1. Introduction
The word “dendrimer” was created by Tomalia et al. [1] to name a new class of hyperbranched polymers, constituted of branched monomers associated stepwise and not by polymerization reactions. Most dendrimers are synthesized by a divergent process, starting from a multifunctional core, which is modified to become suitable for reaction with a branched monomer. Such process affords the first generation of the dendrimer. If both steps are quantitative, they can be repeated, by modifying the terminal functions of the dendrimer, to render it suitable for the reaction with the branched monomer, affording the second generation of the dendrimer (Scheme 1). Quantitative reactions are the key-point for the synthesis of dendrimers [2]. Each time the number of terminal functions is multiplied, generally by two [3] or three [4], a new generation is created. When the number of generations increases, it becomes more and more difficult to draw the full structures, thus dendrimers can also be represented by a linear structure, with parentheses after each level of branching points, associated to a number which corresponds to the number of divergent ramifications for the branching point considered. Both the full structure and the linear structure of the second-generation dendrimer are represented in Scheme 1. Dendrimers have a bowl-shaped structure, in particular for high generations. There is a limit to the number of generations attainable with dendrimers, which depends on the length of the branches [5]. Indeed, the surface available to accommodate the terminal functions increases more slowly than the number of terminal functions. Polyamidoamine (PAMAM) dendrimers [6] and poly-L-lysine dendrimers [7] were the first dendrimers synthesized up to the tenth generation. Polyphosphorhydrazone (PPH) dendrimers [8], which will be the main topic of this review, were synthesized up to generation 12 [9], and were, for a long time, the largest type of dendrimers ever synthesized. Relatively recently, generation 13 was obtained with polytriazine dendrimers [10].
Divergent synthesis of dendrimers. Generation 2 is represented twice: both the full structure, and the corresponding linear representation with parentheses after each branching point.
The presence of a large number of functional groups, easily accessible on the surface, and potentially modifiable at will, make dendrimers unique objects in the field of nanotechnologies. Indeed, dendrimers have a nanometric size, but contrarily to classical metal nanoparticles of the same size [11], which pertain to hard matter, dendrimers pertain to soft matter, as micelles and liposomes [12]. Modification of the terminal functions of diverse families of dendrimers has already opened the way towards many potential applications in the fields of catalysis, nanomaterials, biology [13], and also sensors, or electronic, to name a few [14].
Most dendrimers comprise a nitrogen atom at each branching point. This is the case of the PAMAM dendrimers [15], and poly-L-lysine dendrimers [16], but also of polypropyleneimine (PPI) dendrimers [17]. Besides, dendrimers constituted of more inorganic elements such as phosphorus [18] and silicon [19] particularly carbosilane [20] are also known and have been reviewed [21, 22]. The case of phosphorus is particularly attractive for several reasons, due to the richness of its chemistry [23], the sensitivity and the wide window of 31P NMR [24], as well as the potential biocompatibility, as phosphates are for instance the cement of DNA.
In this review, we will present the synthesis of polyphosphorhydrazone dendrimers, and then selected examples of their properties in the field of catalysis, to illustrate the dendritic effect, the recycling, the decreased leaching, and the entrapping of nanoparticles. In a second part, other green approaches of catalysis with dendrimers will be displayed, such as catalysis in water, switchable catalysis, and organocatalysis. An overview of the different types of catalysis carried out with phosphorhydrazone dendrimers has been published [25], but no emphasis on their specific properties has been reported.
2. Synthesis of polyphosphorhydrazone (PPH) dendrimers
Two steps are used for the synthesis of polyphosphorhydrazone (PPH) dendrimers, as for most dendrimers. The core bears P–Cl functions in most cases, based either on the thiophosphoryl trichloride P(S)Cl3 (1a-G0) [8] or the hexachlorocyclotriphosphazene N3P3Cl6 (2a-G0) [26]. Starting from the core, the first step is the reaction with 4-hydroxybenzaldehyde (AB) in basic conditions, either using NaH or cesium carbonate. The second step is the condensation of the aldehydes of 1b-G0 or 2b-G0 with the thiophosphorhydrazide H2NNMeP(S)Cl2 (CD2) used as branched monomer, and obtained by reaction of methylhydrazine with P(S)Cl3 at low temperature (−60 °C). This second reaction permits to double the number of chlorides compared to the core 1a-G0 or 2a-G0, and thus affords the first generation of the dendrimer 1a-G1 (Scheme 2A) or 2a-G1 (Scheme 2B). Both steps are quantitative, thus they can be used again to get the second generation 1a-G2 or 2a-G2, then the third 1a-G3 or 2a-G3, and so on. By starting from N3P3Cl6 as the core, by using the same principle, the 8th generation 2b-G8 can be obtained (Scheme 2B) [27], whereas the 12th generation 1a-G12 can be obtained when P(S)Cl3 is used as the core (Scheme 2A) [9].
Synthesis of polyphosphorhydrazone dendrimers, starting from either P(S)Cl3 (A) or N3P3Cl6 (B) as core.
Several other methods to prepare phosphorus dendrimers have been elaborated, often based on the Staudinger reaction [28] between phosphines and azides, in particular using thiophosphoryl azides [29], for the synthesis of highly sophisticated dendritic structures [30]. However, this family of phosphorus dendrimers has not found any practical use up to now, and the selected properties of PPH dendrimers in the field of catalysis will only be described.
3. Specificities of dendrimers as catalysts
The very first examples of dendritic catalysts, were a silane dendrimer functionalized with aryl nickel complexes, which was used in the Kharasch addition of polyhalogenoalkanes to carbon–carbon double bonds [31], and a small polyphosphine dendrimer complexing palladium used in the electrochemical reduction of CO2 to CO [32].
Different types of ligands have been grafted very early on the surface of PPH dendrimers, in particular phosphines [33] for the complexation of metals such as gold, enabling the characterization by High Resolution Transmission Electron Microscopy (HRTEM) [26], but also the complexation of iron and tungsten [34], palladium, platinum and rhodium [35], ruthenium [36], and zirconium [37] but none of them were used as catalysts. The first examples of PPH dendrimers used as catalysts concerned palladium and ruthenium complexes which were utilized in Stille couplings, Knoevenagel condensations, and Michael additions [38]. Other examples of PPH dendritic catalysts concerned Pd complexes for asymmetric allylic alkylations [39], and C–C cross-coupling reactions [40].
3.1. Dendritic effect in catalysis
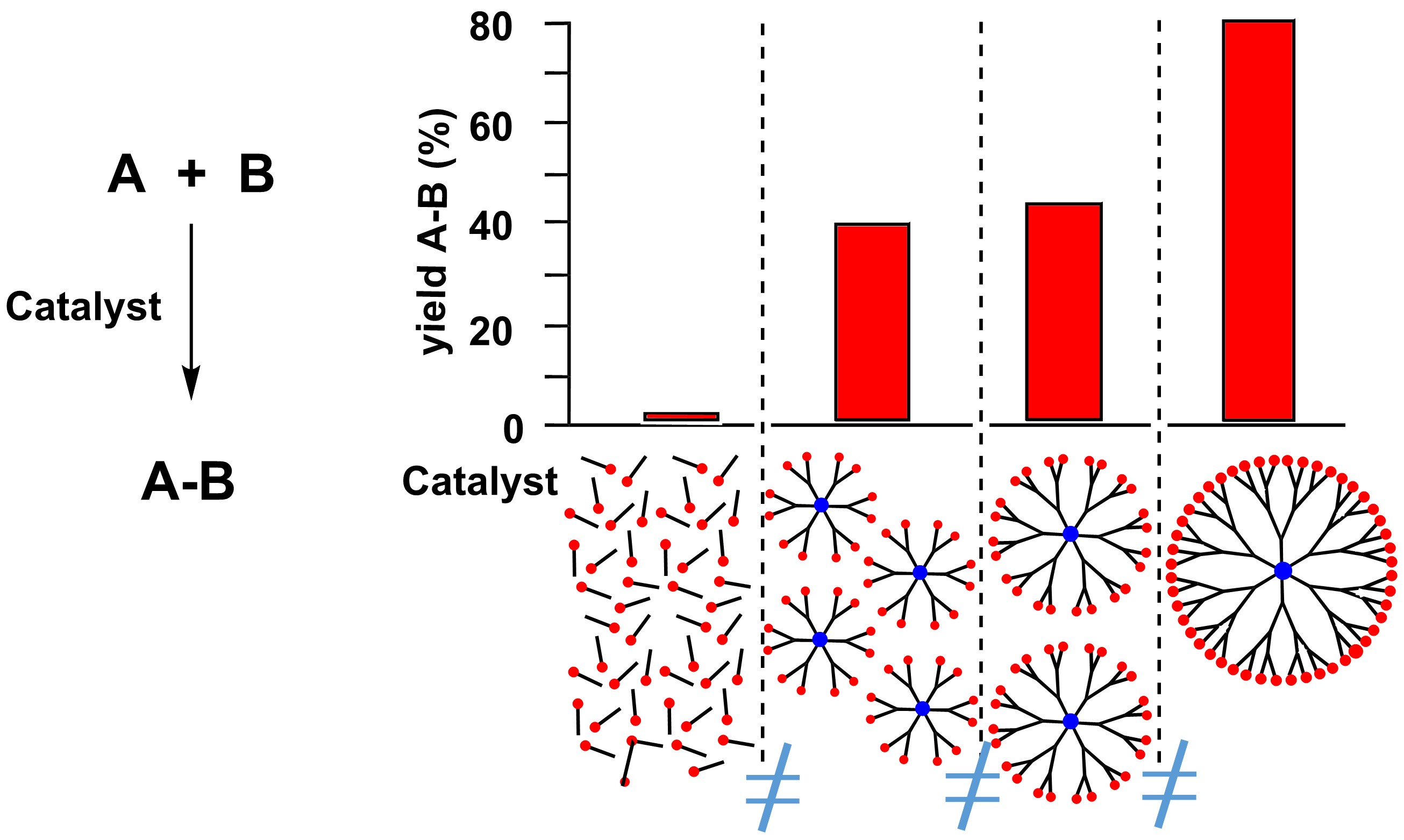
One of the most intriguing properties of dendrimers is the so-called “dendritic effect” [41]. This effect is observed when a functional group behaves differently when it is alone or linked to a dendrimer, and the effect is also depending on the generation of the dendrimer. This effect is essentially unexplained, even if it is believed that it is related to the multivalency effect, which is well-known in biology [42]. In the case of catalysis, a spectacular example of dendritic effect concerned the ester hydrolysis catalyzed by peptide dendrimers composed of histidine–serine. The generation 4 peptide dendrimer was 140,000-fold more efficient than the reference catalyst for ester hydrolysis, with a 4500-fold acceleration per histidine side-chain [43]. In the case of PPH dendrimers, another intriguing example of the dendritic effect concerned pyridine-imine ligands used for the complexation of copper. The monomer and generations 1 to 3 of the PPH dendrimers were synthesized, and used as catalysts for O- and N-arylation as well as for vinylations. The most spectacular example concerned the arylation of pyrazole, using the same quantity of copper (10 mol%) for all the generations used. The efficiency of 1 equiv of generation 3 (2c-G3) was compared to that of 2 equiv of generation 2 (2c-G2), or 4 equiv of generation 1 (2c-G1), or 48 equiv of monomer (2c-M), as in all the examples displayed in this review (Scheme 3). The monomeric catalyst 2c-M was found almost inactive as only 2% yield in 1-phenylpyrazole 3 was obtained when pyrrole was treated with phenylbromide. The yield in 3 was increased with generation 1 (40% yield), generation 2 (44% yield), and the best yield in 3 was obtained with generation 3 (80% yield) [44].
Illustration of the “dendritic effect” with copper complexes of pyridine-imine ligands used for the arylation of pyrazole, using the same quantity of copper.
A strong positive dendritic effect has been also observed in both the yield and the enantioselectivity of Rh-catalyzed [2+2+2]-cycloadditions with dendritic phosphoramidite chiral ligands, leading to axially chiral biaryl compounds. As previously, the same quantity of catalytic sites is used in all cases [45] (Scheme 4). The monomer complex 2d-M-Rh and the branch complex 2d-B-Rh have practically no activity and no selectivity in the synthesis of the biarylic compound 4. On the contrary, all the dendrimers are active, and display an increasing efficiency on going from the first generation 2d-G1-Rh to the third generation 2d-G3-Rh, in both yield and enantioselectivity.
Catalyzed [2+2+2]-cycloadditions using monomeric (2d-M), branch (2d-B), and dendritic (2d-G1 to 2d-G3) Rh complexes. Influence of the generation on the yield and the enantioselectivity.
Other examples of positive dendritic effects have been observed in the isomerization of 1-octan-3-ol catalyzed by dendritic Ru-complexes [46]. However, it should be emphasized that a positive dendritic effect is not always observed with dendrimers. In some cases, there is no change when using a dendrimer compared to a monomer [47], or there is only a slight difference, which depends on the reagents used [48]. In some cases, the absence of dendritic effect is due to the cleavage of the catalytic entity from the dendrimer when using hard conditions [49]. Some negative dendritic effects have been also reported [50], but there is probably a bias in the reported number of positive dendritic effects, as most negative results have never been published.
3.2. Recovery and reuse of dendritic catalysts
Another important benefit of using dendritic catalysts is the possibility to recover and reuse them in several catalytic runs. The main reason is that dendrimers are quite large compared to the reagents and products, and thus they can be less soluble in some solvents than small molecules. This is in particular the case of phosphorhydrazone dendrimers, which are soluble in several organic solvents, but not in diethylether. Thus, the dendritic catalyst can be recovered by precipitation in ether. This concept has been applied several times generally for three runs (see for example: [38, 39, 45, 48]), but such property was applied in particular to the 4th generation dendrimer 2e-G4 bearing terpyridine terminal functions, suitable for the complexation of scandium. This dendrimer was used in Friedel–Crafts acylations, under microwave irradiation. It is worth mentioning that 12 runs can be achieved, with different substrates at each run, and the efficiency of this dendrimer was still very high even after 12 runs [51] (Scheme 5).
Generation 4 dendrimer complexing scandium used as catalyst in Friedel–Crafts acylation, under microwave irradiation. Recovery and reuse of the dendritic catalyst in 12 runs, using different substrates at each step. Blue arrows indicate the recycling.
Another example of the dendritic catalyst recovery concerned a biphasic experiment (water/heptane 1:1), utilizing dendritic ruthenium complexes of PTA (1,3,5-triaza-7-phosphaadamantane) [52] in the isomerization of 1-octan-3-ol 5. The dendrimers are soluble in water, whereas the reagents and products are soluble in heptane. Catalysis occurred at the interface, upon strong stirring. After stopping the magnetic stirring, two phases are rapidly formed and easily separated, the organic phase containing the products, and the water phase containing the dendritic catalysts. A positive dendritic effect on the yield was observed from the monomer 2f-M (38% conversion of 5) to the third generation 2f-G3 (98% conversion of 5). The water phase was recovered and reused in another run, including for the first generation 2f-G1, which was efficiently reused for 3 runs [46] (Scheme 6).
Water-soluble dendrimers used in catalyzed biphasic isomerization of allylic alcohols to ketones. Positive dendritic effect, and recycling of 2f-G1 by phase separation.
The third recycling method used to recover the dendritic phosphorhydrazone catalysts is very original, as it uses magnetic nanoparticles as supports of the dendritic catalysts, which are easily recovered with a magnet [53]. The very first example concerning the use of dendritic catalysts in such process was obtained with generations 0 (6-G0) and 1 (6-G1) of PPH dendrimers having phosphine palladium complexes on the surface and a single pyrene linked to the core. At room temperature, the pyrene interacts by π-stacking with a few graphene layers encapsulating the magnetic cobalt nanoparticles. The efficiency of the π-stacking decreased when heating, thus catalysis with these dendritic catalysts is performed in solution. After completion of the catalysis, the reaction medium is cooled down to room temperature, the dendrimers go back to the graphene entrapping magnetic nanoparticles, which are easily recovered using a magnet, as illustrated in Scheme 7. Such process has been applied to Suzuki couplings of boronic acids with various aryl bromides, and in particular for the synthesis of an anti-inflammatory drug, Felbinac, which was isolated in 100% yield even after 12 runs [54].
Magnetic cobalt nanoparticles covered by graphene interacting with a pyrene catalytic dendron at room temperature, and used for the synthesis of Felbinac. Recovery using a magnet was carried out 11 times.
3.3. Decreased metal leaching
Leaching of metals during catalytic processes is an important problem for both the loss of precious/expensive metals, and the difficult purification of products, especially for the pharmaceutical industry [55]. As shown in the previous paragraphs, it is possible to recover and reuse dendritic catalysts with a good efficiency even after several runs, thus it is presumed that leaching should be reduced when using dendrimers compared to monomers, but it might also depend on the type of ligands. Two families of dendrimers (monomer, generations 1 and 3) were synthesized, decorated with either triphenylphosphine (2g-MPPh3, 2g-G1PPh3, 2g-G3PPh3) or thiazolyldiphenyl phosphine (2h-MthiazolP, 2h-G1thiazolP, 2h-G3thiazolP). They were involved in Suzuki coupling reactions, after complexation of palladium. Both families demonstrated a better efficiency of the first generations, compared to the corresponding monomers (Scheme 8). Thus, their recycling was studied. It was shown that the 1st generation dendrimer decorated with Pd complexes of thiazolyl phosphine (2h-G1thiazolP-Pd) was efficiently recovered and reused, without loss of activity for at least 5 runs. On the contrary, the efficiency of the 1st generation dendrimer decorated with Pd complexes of triphenylphosphine (2g-G1PPh3-Pd) decreased rapidly, and disappeared after 3 runs. To elucidate this difference, palladium leaching with the monomers and the 1st generation dendrimers was measured by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Leaching was found very high with both monomers, reduced for the triphenylphosphine linked to the dendrimer, and undetectable for the thiazolyl phosphine linked to the dendrimer (Scheme 8). Such a low level of palladium leaching is very rare for Suzuki reactions in homogeneous conditions, and explains the efficiency of the recovery and reuse of the dendritic catalyst [56].
Two types of dendrimers suitable for the complexation of palladium, and used in Suzuki couplings. Recycling was only efficient with the dendrimer bearing the thiazolyl phosphine complexes, thanks to undetectable palladium leaching.
3.4. Entrapping catalytic nanoparticles
Beside the grafting of discrete complexes on the surface of dendrimers, examples of dendrimers incorporating metal nanoparticles used for catalysis have been largely explored [57]. Most generally, the starting complex has to be reduced to generate the nanoparticles and to stabilize them, by entrapping them inside the dendrimers or in between dendrimers. A series of phosphorhydrazone dendrimers was functionalized with 15-membered triolefinic triazamacrocycles, generation 0 built from P(S)Cl3 as core (1i-G0) and generations 0, 1 and 4, built from N3P3Cl6 as core (2i-G0, 2i-G1, and 2i-G4). These macrocycles are suitable for complexing Pd(0), issued from Pd2(dba)4 (dba = dibenzylidene acetone). The use of a stoichiometric amount of palladium (1 Pd per macrocycle) induces the formation of discrete complexes, whereas the use of an excess of Pd induces the formation of Pd(0) nanoparticles, stabilized by the dendrimers. The average diameters of the nanoparticles are between 3 and 5 nm, irrespective of the size (generation) of the dendrimers. Both the discrete complexes and the nanoparticles stabilized by the dendrimers were tested as catalysts in Heck reactions, using the same quantity of palladium in all cases. Generation 0 built from the P(S) trifunctional core (compound 1i-G0) was found the most efficient after 4 h, with both the discrete complex and the nanoparticles. However, no large differences were observed after 24 h. In both cases, recycling was attempted 5 times (Scheme 9). The most important differences were observed after 4 h. The discrete complex is easily recovered and reused with the same efficiency. Surprisingly, the nanoparticles complexed by the generation 0 dendrimers (1i-G0) displayed an increasing activity with the number of recycling runs, with a yield in butyl cinnamate after 4 h increasing from 36% after run 1 to 98% after run 6. This contra-intuitive result is due to the decrease of the nanoparticles size, with an average size from 4.1 nm at the first run to 2.3 nm at the sixth run [58].
Macrocycles on the surface of dendrimers, used for complexing palladium as discrete complexes or as palladium nanoparticles. Heck coupling reactions, and recycling.
Another way to generate catalytic nanoparticles consisted in milling, under air, ruthenium chloride (RuCl3), sodium borohydride (NaBH4) and polyphosphorhydrazone (PPH) dendrons of generations 0 to 2, having an alkyl chain (C12 or C16) at the focal point and triarylphosphines on the surface (compounds 7-G0, 7-G1, and 7-G2). Ru nanoparticles obtained in these conditions, were then used as efficient catalysts in the hydrogenation of styrene, to afford ethylbenzene 8 (Scheme 10). No effect of the alkyl chain length on the efficiency was observed. However, a positive dendrimer effect was observed, as the Ru particles stabilized with the second generation dendron (Ru@7-G2) were the most efficient (85% yield with generation 0; 96% yield with generation 2). This is probably due to the fact that the surface of the nanocomposite systems is less hindered when higher generation dendrons are involved, as illustrated in Scheme 10 [59].
Dendrons for the synthesis of Ru nanoparticles. Catalysts for the hydrogenation of styrene.
4. Additional green approaches of catalysis
The different specificities of dendrimers in catalysis shown in Section 3 are all relevant for green chemistry processes by decreasing the leaching of metal, and by decreasing either the time or the temperature of the reaction, reusing the catalyst to decrease the quantity of metal needed, and simplifying the purification of the products. Other types of green approaches are more classical and are known also with monomeric catalysts.
4.1. Catalysis in water
Water can be considered as a green solvent, compared to classical organic solvents. As the internal structure of the phosphorhydrazone dendrimers contains numerous aromatic cycles, they are rather hydrophobic, and can be soluble in water only if the terminal functions are hydrophilic, bearing either positive charges such as ammoniums [60, 61, 62] or negative charges such as carboxylates [63, 64, 65], phosphonates [66, 67] or sulfonates [68], or neutral polyethyleneglycol (PEG) [69, 70].
As shown in Scheme 6, the positive charge on the PTA (PhosphaTriazaAdamantane) ligand induces a solubilization of the dendrimers in water and at the interface between water and heptane [46]. Generations 0 (2f-G0) to 3 (2f-G3) of the same family of PTA dendrimers have been also investigated, both as catalysts for the hydration of phenylacetylene in H2O/iPrOH, and for their interaction in phosphate buffer (excepted 2f-G3) with supercoiled DNA, to afford relaxed DNA [71].
Scorpionates such as tris(pyrazolyl) ligands, have been grafted to the surface of a 1st generation dendrimer 2j-G1, and used for the complexation of palladium. Complexes of the dendrimer (2j-G1-Pd) and of the corresponding monomer (2j-M-Pd) have been used as catalysts in both Sonogashira and Heck C–C cross-coupling reactions in a H2O/CH3CN (1:6) mixture (Scheme 11) [72]. Monomer 2j-M-Pd and dendrimer 2j-G1-Pd complexes afford analogous efficiency in the Sonogashira coupling, whereas the dendrimer 2j-G1-Pd is more efficient than the monomer 2j-M-Pd in Heck coupling.
Monomer and dendrimer bearing scorpionates for the complexation of palladium. Sonogashira and Heck coupling reactions.
4.2. Switchable catalysts
Redox-switchable catalysis concerns an emerging field of research, in which a redox-active functionality is incorporated within a ligand framework. As the redox process influences the electron-donating ability of the ligands, the coordinated metal centers can be influenced in situ, thus modifying the catalytic activity [73]. It has never been applied to dendritic catalysts before our work. Ferrocene is a widely used redox-active group, which displays a high degree of reversibility. Furthermore, it can be easily functionalized and has been frequently grafted to dendrimers, including to phosphorus dendrimers [74]. The presence of an additional functionalization such as phosphine complexes of palladium, enabled their use in asymmetric allylic substitution reactions [47]. Later on, a monomer and a 1st generation dendrimer (2k-G1) were functionalized with unsymmetrically disubstituted 1,1′-ferrocenylphosphine, in which one cyclopentadienyl (Cp) ring is substituted with a phenol for the grafting to the dendrimer, and the other Cp is substituted with an aryl phosphine. The phosphines were then used for complexing ruthenium, and the complexes efficiently catalyzed the isomerization of 1-octen-3-ol to 3-octanone. Upon oxidation of the ferrocene (2k-G1-Red) to ferrocenium (2k-G1-Ox), the experimental rate constant dramatically decreased, due to the precipitation of the oxidized dendritic catalysts 2k-G1-Ox in the medium. Upon reduction to 2k-G1-Red, the dendritic catalysts became again soluble, and the rate constant dramatically increased, displaying an ON/OFF/ON switching process (Scheme 12) [75]. This family of redox-switchable dendrimers was expanded up to generation 3, and with the use of either no linker or a biphenyl linker between the ferrocene and the phosphine [76].
Dendrimer bearing redox switchable phosphines complexing ruthenium, and its use as catalyst in the isomerization of 1-octen-3-ol to 3-octanone. Influence of the oxidation and reduction of the ferrocenes on the catalytic rate.
More recently, the same switching process was successfully applied to dendrimers bearing chiral ferrocenyl phosphines as terminal functions for ruthenium-catalyzed transfer hydrogenation [77] of acetophenone [78].
4.3. Organocatalysis
Organocatalysis, i.e. catalysis in the absence of any metal, is particularly attractive in relation to the principles of “green chemistry”, being both cheaper and safer. The use of dendrimers as organocatalysts has been recognized early and reviewed [79]. Phosphorhydrazone dendrimers have been used as supports of known organocatalysts in several cases. In a first example, small polymers linked to the surface of cobalt nanoparticles 2l-NP (identical to those used in Scheme 7) as well as dendrimers of generations 1 to 3 (2l-G1, 2l-G2 and 2l-G3) were functionalized with the Jørgensen–Hayashi catalyst [(S)-α,α-diphenylprolinol trimethylsilyl ether]. Both families of compounds were used as catalysts in the Michael additions of a wide range of aldehydes to different nitroolefins. The addition of propanal to β-nitrostyrene was particularly studied to demonstrate the recycling ability (Scheme 13). Among the dendrimers, generation 3 (2l-G3) was the most efficient at the first run, and remained highly efficient over 4 runs. The cobalt nanoparticles covered with the catalysts 2l-NP were found as efficient as the dendrimer 2l-G3, but the recycling was surprisingly poorly efficient. Indeed, at run 4, the catalytic efficiency of the dendrimer 2l-G3 was still 98%, whereas that of the nanoparticles 2l-NP was only 34% [80].
Dendrimers and cobalt nanoparticles as support of the Jørgensen–Hayashi organocatalyst. Coupling of propanal to β-nitrostyrene. Comparison of the efficacy in recycling experiments.
Cinchona alkaloids are natural products, which derivatives have been used in many catalytic asymmetric reactions [81]. One of these derivatives, (+)-cinchonine, has been grafted on the surface of phosphorhydrazone (PPH) dendrimers in two different ways for two different purposes. In the first attempt, (+)-cinchonine was modified with a phenol, to be reacted with the P(S)Cl2 terminal functions of the dendrimers of generations 1 (2m-G1) and 4 (2m-G4). A branch derivative (2m-B) was also synthesized as a model compound bearing two (+)-cinchonine derivatives (Scheme 14). These compounds were tested in the α-amination of several β-dicarbonyl compounds. The reactions were very fast, even with only 4 mol% of catalyst (to be compared with 20 mol% in the case of the monomer 2m-M), and went to completion in 2 min at 25 °C, and in 10 min at −25 °C with the smallest compounds (branch 2m-B, and 1st generation dendrimer 2m-G1). The best enantiomeric excesses were obtained with the branch and the 1st generation dendrimer. Recycling experiments were successfully carried out with the 1st generation 2m-G1, and ten runs were carried out without loss of activity and enantioselectivity. The scope of the catalytic enantioselective amination of various active methylene compounds was then evaluated, using cyclic and noncyclic esters, cyclic β-diketones and opened chain esters [82].
(+)-Cinchonine derivative linked to a monomer (2m-M), a branch (2m-B) and to generation 1 of dendrimer (2m-G1), and their use as organocatalysts for the α-amination of β-dicarbonyl compounds.
The second way to graft (+)-cinchonine required the modification of the surface of the dendrimers in three steps from the aldehydes, e.g. reduction with BH3⋅SMe2, transformation to benzylic chloride with SOCl2 [83], and reaction with NaI to give benzyl iodide terminal functions. The next step was the quaternization of the nitrogen of the quinuclidine of (+)-cinchonine, containing different R groups in the O-9 hydroxy group of the cinchonine unit. A small quantity (0.1 mol%) of these functionalized dendrimers 2n-G1a-d was then used as a phase transfer catalyst in the asymmetric alkylation of a glycinate Schiff base with benzyl bromide, at 25 °C, 0 °C, and even −20 °C (Scheme 15). To simplify the analysis, the resulting imines 9 were derivatized to the corresponding trifluoroacetamides 10 by their hydrolysis with citric acid, followed by the protection of the generated primary amines by reaction with trifluoroacetic anhydride. The best results concerning the yield and the enantiomeric excess were obtained for 2n-G1b (R = allyl). This dendrimer was also used in recycling experiments, at 0 °C. Five runs were carried out, and yields were similar for each run, but a slight decrease of ee was observed. The scope of the catalytic enantioselective alkylation of the N-(diphenylmethylene)glycine tert-butyl ester with various alkylating agents was then also evaluated. Good yields (76–87%) and enantioselectivities (76–89%) were obtained in most cases [84].
Alkylation of the nitrogen of the quinuclidine of (+)-cinchonine for the grafting to the 1st generation dendrimer. Phase transfer catalyst in the asymmetric alkylation of a glycinate Schiff base with benzyl bromide. Influence of the temperature and the R substituent. Recycling experiments with 2n-G1b.
5. Conclusions
We have shown in this review different aspects of the use of dendrimers in catalysis. Very positive influences on the yield, the enantioselectivity, the easy recovery and reuse, make dendrimers especially attractive in the field of catalysis. However, it should be emphasized that catalysis is only a part of the fields that benefit from the use of dendrimers. The PPH (polyphosphorhydrazone) dendrimers in particular gave birth to two start-ups, one in the field of materials, in particular biosensors for diagnosis [85, 86], and one in the field of biology, in particular an anti-inflammatory drug [87, 88]. Thus, there is still plenty of room to expand the use of dendrimers in general, and more specifically of PPH dendrimers.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.





 CC-BY 4.0
CC-BY 4.0