ±0.3 | - | - | - | - |
| Isopentyl benzoate | w | 1.3 ±0.1 | 2.4 ±1 | 0.0 ±0 | 0.3 ±0.4 | - | - | - | - |
| 3-Methyl-3-butenyl benzoate | x | 1.2 ±0.3 | 2.0 ±0.8 | 0.2 ±0.2 | 0.4 ±0.6 | - | - | - | - |
| X-Undecen-2,10-dione | y | 2 ±0.6 | 0.8 ±0.4 | 4.5 ±2.1 | 4.7 ±1.8 | - | - | - | - |
| Undecen-2,10-dione | z | 1.6 ±0.5 | 0.5 ±0.3 | 1.3 ±0.8 | 0.4 ±0.5 | - | - | - | - |
Experimental treatments: SH, stressed hornet; WH, wounded hornet.
While the 26 compounds were all present in the pre-wintering gynes and foundresses, 3 were absent from the workers (g, m, and w; Table 2), and 1 was absent from the queens (m; Table 2). In the queens and foundresses, two compounds, 4,8-dimethyl-7-nonen-2-one (j) and compound s, accounted for more than 58% and 42% of the overall profile, respectively. In the workers and pre-wintering gynes, the two most common compounds were 4,8-dimethyl-7-nonen-2-one (j) and nonan-2-one (c); they accounted for 43% and 42% of the overall profile, respectively.
PCAs were carried out on these 26 compounds. Two rounds of analysis were performed. The first explored differences among pre-wintering gynes, foundresses, and queens (n = 7, 6, and 6 pools, respectively, Figure 4). The first and second axes accounted for 40% and 21% of the total variance, respectively, and revealed a marked separation among the three groups. This clustering was confirmed by the K-means analysis, in which the foundresses and queens were assigned to distinct groups. Only one pre-wintering gyne (G2) was incorrectly placed in the queens’ group. The queens stood apart from the other two groups based on the presence of three compounds: acetophenone (a) and two unknown compounds (f and s). The foundresses formed a distinct group based on the presence of four compounds: citronellyl acetate (r), geranyl acetate (t), isopentyl benzoate (w), and isoprenyl benzoate (x, i.e., 3-methyl-3-butenyl benzoate). The second round of analysis added profile information for the workers (n = 15 pools) (Figure 5). No differences were seen between workers and pre-wintering gynes, a result confirmed by the K-means analysis resulting in no group separation.
(A) Principal component analysis results and (B) correlation circle plot for the venom gland profiles of queens, foundresses, and pre-wintering gynes. Q: queens (n = 6), F: foundresses (n = 6), and G: pre-wintering gynes (n = 7). The ellipses correspond to the 95% confidence interval of the data. Compounds found closer to the circumference of the circle convey greater amounts of information.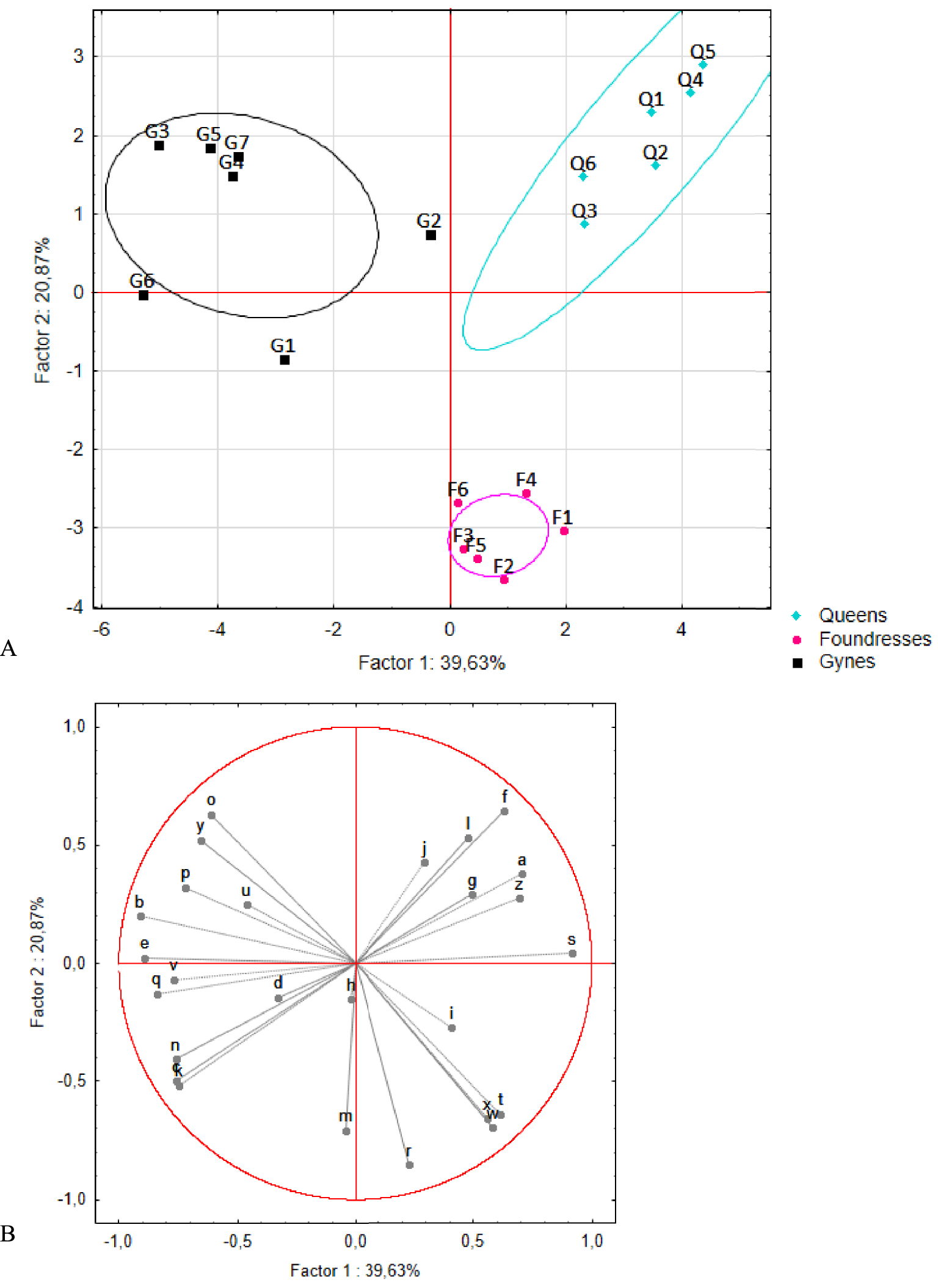
(A) Principal component analysis and (B) correlation circle plot for the venom gland profiles of queens, foundresses, workers, and pre-wintering gynes. Q: queens (n = 6), F: foundresses (n = 6), W: workers (n = 15), and G: gynes (n = 7). The ellipses indicate the 95% confidence interval of the data.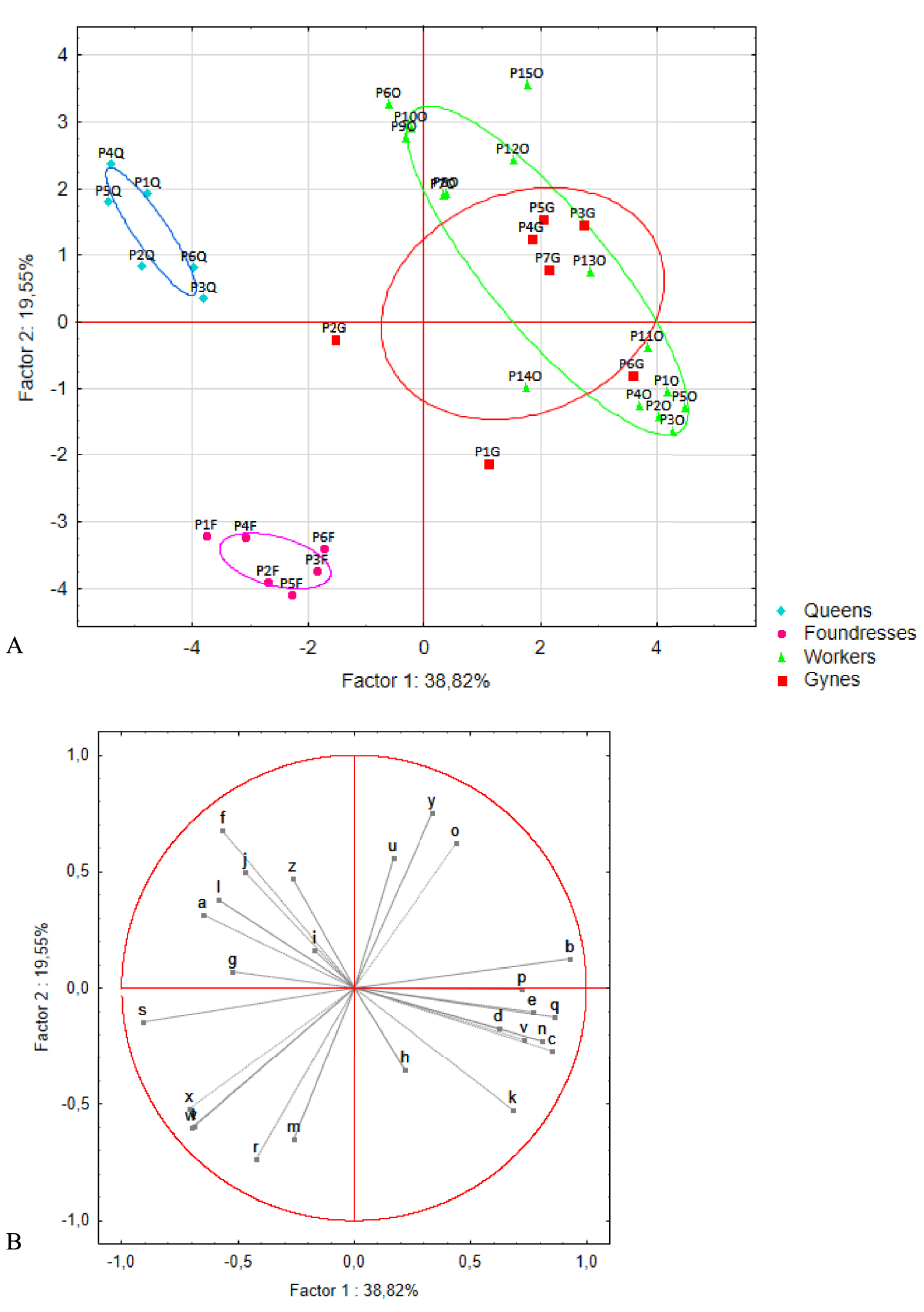
The fiber-based extractions revealed that the workers and pre-wintering gynes released 13 compounds under stressful conditions (SH and WH). All the compounds were present in the solvent extract obtained from the venom glands (Table 2). Two compounds accounted for more than 50% of the total profile: 4,8-dimethyl-7-nonen-2-one (j) and the unknown compound s. A PCA was carried out on the profiles of the workers (n = 16 pools) and pre-wintering gynes (n = 8 pools) subject to the SH and WH treatments. The first and second axes accounted for 42% and 22% of the total variance, respectively. The analysis did not show any clear differences based on caste or treatment (Figure 6).
Principal component analysis of the 13 compounds identified via PDMS-SPME after workers and pre-wintering gynes were experimentally stressed (SH treatment, G: n = 4 and W: n = 8) or wounded (WH treatment, G: n = 4 and W: n = 8). The ellipses indicate the 95% confidence interval of the data.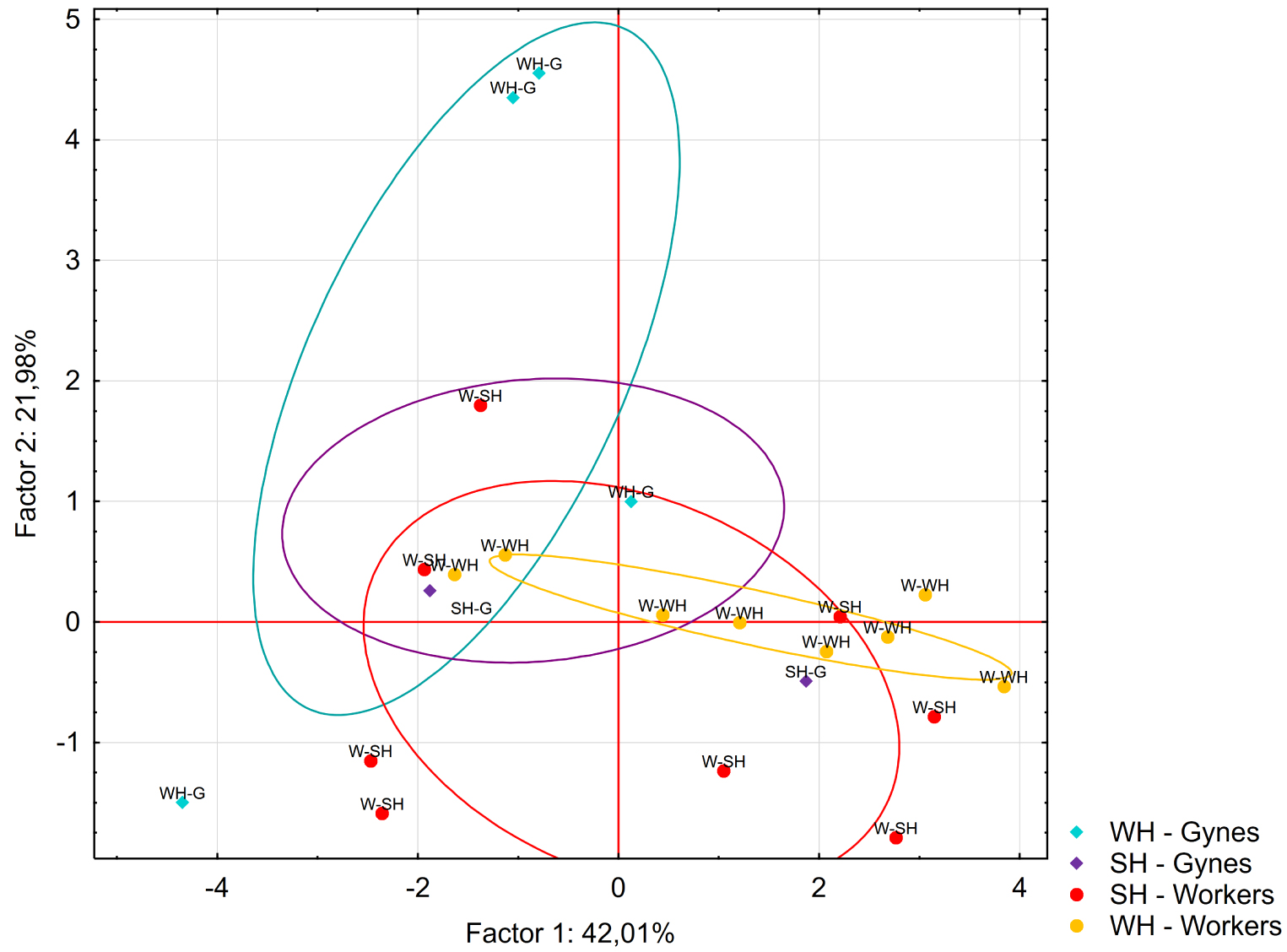
4. Discussion
As insects evolved to form organized societies, it became essential for them to develop communication systems for sharing diverse messages [48]. Eusocial insects most commonly employ chemical communication [49], and it can be challenging to study the cues and signal patterns involved in different behaviors (e.g., recruitment, dispersal, aggressiveness, or aggregation) [30, 35, 50]. Chemical signals, like pheromones, can rapidly and efficiently disseminate information and are used by all eusocial insects [51, 52]. Volatile pheromones are common and may convey information across great distances [33, 52, 53]. Previous studies identified 19 compounds with chain lengths ranging between 7 and 12 carbons in workers of both V. v. auraria and V. v. nigrithorax [26, 27, 42, 43]. The majority were ketones, the most phylogenetically widespread class of alarm pheromones [52]. In the study presented here, 9 new compounds were identified among the 13 compounds found, which displayed the same range of chain lengths as in previous research (Table 1). To our knowledge, this is the first time that three of them (β-citronellol methyl ether [l], 2-nonanyl acetate [k], and 2,9-decanedione [u]; Table 2) could been identified as potential semiochemicals or as molecules involved in toxic actions. Other compounds that we observed are known to have communication functions in Hymenoptera. Geranyl acetate (t) is used by three Bombus species [54], and citronellyl acetate (r), isoamyl isovalerate (e), and isoamyl senecioate (g) occur in the European hornet Vespa crabro [55]. Undecan-2-one (q) occurs in Dolichovespula maculate [56] and Polistes species [39]. Nonan-2-one (c), 4,8-dimethyl-7-nonen-2-one (j), and undecan-2-one (q) are known to elicit different levels of alarm in Vespa orientalis [57]. Thus, these compounds may be common in Vespidae. Some compounds have also been found in other insects. For example, phenethyl acetate (m) is an attractant in Diachasmimorpha longicaudata [58] and is part of a pheromone released by the milkweed bug Neacoryphus bicrucis [59]. Others, such as isopentyl benzoate (w) and isoprenyl benzoate (x), are volatiles in plants [60, 61]. The presence of undecan-2-ol, in contrast to previous research in V. v. auraria [34] and V. v. nigrithorax [57], was not observed. Since a reference sample of undecan-2-ol was also injected, using the same method, and present in the profile, we are confident that the absence of this compound does not reflect a dehydration or thermolysis artifact in our GC-MS injector.
This same previous research [42, 43] discovered that four compounds were common in the venom glands of V. velutina workers: nonan-2-one (c), 4,8-dimethyl-7-nonen-2-one (j), X-undecen-2-one (n), and undecan-2-one (q). In our study, however, the main compound observed in all castes was 4,8-dimethyl-7-nonen-2-one (21–28%, liquid extraction), not nonan-2-one. Nonan-2-one was the second most common compound in workers, pre-wintering gynes, and foundresses but not in queens (relative representation: 4%). Instead, the second and third most common compounds in queens were compound s (23%, Figure 3) and undecen-6-one (o), respectively. In Cheng et al. [42], Vespa v. auraria collected did not display the peak for compound s. Nevertheless, compound s might correspond to the unidentified peak placed after the citronellyl acetate in both Vespa v. auraria (seen by [62]) and V. v. nigrithorax (seen by [43]). Additionally, compound quantity differed between our work here and that of [42]. For example, in V. v. auraria, Cheng et al. [42] found that X-undecen-2-one (n) and undecan-2-one (q) were the second and third most common compounds, respectively; in our study, however, they were present at far lower levels (2.6–9.3%, liquid extraction). In short, our results differ quantitatively and qualitatively from those of both [42] and [43].
Considering that undecan-2-ol was absent from our results and that we observed a different set of common compounds (i.e., like citronellyl acetate in the French and Spanish hornet populations), it could be that the hornet’s chemical blends are changing at the population scale, as has been seen in some ants [63, 64, 65]. Whether such shifts between and within subspecies could arise from isolation by distance or difference in evolutionary lineages is a question that merits further exploration.
In the venom gland profiles, there were clear differences among the gynes, queens, and foundresses. This result indicates that the chemical signature of the female venom gland changes over the course of life history: from pre-wintering gynes (females without nest) to foundresses (mated females with small nest and some eggs) to queens (mated females with workers). However, no differences between the pre-wintering gynes and workers profiles were observed. This result shows that pre-wintering gynes start off with profiles similar to those of workers but that those profiles then change over time. The most obvious change could be seen in compound s: its mean relative levels were 5% in workers versus 7, 15, and 23% in pre-wintering gynes, foundresses, and queens, respectively. Unfortunately, no match was found for compound s in any of the available databases. However, this compound did have a fragmentation spectrum highly similar to that of citronellyl acetate (r): fragments 137 and 152 were present, and fragment 138 was almost completely absent.
The differences among the females raise questions about the hornet’s chemical ecology. The blend observed could act as an alarm pheromone for pre-wintering gynes, which occur in their colony of origin and can play a defensive role alongside workers. However, the queens displayed a different compound ratio, which could mean that the blend had a more queen-specific function. Indeed, this compound ratio could serve as a specific signal from the queen, potentially informing the colony’s workers that their queen has a problem or is in danger. Alternatively, it could act as a signal of queen presence and health. Indeed, research on wasps, bees, and ants has shown that reproductives may chemically communicate their fertility [66]. Consequently, it could be that compound s is a signal generated by mature egg-laying queens. Further study is needed to explore this hypothesis in V. v. nigrithorax.
Previous studies identified 12 compounds in the crushed venom glands of workers (V. v. nigrithorax; [43]) and in living hornets (V. v. auraria; [42]) using blue PDMS fibers (Supleco). Here, we used SPME and liquid extracts to characterize the compounds released by the venom glands of V. velutina hornets as a result of experimental stress or wounding. 13 out of 26 compounds in this study were identified in living hornets using red PDMS fibers (Supleco). We found 4,8-dimethyl-7-nonen-2-one (32–38%; j) to be the most common compound, followed by compound s. The results were unaffected by either caste (worker versus pre-wintering gyne) or treatment. When stressed, both groups of hornets emitted an alarm signal that was the same as the one emitted when facing a life-threatening event. Eight of the compounds associated with this signal had been previously observed in both V. v. auraria and V. v. nigrithorax [42, 43]. Recent work by Rodriguez-Flores et al. [26] identified 8 compounds in queens (n = 4) and workers (n = 3) using HS-SPME/GC-MS [29]. Among them were nonane-2-one, undecan-2-one, and 4,8-dimethyl-7-nonen-2-one, which have been seen in all studies on the hornet’s chemical ecology; in contrast, nonanal, decanal, geranyl acetone, and 4,8-dimethyl-1,7-nonadiene were not seen [26]. The differences between our results and previous research could be explained by SPME fiber type, collection materials, and/or chromatography methods and machine systems.
This study represents an important step towards developing pheromone-based traps. Indeed, the next step is to identify all the components of V. v. nigrithorax pheromones, including compound s, which might be important in queen signaling. A useful approach could be to assess the effects of each major compound, first individually and then in proportionally accurate blends. Indeed, certain compounds may only elicit a strong response when they occur in blends, as is the case with alarm pheromones in honey bees [67] or sex pheromones in moths [68]. Moreover, the precise compound ratio of blends could be of major importance. It is also essential to test the effects of any target compounds on bees. Given that alarm pheromones are produced during both defensive and offensive situations, they could also serve as a kairomone for the hornet’s prey. Indeed, bees may detect certain components of hornet alarm pheromones and modify their behavior accordingly.
Conflict of interest
The authors declare no competing interests.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Ethics approval
The study does not require ethics approval since the yellow-legged hornet is classified as an invasive species; all research was carried out in compliance with the relevant national guidelines.
Code availability
Not applicable.
Authors’ contributions
The experiments and data collection were carried out by LB, JG, MH, and ED. The chemical assays were carried out by CL, AGB, LB, and AK. Formal Analysis: LB. The first draft of the manuscript was written by LB. The manuscript was revised and edited by LB, CL, AGB, and ED. Funding was obtained by ED. All the authors read the manuscript and gave their approval for submission. Conceptualization & Project Administration: ED.
Consent to participate
Not applicable.
Consent for publication
All the authors have approved the content of this paper and have agreed to the “Special Issue: Chemical Ecology – Chemical Mediation in the Environment” Comptes Rendus de Chimie submission policies. This manuscript has not been published previously and is not under consideration for publication elsewhere.
Funding
This study received funding from the Centre-Val de Loire region, in the form of French regional grants for our FRELON and FRELON 2 projects, as well as from Veto-pharma and the French administrative department of La Manche. The TQ-MS was funded by the region Centre-Val de Loire (APR-IA 2012).
Acknowledgments
Thanks to Rémi De La Burgade for the advice and access to the chemistry platform. This study received funding from the Centre-Val de Loire region, in the form of French regional grants for our FRELON and FRELON 2 projects, as well as from Veto-pharma and the French administrative department of La Manche. We are grateful to everyone who helped locate the hornets’ nests. We would like to thank the chemical ecology platform of the IRBI (UMR7261 CNRS, University of Tours) for the services and facilities. Our thanks also go out to Ignacio Ruiz for the help in the field and Jessica Pearce for the English-language editing.




 CC-BY 4.0
CC-BY 4.0