1. Introduction
According to recent data from IPBES (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services), 60% of species extinctions are due to Invasive Alien Species (IAS) [1]. These latter constitute one of the five main causes of biodiversity loss on a global scale and cost 400 billion dollars each year. IAS profoundly and negatively affect biodiversity, economy, food security, water security, and human health. Among them, the Japanese knotweed, or Fallopia japonica, is one of the most fearsome plant species. It is widely established in wetlands in Europe, Oceania, North and South America [2]. The uncontrolled development of F. japonica strongly impacts the biological diversity of wetlands, on uses, the weakening of banks and hinders flood management. Arundo donax, also called the giant reed and present in temperate and tropical areas [3], is another demonstrative example. Its long, fibrous, interconnected root mats constitute a frightening obstacle to ecosystems and affect the functioning of wetlands. The growth of A. donax is 2–5 times faster than native competitors, and its vegetative reproduction allows it to quickly invade new areas and completely supplant native vegetation. Finally, A. donax is extremely flammable, increasing the likelihood and intensity of wildfires.
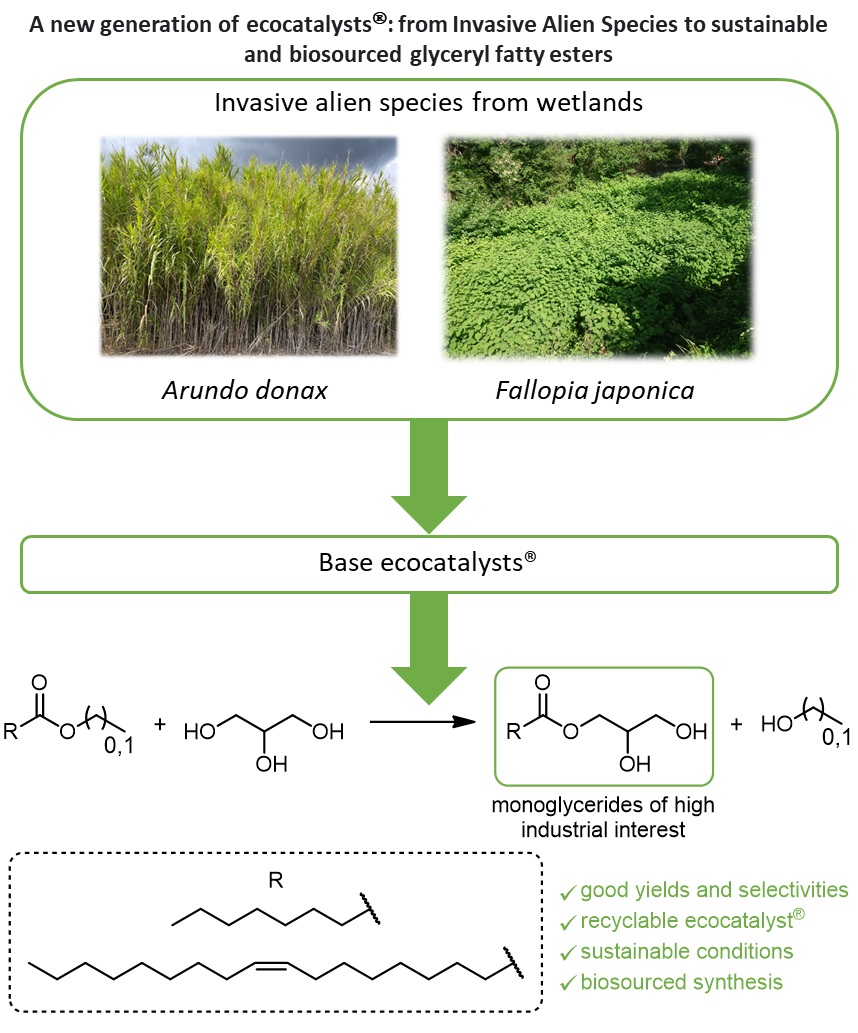
While they constitute the largest reservoirs of carbonaceous organic matter, wetlands contribute to mitigating extreme climatic phenomena (floods and droughts), water purification, and preserving biodiversity. Thus, the rapid disappearance of wetlands, which no longer ensure their regulatory action, is worryingly reducing the resilience of neighbouring ecosystems. Over the past ten years, wetland managers have made considerable efforts to try to eradicate IAS. The technique is frequently based on the extraction of rhizomes. This method is destructive, expensive and has a major limitation: the slightest residue of rhizomes or roots can grow uncontrollably. The various eradication operations seem so far illusory. Today, it is more realistic to study the implementation of solutions to refrain the proliferation of IAS rather than aiming for their eradication. Thus, for example, a less destructive management of the proliferation of invasive species, F. japonica and A. donax, is being studied: rather than extracting the rhizomes at the cost of significant and inconclusive efforts, repeated mowing of the aerial parts could deplete the plant. This technique affects the vegetative dynamics of the species and allows native species to recreate habitats. These experiments must be designed over several years to assess the process efficiency of management control for each species. It is therefore essential to find an economically viable valorization of the harvested parts to support the ecological study. To do so and particularly to limit IAS in wetlands over the long term, the Grison group is studying the transformation of harvested aerial parts into essential raw materials for sustainable chemistry (Figure 1). Their particular physiology and vegetative dynamics bear witness to an unusual mineral composition [4]. They complete the family of biosourced metal catalysts, called ecocatalysts® [5, 6, 7, 8].
Recently, we showed that ecocatalysts® derived from F. japonica were composed of fairchildite, a double carbonate of potassium and calcium, K2Ca(CO3)2. This original structure led to very good results in the Michael addition of di-activated methylene to cyclic enones [9]. The study was here extended to a reaction of industrial interest, the transesterification of alkyl esters with glycerol. This new application led to the green and biosourced synthesis of emollients and non-ionic emulsifiers, glyceryl caprylates and oleates. The structure and reactivity of ecocatalysts® derived from F. japonica and A. donax were compared. After a detailed study of the different parameters influencing the conversion and selectivity of transesterification reactions, the design of an experimental plan was carried out to optimize the reaction conditions. The method was then studied on larger quantities through a scale-up study.
From the management of IAS in wetlands to green synthesis of glyceryl esters. (a) F. japonica and (b) A. donax [10]; (c, d) massive harvests of aerial parts; (e) transformation of aerial parts into ecocatalysts®; (f) synthesis of glyceryl esters using ecocatalysts®. Pictures taken by the authors.
2. Results and discussion
2.1. Characterisation of ecocatalysts®
Ecocatalysts®, Eco1-E-FA-JA and Eco1-E-AR-DO, were prepared from aerial parts of F. japonica and A. donax, including stems, leaves and petioles. The harvested parts were ground and thermally treated under airflow, directly characterised by MP-AES and XRPD and used as catalysts for the transesterification of methyl and ethyl fatty esters.
2.1.1. Elemental composition of ecocatalysts®
Metallic compositions of ecocatalysts®, resulting from the thermal treatment of aerial parts of F. japonica (Eco1-E-FA-JA) and A. donax (Eco1-E-AR-DO), were studied by MP-AES analyses. Assays were performed in triplicate for each sample to determine the standard deviation of the measurement (Table 1). The results showed that potassium was the major mineral element in each ecocatalysts®. These values were significantly higher than the average values of native plants present in the wetlands where the harvests were carried out (potassium content usually around 3–5%). It should be noted that Eco1-E-FA-JA exhibited a high magnesium level, which is consistent with the high level of chlorophyll in the leaf biomass of F. japonica [11].
Elemental composition of Eco1-E-FA-JA and Eco1-E-AR-DO determined by MP-AES
| Ecocatalysts® | Composition (wt% (%RSD)) | |||||
|---|---|---|---|---|---|---|
| Ca | Fe | Mg | Na | K | Al | |
| Eco1-E-FA-JA | 9.09 | 0.28 | 3.90 | 0.10 | 30.16 | 0.13 |
| (0.57) | (4.33) | (0.5) | (0.66) | (0.7) | (3.52) | |
| Eco1-E-AR-DO | 2.71 | 0.05 | 1.49 | 0.12 | 37.16 | 0.03 |
| (2.29) | (2.93) | (4.07) | (0.63) | (0.48) | (1.10) | |
2.1.2. XRPD analysis of ecocatalysts®
The structures of Eco1-E-FA-JA and Eco1-E-AR-DO were investigated by XRPD (Table 2, Figures S1 and S2 in ESI). The two ecocatalysts® presented mostly crystalline potassium salts; neutral crystalline potassium salts were similar but the basic ones differed. Indeed, Eco1-E-AR-DO exhibited K2CO3, while Eco1-E-FA-JA revealed the presence of KMgPO4⋅6H2O, crystallized as struvite-K. Surprisingly no crystalline phase of fairchildite, K2Ca(CO3)2, could be detected within Eco1-E-FA-JA, as it had been previously observed [9]. The difference of structure between the two ecocatalysts®, which both derived from the same plant species F. japonica, might be due to different locations and dates of collection. Nevertheless, the presence of struvite-K was of high interest since phosphates possess stronger basic properties than carbonates.
Crystalline species of potassium salts Eco1-E-AR-DO and Eco1-E-FA-JA determined by XRPD and pH of a suspension of ecocatalysts® in water
| Ecocatalysts® | Crystalline species | pH∗ | |||||
|---|---|---|---|---|---|---|---|
| KCl | K2SO4 | K2CO3 | K2Ca(CO3)2 | KMgPO4⋅6H2O | CaCO3 | ||
| Eco1-E-AR-DO |
 |
 |
 |
- | - | - | 12 |
| Eco1-E-FA-JA |
 |
 |
- | - |
 |
 |
11.5 |
∗pH was measured after stirring 100 mg of ecocatalyst® in 1 mL of distilled water for 15 min and decanting.
The basicity of the two ecocatalysts® was hence assessed and gave high pH values of 12 for Eco1-E-AR-DO and 11.5 for Eco1-E-FA-JA (Table 2).
These two catalysts being therefore slightly different, it was particularly interesting to compare their reactivity in the basic-catalyzed transesterification of triglycerides.
2.2. Application of the ecocatalysts® in the transesterification of triglycerides
The preparation of glyceryl esters is based on three possible strategies (Scheme 1):
General routes for the synthesis of glyceryl esters from literature and envisaged strategies for the transesterification of alkyl esters with glycerol using ecocatalysis.
∙ Hydrolysis of a triglyceride.
(Strategy 1): This strategy may seem attractive because it is direct. However, the hydrolysis of a triglyceride into a monoglyceride is very difficult to control as hydrolysis is increasingly rapid as the fatty chains of the triglyceride are hydrolyzed [12, 13]. The literature is therefore mainly based on enzymatic hydrolysis using specific lipases, which are more suited to this situation [14, 15]. However, the selectivities remain moderate (>50%) and the reactions are described on small quantities of substrates.
∙ Esterification of an activated fatty acid.
(Strategy 2): This sequenced method requires the prior hydrolysis of the triglyceride into fatty acid, followed by the activation of the acid to carry out the esterification. Various activation methods are described: activation of the fatty acid partner by Brønsted acids such as APTS [16], Zn or Ti salts supported on zeolite [17, 18], tungsten nanoparticles in ionic liquid [19], cobalt salts supported on hydroxyapatite [20], activation by enzymes [21, 22, 23], formation of fatty acid chlorides [24, 25, 26, 27, 28], or acid anhydrides [29, 30]. Using glycerol as a nucleophile leads to unsatisfactory results despite the variety of activation agents (drastic conditions in terms of temperature and/or reaction time), and masked forms of glycerol have been described to improve conversion and selectivity. Thus, isopropylidene glycerol [30, 31], glycidol [32, 33, 34, 35, 36], epichlorohydrin [37] or 3-chloro-1,2-propanediol [38] have been reported. This strategy is hardly compatible with the principles of green chemistry (protection/deprotection sequences of the masked form of glycerol, toxic reagent, moderate or even low overall yields).
∙ Transesterification of a methyl or ethyl fatty ester.
(Strategy 3): This strategy is probably the best compromise between efficiency and number of reaction steps. The key intermediate here is the fatty methyl ester (eventually an ethyl ester), widely described since the emergence of biodiesel. However, only a few examples of monoglyceride formation from fatty esters are described in the literature. Among them, the preparation of glyceryl oleate and caprylate has been reported using sodium carbonate for 5 h at 180 °C [39]. The yield is not specified, but the reaction conditions show that using alkaline carbonates makes it possible to facilitate the transesterification under reasonable reaction conditions in terms of duration and temperature. Our recent work showed that the ecocatalyst® derived from F. japonica presented a reactivity that could be higher than the alkaline carbonates reactivity [4]. These encouraging results led us to revisit this third strategy through the transesterification of methyl and ethyl esters with glycerol in caprylic and oleic series (Scheme 1).
2.2.1. Optimization of the transesterification conditions of methyl caprylate 1 with glycerol
The preparation of glyceryl caprylate 2 by transesterification of methyl caprylate 1 with glycerol was chosen as the first model reaction. The solvent-free reaction was promoted under microwave activation. Glycerol plays the role of nucleophile as well as a heating source absorbing microwaves (Figure 2). A fine study of the reaction conditions was carried out with Eco1-E-AR-DO. The influence of four parameters were investigated on the conversion and the selectivity of the reaction: temperature, reaction time, catalyst loading and the quantity of glycerol. The detailed results are summarised in the Table S1 of the ESI. Given the number and range of experiments, Pearson’s correlations were calculated to statistically analyze these data (Figure 2a). Correlation values greater than 0.50 and p-values less than 0.05 were considered (in bold in Figure 2a). The correlation matrix showing only the correlation of the four parameters and the two responses of the system highlights two statistically significant correlations: conversion rate and selectivity with the catalytic loading and the quantity of glycerol. Both conversion rate and selectivity were positively correlated to the catalytic loading. The influence of glycerol was more complicated: conversion rate was negatively correlated to the quantity of glycerol while selectivity was positively correlated. The correlation coefficient of −0.44 between the conversion rate and the quantity of glycerol was considered, although it does not meet the selectivity criteria. The use of ten equivalents of glycerol was thus chosen, which was a compromise to achieve a sufficient conversion rate without affecting the selectivity. Experimental conditions that take into account these initial results have been tested. Indeed, the transesterification of methyl caprylate 1 with glycerol (10 equiv) at 150 °C for 1 h using Eco1-E-AR-DO (0.5 equiv in potassium) led to an excellent yield (Figure 2b). The use of Eco1-E-FA-JA led to excellent results as well (92% of conversion and 96% of selectivity).
(a) Pearson’s correlation matrix of the experimental variables from 16 experiments for determining the key parameters of the transesterification of methyl caprylate 1 with glycerol using Eco1-E-AR-DO, at a small scale (values in bold correspond to a p-value < 0.05); (b) best conditions found at different scales. ∗methyl caprylate 1 (1 equiv), glycerol (10 equiv) and ecocatalyst® (0.5 equiv in potassium), ¥conversion and selectivity were established using an HPLC-QDa calibration curve; (c) recycling studies of Eco1-E-AR-DO at the intermediate scale.
The recyclability of Eco1-E-AR-DO was then studied on a larger scale (8.5 mmol), called the intermediate scale, to allow distillation of the crude mixture. Distillation of a mixture of glycerol and glyceryl caprylate 2 under reduced pressure (145 °C, 0.04 mbar) was used to recover the ecocatalyst® as the distillation residue. Eco1-E-AR-DO was reactivated by thermal treatment and reused, giving comparable yields during at least 4 reaction cycles (Figure 2c).
The MW-transesterification was scaled up on a larger scale (85 mmol), called the large scale, using a specific set-up. The reaction was performed with Eco1-E-AR-DO on a pilot MW 1 L reactor equipped with a distillation apparatus. MeOH was distilled to shift the equilibrium while the temperature of the reaction mixture was maintained at 160 °C, with a high absorbance of MW power. Good conversion (81%) and selectivity (81%) could be preserved at such a scale.
Depending on the scale of the reaction, the method used here led to yields ranging from 66% to 89%. In the literature, the transesterification of methyl caprylate 1 with glycerol was described using a Ba–Al oxides catalyst at 140 °C for 16 h, leading to a 96% yield [40]. This reaction required a long reaction time and the use of a Ba–Al oxide catalyst, which is questionable from an environmental point of view [41], while our method relied on a biosourced catalyst and a shorter reaction time which could be decreased to 1 h or 2 h according to the reaction scale.
Two work-up conditions were studied to separate glycerol and glyceryl caprylate 2 (Figure S3 in ESI). The first one relies on a conventional treatment by adding water to remove glycerol, followed by a liquid-liquid extraction of glyceryl caprylate 2 with Me-THF. Glyceryl caprylate 2 (100 g) could be obtained with high purity (less than 1 wt% of glycerol) (Table 3).
Industrial specifications [42] and properties of glyceryl caprylate 2 obtained by extraction with H2O/Me-THF
| Industrial specifications | Sample properties | |
|---|---|---|
| Acid value (mg KOH/g) | <2.5 | <2.5 |
| Peroxide value (m equiv/kg) | <1 | 0.6 |
| Free glycerol (%) | <2.5 | <1 |
| Monoglycerides (%) | >80 | >90 |
| Diglycerides (%) | <20 | <10 |
| Triglycerides (%) | <5 | <0.1 |
| Methanol (ppm) | - | <0.4 |
Based on a cloud point experiment, an alternative work-up was investigated to avoid the use of solvent and prevent waste formation. Glyceryl caprylate 2 was added to a brine solution, and the mixture was stirred at 60 °C. Cloud points were determined visually by noting the formation of two phases: brine water/glycerol and glyceryl caprylate 2, which were easily separated by hot decantation. This method led to an excellent separation with only 4 wt% of glycerol.
2.2.2. Extension of the method to the transesterification of methyl oleate 3 with glycerol
The best conditions obtained during the synthesis of methyl caprylate 1 (150 °C, 10 equiv glycerol, 0.5 equiv in potassium, 1 h, microwave activation) were transposed to the transesterification of methyl oleate 3. However, the conversion rate collapsed to 5%, probably due to the lengthening of the carbon chain. Eco1-E-FA-JA appeared far more efficient to achieve this reaction with significant conversion (Figure 3b).
(a) Pearson’s correlation matrix of the experimental variables from 16 experiments for determining the key parameters of the transesterification of methyl oleate 3 with glycerol using Eco1-E-FA-JA (values in bold correspond to a p-value < 0.05). (b) Transposed conditions from transesterification of methyl caprylate 1 and examples of conditions used for the Pearson’s correlation matrix, ∗methyl oleate 3 (17 mmol, 1 equiv), glycerol (10 equiv) and ecocatalyst® (0.5 equiv in potassium), MW activation, ¥conversions and selectivity were established using an HPLC-ELSD calibration curve.
A new set of experiments was then conducted to improve the conversion rate and the selectivity by studying the reaction time, the temperature and the catalytic loading (Figure 3a). Results were again statistically analyzed by calculating Pearson’s correlations, which showed time and temperature were positively correlated to the conversion rate (correlation coefficient > 0.5 and p-value < 0.05). Moreover, the conversion was negatively correlated to the selectivity. For instance, an increase of the temperature led to a conversion rate of 88% but with a selectivity of only 45% selectivity, which can be explained by the beginning of glycerol polymerization.
A design of experiment (DoE) approach was carried out to determine the optimal settings of the transesterification reaction parameters for the production of glyceryl monooleate 4 [43].
The experimental space, shown in Table 5, was defined by the upper and lower limits of three parameters: the temperature of the reaction (160–170 °C), which was limited to 170 °C to avoid the glycerol polymerization side reaction, the reaction time (40–80 min) and the quantity of Eco1-E-FA-JA (0.25–0.5 equiv in K) (Table 5). The studied response was the yield in glyceryl monooleate 4. A design matrix was generated using Modde 13.0 software according to full fractional design, resulting in 11 experimental points that included 23 factor points and three replications at the middle point to evaluate the reproducibility. A polynomial model (see Figure 4b for the corresponding equation) for the glyceryl monooleate 4 yield was obtained with a satisfying R2 (0.954) and an acceptable fit between prediction and experimental data (Q2 = 0.800). Figure 4a highlights that increasing the reaction time and quantity of Ecocatalyst® significantly positively impacts glyceryl monooleate 4 synthesis. The other studied parameters have no significant impact. As shown by the contour plots of Figure 4b, the hotspot is located in the top right-hand area (i.e. Eco1-E-FA-JA at 0.5 equiv in potassium and 170 °C), these optimum conditions allowing the prediction of a theoretical yield of 77%. These optimized experimental parameters were then tested in triplicate, leading to a repeatable yield of 82%, slightly higher than the yield predicted by the DoE model.
(a) Regression coefficients of the model and (b) the associated model equation with normalized coefficients; (c) response contour of yield for the transesterification of methyl oleate 3 with glycerol; (d) theoretical and experimental yields obtained using optimum conditions determined by DoE. ¥Yields in glyceryl oleate 4 were established using an HPLC-ELSD calibration curve.
This result can be advantageously compared to the literature data regarding performances and soft reaction conditions (Table 4). The same reaction was described either with glycerol or using isopropylidene glycerol. The use of glycerol relied on chemical activation by P1 phosphazene [44] or Mg oxides [45] at high temperature or during long reaction time. Isopropylidene glycerol was used in enzymatic catalysis in a hazardous solvent (hexane) and required a strong acid-catalyzed deprotection step [46]. The solvent-free way of synthesis presented here, which needs low reaction times and the use of an eco-friendly catalyst, is particularly attractive from a sustainable point of view.
 |
Reactant | Conditions | Yield in 4 | Ref. |
|---|---|---|---|---|
| Glycerol | Ecocatalyst® | 170 °C (MW), 1.3 h without solvent | 82% | Present work |
| Glycerol | Phosphazene P1 | rt, 16 h in DMSO | 87% | [44] |
| Glycerol | Mg salts | 220 °C, 5 h | >60% | [45] |
| Isopropylidene glycerol | Lipase EC 3.1.1.3 | (1) 65 °C, 48 h in hexane | 88% | [46] |
| (2) HCl (50% v/v) |
Factors and their values used for the experimental design
| Factors | Levels | |
|---|---|---|
| −1 | +1 | |
| Temperature | 160 °C | 170 °C |
| Time | 40 min | 80 min |
| Eco1-E-FA-JA (equiv K) | 0.25 | 0.5 |
2.2.3. Transesterification of ethyl caprylate 5 by glycerol
The transesterification of ethyl caprylate 5 by glycerol presents a specific interest in producing 100% biosourced monoglyceryl esters. Indeed, ethyl caprylate 5 is derived from the transesterification of triglyceride by ethanol rather than by methanol, which is petroleum-based and neurotoxic. However, ethyl caprylate 5 is less reactive than methyl caprylate 1. The transesterification of ethyl caprylate 5 by glycerol therefore required a specific study. The strategy previously used for methyl caprylate 1 and oleate 3 was transposed identically. First, the influence of the time reaction, temperature and catalyst loading on the conversion rate and the selectivity was investigated using Pearson’s correlations (Figure 5a). The correlation matrix highlights that selectivity shows negative correlations with the reaction time and the catalytic loading, suggesting that decreasing the quantity of ecocatalyst® increases the selectivity (Figure 5b). The reaction time and catalytic loading are positively correlated with the conversion rate and temperature is positively correlated with the selectivity, but their values of correlation coefficient are not statistically significant (p-value > 0.05).
(a) Pearson’s correlation matrix of the experimental variables from 12 experiments for determining the key parameters of the transesterification of ethyl caprylate 5 with glycerol (values in bold correspond to a p-value < 0.05); (b) examples of conditions used for Pearson’s correlations. ∗Ethyl caprylate 5 (17 mmol, 1 equiv), glycerol (10 equiv) and ecocatalyst®, under MW activation, ¥conversions and selectivity were established using HPLC-ELSD and HPLC-PDA (𝜆 = 211.3 nm) calibration curves.
The optimal reaction conditions of ethyl caprylate 5 transesterification were then determined by DoE with regards to three key parameters: the temperature of the reaction (150–160 °C), the reaction time (15–30 min) and the loading of ecocatalyst® Eco1-E-FA-JA (0.025–0.05 equiv). The response was the yield in glyceryl monocaprylate 2. The same simple systematic design (full factorial design with 11 experimental points) was used to estimate the main effects and potential interactions. The coefficient values of the polynomial response model (R2 = 0.905 and Q2 = 0.677) are all significantly positive, except for the interaction between temperature and reaction time, meaning that an increase in the temperature, time and catalyst tends to increase the yield in glyceryl monocaprylate 2 (Figure 6a and b). Figure 6c shows that the hotspot—again located at the top right-hand zone (i.e. Eco1-E-FA-JA at 0.05 equiv in potassium and 160 °C)—indicates that these optimum conditions predict a theoretical yield of 70%.
(a) Regression coefficients of the model and the associated model equation with normalized coefficients; (b) response contour of yield for the transesterification of ethyl caprylate 5 with glycerol ¥isolated yield.
This prediction was confirmed experimentally, with an isolated yield of 67% of glyceryl monocaprylate 2, slightly lower than the yield predicted by the 23 full factorial design model.
In an attempt to obtain better results, the increase of each parameter independently was tested. It led to similar results, with no increase of the yield (Table S9). To our knowledge, no similar synthesis were reported in the literature except in a patent, which claims the possibility of carrying out the same reaction by enzymatic catalysis, but without quantitative performance indicators [47].
3. Conclusion
In conclusion, we exploited two wetland Invasive Alien Species (IAS), Arundo donax and Fallopia japonica, by transforming these unwanted plant species into valuable resources, ecocatalysts®. Their characterization showed an interesting mineral composition favoring transesterifications in a basic medium. These ecocatalysts® are eco-friendly and harmless catalysts, which have proven to be very effective for the preparation of glyceryl caprylate and oleate. Using statistical tools such as Pearson’s correlations and DoE, good to excellent selectivities and yields were obtained under microwave activation, without solvent and with a high degree of naturalness. Finally, this study encourages the valorization of IAS, which aims to explore disruptive solutions to support the management of IAS through their transformation into new useful eco-materials for sustainable chemistry processes.
4. Experimental part
4.1. Preparation of ecocatalysts®
Aerial parts of F. japonica were harvested in April 2023, in the wetlands of Clapices, Aulas (South of France) by BIOINSPIR company. Aerial parts of A. donax were harvested in November 2021, in Montferrier-sur-Lez (South of France). Ecocatalysts® were prepared from aerial parts of the harvested plants, which were first dried in an oven at 30 °C, ground and then thermally treated in an oven under airflow. The following temperature program was used: 20 °C–350 °C in 1 h, 350 °C–550 °C in 1 h, then 4 h at 550 °C.
4.2. Characterization of ecocatalysts®
Mineral composition of the ecocatalysts® was determined using an Agilent 4200 Microwave Plasma-Atomic Emission Spectrometer (MP-AES) coupled with an SPS4 autosampler. The samples were digested in 6 mL of reversed aqua regia [hydrochloric acid (37%)/nitric acid (65%) = 1:2] under an Anton Paar Multiwave Go microwave-assisted digestion, with the following program: 20 °C–164 °C in 20 min then 10 min isothermal at 164 °C. Samples were filtered and then diluted to 0.2 g⋅L−1 in nitric acid (1%). Three blanks were recorded for each step of the dilute procedure. Three analyses were carried out for each sample to determine the standard deviation of the measurement. X-ray diffraction (XRD) analyses were performed on samples dried at 110 °C for 2 h by using a BRUKER diffractometer (D8 advance, with a Cu K𝛼 radiation 𝜆 = 1.54086 Å) equipped with a LynxEye detector.
4.3. Procedure for the preparation of glyceryl monocaprylate 2 from methyl caprylate 1
4.3.1. Small scale procedure
Methyl caprylate 1 (0.85 mmol), glycerol (8.5 mmol) and Eco1-E-AR-DO (0.425 mmol of potassium) were stirred in a 10 mL microwave reactor. A microwave oven (CEM Discover 2.0) was programmed as follows: temperature gradient from rt to 150 °C in 2 min, then constant temperature for 1 h. The reaction mixture was filtered using a sintered glass filter (porosity 4) and washed 3 times with isopropanol. The reaction was placed in a 100 mL volumetric flask and analyzed by HPLC-QDa by ion extraction in Single Ion Recording (SIR) mode.
4.3.2. Intermediate scale procedure
This procedure allowed the study of the ecocatalyst® recyclability. Methyl caprylate 1 (8.5 mmol), glycerol (85 mmol) and Eco1-E-AR-DO (4.25 mmol of potassium) were stirred in a 20 mL microwave reactor. A microwave oven (CEM Discover 2.0) was programmed as follows: temperature gradient from rt to 150 °C in 2 min, then constant temperature for 2 h. The reaction mixture was filtered using a sintered glass filter (porosity 4), washed with isopropanol and analyzed by HPLC-QDa by ion extraction in Single Ion Recording (SIR) mode.
4.3.3. Large scale procedure
Methyl caprylate 1 (595 mmol), glycerol (5.95 mol) and Eco1-E-AR-DO (59.5 mmol of potassium) were stirred in a 1 L flask. A microwave oven (CEM MARS 6) was programmed as follows: temperature gradient from rt to 160 °C in 10 min, then constant temperature for 1 h. During the reaction, the produced methanol was distilled at atmospheric pressure. The reaction mixture was filtered using a sintered glass filter (porosity 4), washed with isopropanol and analyzed by HPLC-QDa by ion extraction in Single Ion Recording (SIR) mode.
4.4. Procedure for the preparation of glyceryl monooleate 4 from methyl oleate 3
In a typical procedure, methyl oleate 3 (17 mmol), glycerol (170 mmol) and Eco1-E-FA-JA (8.5 mmol of potassium) were stirred in a 50 mL flask. A microwave oven (CEM Discover 2.0) was programmed as follows: temperature gradient from rt to 150 °C in 5 min, then constant temperature for 1 h 20. During the reaction, the produced methanol was distilled under a vacuum (20 mbar). The reaction mixture was filtered using a sintered glass filter (porosity 4) and washed 3 times with isopropanol. The reaction was placed in a 250 mL volumetric flask, diluted 8 times and analyzed by HPLC-PDA and HPLC-ELSD.
4.5. Statistical studies for the preparation of glyceryl monooleate 4 from methyl oleate 3
4.5.1. Design of experiments
Using the Modde 13.0 software, a DoE was performed using the following factors to optimize the experimental conditions for the preparation of glyceryl monooleate 4 from methyl oleate 3 using Eco1-E-FA-JA.
A first-order polynomial equation including correlation terms was used to find the relationship between the factors and the response surface (Equation (1)):
| (1) |
The coefficients were determined using Multiple Linear Regressions (MLR). The validity of the model was assessed by analysis of the variance (ANOVA), R2, Q2 and the lack of fit. A full 23 factorial design, with 3 replicated centered points to evaluate the reproducibility, was used to fit the model. This design required 11 experiments. The results are presented in Table S5 of the ESI. The order of the experiments was randomized to avoid bias. The yields were obtained by using a calibration curve in HPLC. To obtain a good fit between the dataset and the polynomial equation, the correlation term Temperature × Catalyst was removed from the initial polynomial equation. The analysis of variance shows a good correlation between the obtained yield and the factors. The p-value of the regression model (p = 0.002 < 0.05) shows that the regression is statistically significant. The lack of fit (p = 0.571 > 0.05) shows that the model has low replicate errors. The model gives a very good coefficient of determination (R2 = 0.954) and a good cross-validation coefficient (Q2 = 0.800).
Factors and their values used for the experimental design
| Factors | Levels | |
|---|---|---|
| −1 | +1 | |
| Temperature | 150 °C | 160 °C |
| Time | 15 min | 30 min |
| Eco1-E-FA-JA (equiv K) | 0.025 | 0.05 |
4.6. Procedure for the preparation of glyceryl monocaprylate 2 from ethyl caprylate 5
In a typical procedure, ethyl caprylate 5 (17 mmol), glycerol (170 mmol) and Eco1-E-FA-JA (0.85 mmol of K) were stirred in a 50 mL flask. A microwave oven (CEM Discover 2.0) was programmed as follows: temperature gradient from room temperature to 160 °C in 5 min, then constant temperature for 30 min. During the reaction, the methanol produced was distilled under vacuum (220 mbar). The reaction mixture was filtered using a sintered glass filter (porosity 4) and washed 3 times with isopropanol. The reaction was placed in a 250 mL volumetric flask, diluted 8 times with isopropanol and analyzed by HPLC-PDA and HPLC-ELSD.
4.7. Statistical studies for the preparation of glyceryl monocaprylate 2 from ethyl caprylate 5
4.7.1. Design of experiments
A DoE was performed using the following factors to optimize the experimental conditions for the preparation of glyceryl monocaprylate 2 from ethyl caprylate 5 using Eco1-E-FA-JA.
As in the previous DoE, a first-order polynomial equation including correlation terms was used to find the relationship between the factors and the response surface (Equation (2)):
| (2) |
The coefficients were determined using Multiple Linear Regressions (MLR). The validity of the model was assessed by analysis of the variance (ANOVA), R2, Q2 and the lack of fit. A full 23 factorial design, with 3 replicated centered points to evaluate the reproducibility, was used to fit the model. Such a design required 11 experiments. The results are reported in Table S5 of the ESI. The correlation terms Temperature × Catalyst and Time × Catalyst were removed from the initial polynomial equation to obtain a good fit between the dataset and the polynomial equation. The analysis of variance shows a good correlation between the obtained yield and the factors. The p-value of the regression model (p = 0.003 < 0.05) and the lack of fit (p = 0.124 > 0.05) confirm the statistical significance. Moreover, the model gives a good coefficient of determination (R2 = 0.905) and an acceptable cross-validation coefficient (Q2 = 0.677).
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Acknowledgements
The authors thank the French National Centre for Scientific Research (CNRS) for financial support (International program between CNRS and University of Arizona). The authors also thank Professor Laurent Debelle for the fruitful discussions concerning the statistical tests carried out in this work.




 CC-BY 4.0
CC-BY 4.0
