1. Introduction
The sector of natural plants is characterized by a great potential which is unexploited to a large extent, due to the still used limited traditional extraction techniques. This factor has slowed down the development of the cultivation of natural plants from which great value bioactive substances can be extracted, for various applications that bring considerable and sustainable social and economic prospects. The demand of medicines from natural plants bioactive ingredients is constantly increasing, hence the need to introduce non conventional extraction techniques, like supercritical CO2 extraction which has shown many important applications [1, 2, 3].
The sustainability aspect in various industries like pharmaceutical and food industries depends to a large extent upon the extraction step which has to be “green”, with low energy consumption, short operating times, compatible with friendly solvents, gives high quality and value extracts of renewable natural substances with a high purity and enables the valorization of the generated residues for instance into fertilizers, promoting circular economy as well.
As an illustration the supercritical CO2 extraction of oil from onion seeds, was considered as a model system in the present study. This choice was mainly guided by the important local cultivation of onions scientifically known as Allium cepa L. and which have been grown for over 4000 years and belong to the Liliaceae family [4, 5]. In recent years, the world production of onions has increased by more than 25% and now reaching about 44 million tons, the second most important crop after tomatoes [5]. They are an essential ingredient in cooking traditions of many countries, widely consumed worldwide due to their distinctive flavor and numerous health benefits [6] and are considered as a medicinal plant that can alleviate or prevent several common illnesses such as atherosclerosis, asthma, bronchitis, and cough. They are also a rich source of biologically active phyto molecules [7] and may exhibit various activities such as antimicrobial, antifungal, antiviral, antioxidant, antidiabetic, antitumor, anti-inflammatory, antihypertensive, ant allergic, and hypolipidemic properties [6, 8]. All these properties have been the main motivating factor for the recent research efforts made to identify new sources of edible oils due to their nutritional, industrial, and medicinal benefits [7], using appropriate separation techniques like the supercritical CO2 extraction where the great majority of research works was based on the use of freeze-dried onion powders and rarely onion seeds [9]. However many researchers are now getting more interested in the bioactivity of onions, through the seeds to minimize the effect of the high water content of onion powder which may lead to low oil extraction yields, making the extracting process delicate [6]. The onion seeds have considerable oil content, with high percentages of linoleic, oleic, and palmitic acids, proteins, alcohols, acids, and esters, as well as sulfur compounds, which aid in controlling cholesterol, diabetes, cancer, etc. and are characterized by important free amino acids and antioxidants contents [4].
The selection of supercritical CO2 extraction for this study was primarily influenced by its environmentally friendly nature, which involves minimizing the utilization and generation of harmful substances while extracting oils from natural plants. This process is categorized as a green chemistry approach. Supercritical fluids are often regarded as eco-friendly, and their supercritical phase can be viewed as an intermediary state that is almost as dense as a liquid and with transport properties, such as viscosity and diffusion, comparable to those of a gas [10]. Among the solvent based extraction processes, supercritical CO2 is commonly favored, especially for oxidation-sensitive, thermolabile, high-value, or minor components, owing to its advantageous blend of mild critical conditions regarding temperature (304.13 K) and pressure (7.38 MPa) [11]. Another major advantage of supercritical CO2 extraction lies in the ease to manipulate the operating parameters, mainly the pressure and temperature to obtain extracts with composition made to measure, promoting sustainability in sensitive fields like food and pharmaceutical sectors.
2. Materials and methods
2.1. Chemicals and materials
Carbon dioxide (CO2) with a purity of 99.99% was supplied by Air Liquide, Algeria, MeOH, Hexane and KOH were purchased from Sigma Aldrich and were of reagent grade; The used equipments were a Shimadzu GCMS-QP2020 instrument, a ROTOFIX 32 centrifuge, a SAYONA SZJ8500-R14 638 CAFE MILL for grinding, a RETSCH sieve shaker and a KERN precision balance.
2.2. Description of supercritical CO2 extraction
A description of the used CO2 extraction method is presented. The used supercritical CO2 extraction machine (Separex -4219) was purchased from Separex Company (Champigneulles, France). The machine is better described schematically by the simple flowsheet shown in the following Figure 1.
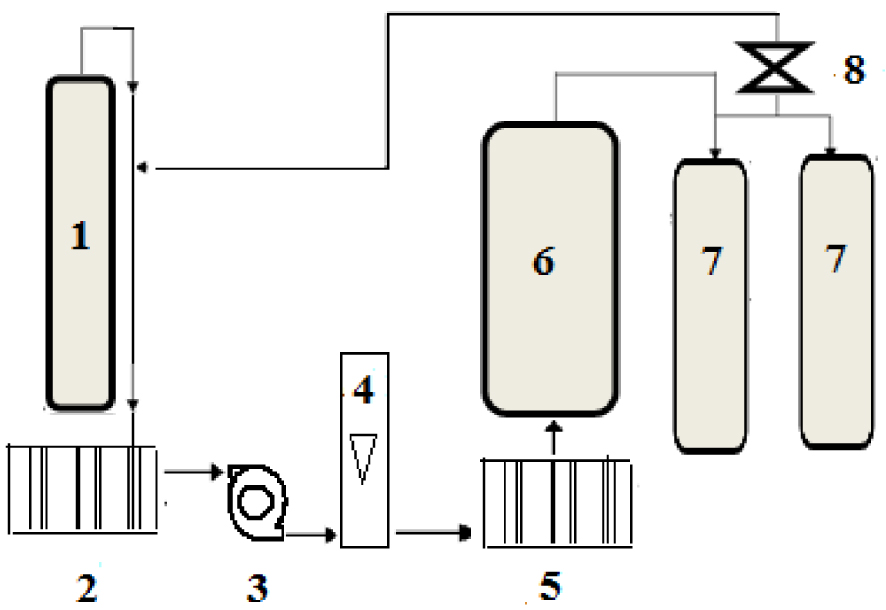
Flowsheet of the supercritical CO2 extraction machine: 1: CO2 tank; 2: cooler; 3: high pressure pump; 5: heater; 6: extractor; 7: separators; 8: expansion valve.
The supercritical CO2 extraction process was carried out acccording to the following steps:
- CO2 from a cylindrical tank is liquified by going through a cooler and then compressed by being pumped by a high pressure pump, making sure that it is at pressure greater than its critical value of 74 bar.
- The CO2 stream going out the high pressure pump goes through a preheater to ensure that its temperature is higher than its crititical value of 31 °C.
- The CO2 stream exiting the preheater is at the supercritical state and it goes into the extractor containing the solid plant material (onion seeds in the present case).
- The contact supercitical CO2—plant solid material in the extractor initiates the mass transfer of the solute (oil) into the supercritical CO2 stream.
- After a certain contact time, the extract phase (oil and supercritical CO2 mixture) goes into two seprators in series where the pressure is highly decreased to induce a separation of the oil from CO2.
- The extracted oil collected from the separators and the flashed CO2 is recycled to be recompressed and brought to the supercritical conditions for a further extraction run.
The operating parameters, mainly temperature, pressure and CO2 flowrate can be controlled and regulated during the process by means of the necessary instrumentation.
2.3. Sample preparation method
The plant onion seeds (Allium cepa L.) were obtained from Oued Souf area in the south eastern Algerian Sahara and were stored at room temperature after removal of all impurities. Then the seeds were crushed into small fragments and passed through a vibrating siever to obtain three different particle diameters of 0.25, 0.55, and 0.85 mm. The supercritical CO2 extraction was performed using the supercritical fluid extraction machine for a constant time of 3 h. A mass of 40 g of ground onion (Allium cepa L.) was fed into the machine to undergo the supercritical CO2 extraction which was fine-tuned using a four-factor Box-Behnken experimental design.
2.4. The applied experimental design
The design of experiments (DOE) was used to methodically alter variables like temperature, pressure, particle size and CO2 flowrate, which may impact any considered specific response or outcome of interest, like the extraction yield for the present case. Controlled manipulations of the considered variables may reveal cause-and-effect relationships and identify the factors that exert the most significant influence on the response variable. Consequently, DOE can statistically enhance the process reliability while reducing the consumption of raw materials and time [12].
The objective of this study was to optimize and investigate the impact of the four mentioned parameters (pressure, temperature, particle diameter and CO2 flow rate), on the extracted oil mass and its quality through the composition. Therefore a BBD (Box-Behnken Design) was used for this purpose. Its choice has been mainly guided by a number of advantages like not requiring a lot of runs for the generation of higher order response surfaces, comparatively to other factorial techniques, determining the interactions between the independent variables, using a reduced experiments number, hence an important time saving. Also for the Box-Behnken design each factor is characterized by three levels, hence the possibility for a second-order model only.
Table 1 shows the actual values of the considered independent parameters in the DOE.
Coded and actual independent variables
| Variables | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Pressure (bar) | 150 | 200 | 250 |
| Temperature (°C) | 35 | 50 | 65 |
| CO2 flow rate (g/min) | 50 | 75 | 100 |
| Particle size (mm) | 0.25 | 0.55 | 0.85 |
2.5. Oil characterization
2.5.1. Preparation of fatty acid methyl ester (FAME)
The onion seed were subjected to supercritical CO2 extraction for 3 h and subsequently the oil converted to methyl ester through trans-esterification. The protocol used for this process involved the use of methanol and hexane, with slight adjustments made to comply with Francisca Salinas’ method which incorporates potassium hydroxide as a catalyst [13].
2.5.2. GC-MS instrument and analytical conditions
The operating conditions of the used GC-MS 2010 plus Shimadzu apparatus are shown in the following Table 2.
The protocol for the GCMS analysis
| Apparatus | GC-MS |
|---|---|
| Injector temperature (°C) | 225 |
| Column temperature (°C) | The oven was preheated to 100 °C and maintained at that temperature for a minute. Subsequently, the temperature was increased to 250 °C and sustained for 15 min. Finally, the oven was kept at this temperature for another 14 min. Overall, the oven was operated for 30 min |
| Injection mode | Split, column flow ratio 10:1 |
| Injection volume | 2 μl with a flow rate of 0.8 ml/min |
| Pressure (kPa) | At the starting temperature of the furnace, the pressure exerted by the column of carrier gas (Helium) at its head was 200 kPa |
Data acquisition and processing was performed by means of specific computer software Minitab and the analyses and interpretation of the results were carried out automatically by the gas chromatography-mass spectrometer (GC-MS). The onion seed oils were separated based on the major components increasing molecular weight, according to the used operating conditions [13].
3. Results and discussion
3.1. BBD analysis results
The process was optimized using the Box-Behnken design that involved a set of 27 experiments. The obtained results showed that the onion seeds oil extraction yield was influenced by the four considered key parameters: temperature, pressure, particle diameter and CO2 flow rate. The obtained yield values ranged from 11.6 to 24.36%, indicating the significance of examining these parameters. A detailed experimental design matrix in terms of the four factors, each at three levels was generated using the BBD approach and the results are shown in Table 3. The response (extraction yield) is expressed as second-order polynomial function of the four independent variables as follows:
| (1) |
The experimental design matrix
| Run | X1 | X2 | X3 | X4 | yexp (%) | ycalc (%) | Residual (%) |
|---|---|---|---|---|---|---|---|
| 1 | 35 | 150 | 0.55 | 75 | 17.876 | 19.013 | −1.137 |
| 2 | 65 | 150 | 0.55 | 75 | 11.604 | 13.097 | −1.493 |
| 3 | 35 | 250 | 0.55 | 75 | 18.047 | 19.256 | −1.209 |
| 4 | 65 | 250 | 0.55 | 75 | 18.253 | 19.820 | −1.567 |
| 5 | 50 | 200 | 0.25 | 50 | 24.320 | 25.041 | −0.721 |
| 6 | 50 | 200 | 0.85 | 50 | 21.199 | 21.937 | −0.738 |
| 7 | 50 | 200 | 0.25 | 100 | 22.241 | 24.206 | −1.965 |
| 8 | 50 | 200 | 0.85 | 100 | 18.09 | 20.082 | −1.992 |
| 9 | 35 | 200 | 0.55 | 50 | 22.017 | 21.620 | 0.397 |
| 10 | 65 | 200 | 0.55 | 50 | 20.664 | 20.609 | 0.055 |
| 11 | 35 | 200 | 0.55 | 100 | 23.161 | 21.940 | 1.221 |
| 12 | 65 | 200 | 0.55 | 100 | 18.473 | 17.599 | 0.874 |
| 13 | 50 | 150 | 0.25 | 75 | 19.854 | 20.666 | −0.812 |
| 14 | 50 | 250 | 0.25 | 75 | 24.350 | 23.291 | 1.059 |
| 15 | 50 | 150 | 0.85 | 75 | 16.411 | 16.194 | 0.217 |
| 16 | 50 | 250 | 0.85 | 75 | 22.625 | 20.535 | 2.090 |
| 17 | 35 | 200 | 0.25 | 75 | 24.039 | 23.051 | 0.989 |
| 18 | 65 | 200 | 0.25 | 75 | 21.974 | 20.321 | 1.654 |
| 19 | 35 | 200 | 0.85 | 75 | 19.371 | 19.382 | −0.011 |
| 20 | 65 | 200 | 0.85 | 75 | 17.408 | 16.760 | 0.648 |
| 21 | 50 | 150 | 0.55 | 50 | 21.407 | 19.921 | 1.486 |
| 22 | 50 | 250 | 0.55 | 50 | 22.695 | 22.894 | −0.199 |
| 23 | 50 | 150 | 0.55 | 100 | 19.902 | 18.066 | 1.836 |
| 24 | 50 | 250 | 0.55 | 100 | 22.210 | 22.059 | 0.151 |
| 25 | 50 | 200 | 0.55 | 75 | 20.968 | 19.830 | 1.138 |
| 26 | 50 | 200 | 0.55 | 75 | 18.174 | 19.830 | −1.656 |
| 27 | 50 | 200 | 0.55 | 75 | 20.455 | 19.830 | 0.625 |
| Optimum | 57 | 250 | 0.25 | 50 | 24.361 | 25.483 | −1.122 |
Table 3 shows that the predicted and experimental yield values were reasonably close, particularly under optimum conditions where the values were 25.483 and 24.361%, respectively.
ANOVA analyses showed that the determined model was weak with a coefficient of determination (R2) equal to 81.37%. The first analysis included the Fisher test, hence the comparison the F values calculated by Minitab software with an F (critical) obtained from the Fisher-Snedecor table using (p − 1) and (n − p) degrees of freedom, for a pre-defined level of significance (0.05). The present model had 15 coefficients, with p − 1 = 14 and n = 27 experiments, resulting in n − p = 12 degrees of freedom. The tabulated F value was 2.62. However, the obtained F value was higher than the tabulated one of 3.74. This confirms the reliability of the proposed model and its predictive capability.
To ensure the validity of the factors in the model, their p-values should be examined and indicate significance when less than 0.05. Upon evaluation, it was discovered that all the terms in the full quadratic model were significant except for the flowrate term, which was found to be insignificant with a p-value of 0.213. The model p-value was calculated as 0.014, while the p-values for temperature, pressure, and particle diameter were 0.024, 0.005, and 0.005, respectively [14].
For a comparison purpose of supercritical CO2, the cold-pressed onion seed oil showed antioxidant properties with a total phenolic content (TPC) of 3.35 mg GAE/g, a higher value compared to those of seed oils from cold-pressed black raspberry, parsley and milk thistle. Furthermore, the cold-pressed onion seed oil yield was found to be 21.1%, higher than the yield of 13.5% shown by Soxhlet extraction [10, 15]. However in the present study and as shown in Table 4, an even higher extraction yield of up to 24.5% was achieved by supercritical CO2 extraction from local onion seeds, at operating conditions of pressure, temperature, diameter, and flowrate of the supercritical CO2 corresponding to the optimum of oil extraction yield and quality, highlighting the significance of ground onion seed oil in various industries, including pharmaceuticals, parapharmaceuticals, cosmetics and food. Also Table 4 shows the comparison of the total phenolic contents (TPC), obtained by the three methods (supercritical CO2, Soxhlet and cold pressing extractions [5, 15]). Clearly the results show that the non conventional supercritical CO2 extraction was much more performing basing on the considered two criteria (extraction yield and TPC), confirming the effectiveness of its sustainability and encouraging its further development. This is in agreement with results of many research works reported in the literature and dealing with different systems such as in [16]. However studies based on oil extraction from onion seeds by supercritical CO2 extraction are very few, according to the literature.
Comparison of onion seeds oil extraction by supercritical CO2, Soxhlet and cold pressing extractions
| Extraction technique | Oil yield (%) | Total phenolic content (mg GAE/g) | Reference |
|---|---|---|---|
| Supercritical CO2 extraction | 24.36 | 36.15 | Present work |
| Soxhlet extraction | 13.50 | 1.84–6.87 | [5] |
| Cold pressed extraction | 21.10 | 3.35 | [15] |
Also these results confirmed the ability of the BBD model in selecting the appropriate range for each parameter.
Generally the composition of extracts obtained through supercritical CO2 extraction is influenced by various factors, such as the plant species and the adopted operating conditions like temperature, pressure, seed size and CO2 flowrate, for the present study. This influence can be seen through the obtained extract properties like their nature, color, viscosity, density, etc. This is confirmed by the three samples of onion seeds oil extracts of Figure 2, where the differences can easily be seen.

Different extracts of onion seeds by supercritical CO2 extraction according to the operating parameters.
The effects of the operating parameters are discussed in details in the next section as follows.
3.2. The influence of the four selected parameters on the oil yield of onion seeds
The solid matrix consisting of onion seeds underwent a mass transfer in contact with the supercritical CO2 in the extractor. This transfer occurred through molecular diffusion due to concentration gradients. Figure 3 shows the oil extraction yield variations with time at given and optimal operating conditions. The curves are showing two phases: a first one corresponding to an important essential oil mass transfer into CO2 due to its availability at the external solid surface, hence a rapid increase of the extraction yield; a second one corresponding to the essential oil exhaust at the external surface and its extraction from the bulk of the solid where the process is dominated by diffusional and internal mass transfer resistances.
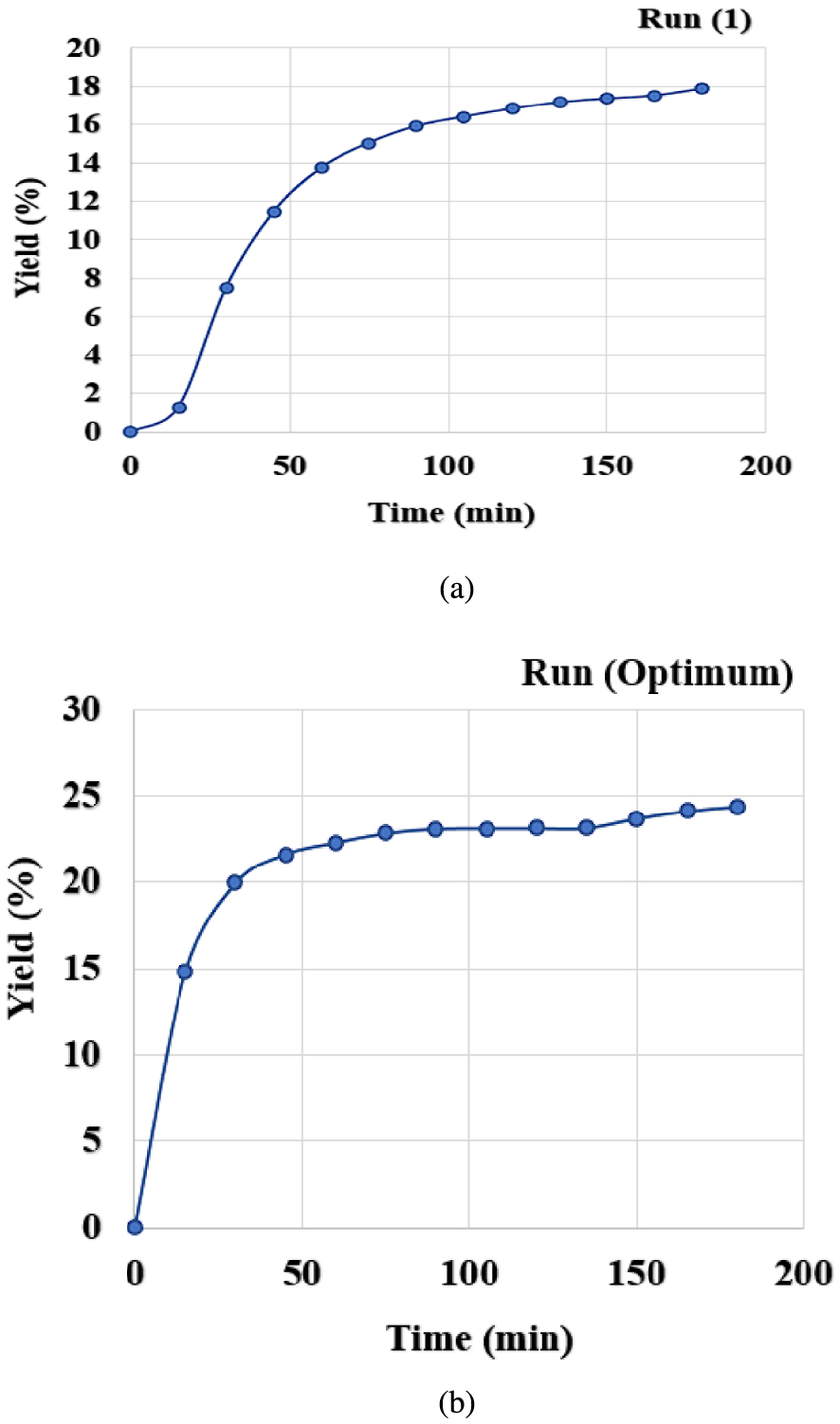
Extraction kinetics of onion seeds (a) at 35 °C, 150 bar, 0.55 mm, 75 g/min; (b) at optimum conditions 57 °C, 250 bar, 0.25 mm, 50 g/min.
The performance of any supercritical fluid extraction process depends upon the operating parameters mainly temperature, pressure, particle size and solvent flowrate. The present study is mainly concerned by two performance criteria: the oil extraction yield and the composition. Therefore it is important to investigate the effect of each parameter on these two criteria and determine operating ranges corresponding to high yield and quality of extracts via the composition.
3.2.1. The effect of pressure on the extraction yield
The effect of pressure on oil extraction yield was considered leading to the observation of various findings. First, at a low temperature of 35 °C, increasing the pressure from 150 to 250 bar, respectively, resulted in nearly constant yields of 17.876–18.047%, corresponding to experimental Runs 1 and 3, respectively. However, at a higher temperature of 65 °C, increasing pressure from 150 to 250 bar in experimental runs 2 and 4, respectively, resulted in a yield increase from 11.604% to 18.253%.
Second, in experimental Runs 13 and 14 at a temperature of 50 °C, a pressure increase from 150 to 250 bar, respectively led to a remarkable increase in oil extraction yield from 19.854% to 24.350% while maintaining a flow rate of 75 g/min and a pressure of 0.25 mm.
Clearly this result shows the great dependency of the solvent power of supercritical CO2 on its density which increases with pressure at constant temperature.
3.2.2. The effect of temperature on the extraction yield
At fixed parameters at [150 bar, 0.55 mm, 75 g/min] for experimental Runs 1 and 2, it was observed that an increase in temperature from 35 to 65 °C led to a decrease in the oil extraction yield from 17.876 to 11.604%, respectively. Similar results were obtained for experimental Runs 17 and 18 at fixed operating conditions of [200 bar, 0.25 mm, 75 g/min] and for experimental Runs 19 and 20 under the conditions of [200 bar, 0.85 mm, 75 g/min], indicating that an increase in temperature had negatively affected the extracted oil mass at pressures of 150 and 200 bar.
Contrary to the previous case, when the pressure increased to 250 bar (experimental Runs 3 and 4 at operating conditions of [250 bar, 0.55 mm, 75 g/min]), the increase of temperature from 35 to 65 °C had no adverse impact on the yield with just a slight yield increase of 0.206%. This indicates that temperature did not affect the oil extracting yield at a the fixed pressure of 250 bar along with other fixed parameters, similarly to the reported supercritical CO2 extraction of Syzygium aromaticum [17].
The impact of temperature on extraction effectiveness relies on the extraction pressure, with a negative effect seen at low pressure. This suggests that the density of CO2 takes precedence over the vapor pressure of the solute. Conversely, at higher pressure, this phenomenon is reversed. Thus, predicting the influence of temperature on the solubility of a substance in a supercritical fluid is more challenging than that of pressure. However, the relationship between temperature and extraction yield is not always simple, and an optimal temperature range may exist before yield drops at higher temperatures. This is due to possible degradation or denaturation of the targeted compound or induced changes to the solvent or solid matrix properties, which can reduce the extraction yield [18].
Finally from the obtained results it can be confirmed that: the solvent power of supercritical CO2 depends mainly on its density which increases with pressure at constant temperature conditions and decreases with temperature at constant pressure conditions. Similar behavior was also observed by Frohlich et al. for the supercritical CO2 extraction of Syzygium aromaticum [17].
3.2.3. Effect of particle size on the extraction yield
For experimental Runs 5 and 6 the considered conditions were [50 °C, 200 bar, 50 g/min] with two particle diameters of 0.25 and 0.85 mm. A decrease in the oil extraction yields from 24.32 to 21.20% for 0.25 and 0.85 mm diameter values, respectively, was observed. This is simply explained by the greater mass transfer area for the smaller diameter. This was also observed for other experimental Runs like, 7, 8, 13, 14, 15 and 16. Therefore the size of particles can be regarded as a key factor that can greatly influence the supercritical fluid extraction yield and the smaller are the particle sizes the higher is the mass transfer area, hence an increase of the diffusion rate and a decrease in the diffusion path, resulting in a better extraction [10].
3.2.4. The effect of flowrate on extraction yield
Fundamentally the flowrate can influence the mass transfer resistance of the solute into the supercritical CO2, hence the saturation of this latter. Therfore the oil extraction yield can be affected by the flowrate of CO2 passing through the solid matrix, where an increase in fluid flowrate can reduce resistance to mass transfer and the fluid may be saturated when leaving the solid matrix. This leads to solute solubility equilibrium, hence a maximum yield.
In terms of the seed morphology, raising the flowrate did not lead to an increase in yield, except for experimental Runs 9 and 10, carried out under identical conditions of [35 °C, 200 bar, 0.55 mm] but with different solvent flowrates. A yield increase from 22.017 to 23.161% was observed with an increase in flowrate, confirming the results reported in previous works [19, 20]. The change in CO2 flowrate induced noticeable changes in the viscosity and the color of the extracts. Therefore in terms of the overall interpretation of the experimental design and identification of the effects of each parameter, qualitative analyses were needed to confirm its impact on oil composition, as shown in Figure 4. In terms of the effect on the extraction process, Figure 5 demonstrates that the yield increased with a decrease in temperature from 65 to 35 °C, an increase in pressure from 150 to 250 bar, a decrease in diameter from 0.85 to 0.25 mm and an increase/decrease in flowrate from 75 to 100 g/min and 75 to 50 g/min, respectively. The lowest yield was observed at a flow rate of 75 g/min, while the highest ones were achieved at 50 and 100 g/min.
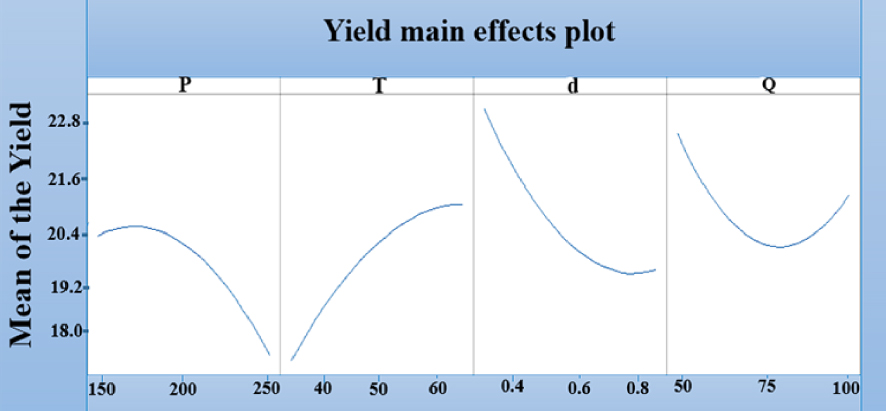
Yield main effects plot y (%).
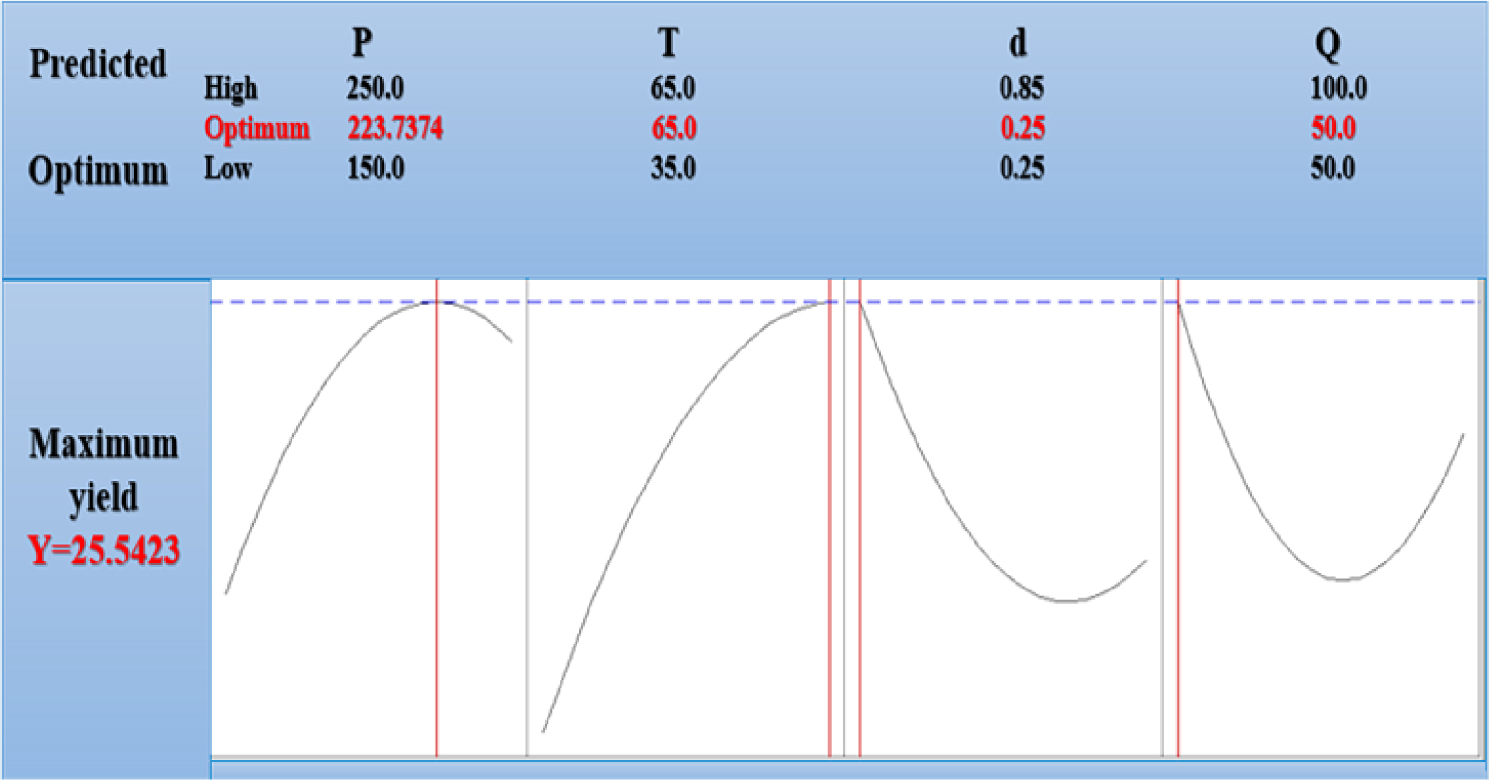
Optimum conditions for supercritical CO2 extraction of onion seeds oil.
Furthermore, the accuracy of this interpretation can be checked through the requirements derived for conducting the most efficient experiment, easing the retrieval of the highest possible yield.
3.3. Qualitative and quantitative analyses
Gas chromatography-mass spectrometry (GC-MS) is a powerful analytical technique that combines two separate methods, gas chromatography and mass spectrometry, to efficiently separate and identify qualitatively and quantitatively complex chemical compound mixtures [21].
This method has found widespread applications in the pharmaceutical industry for various purposes, including analytical research and development, quality control, quality insurance, production, bulk drugs, and formulations [22].
The separation of compounds according to their physical and chemical characteristics is demonstrated in the chromatogram, whereas the molecular weight and structure of the compounds are revealed through information provided by the mass spectrum [23, 24].
One of the main features of supercritical fluid extraction is that the composition of the extracts is greatly dependent upon the operating conditions like the pressure, temperature, particle size and flowrate for the present study, hence the number of the different resulting chromatograms shown in Figure 6 shows just a chromatogram example whereas all the other obtained ones are provided as Supplementary Material, to avoid a discontinuity in the main text. These chromatograms were used to find the respective compositions of each extract and obtain significant insights from the data, by carefully interpreting the set of information resulting from GCMS analysis.
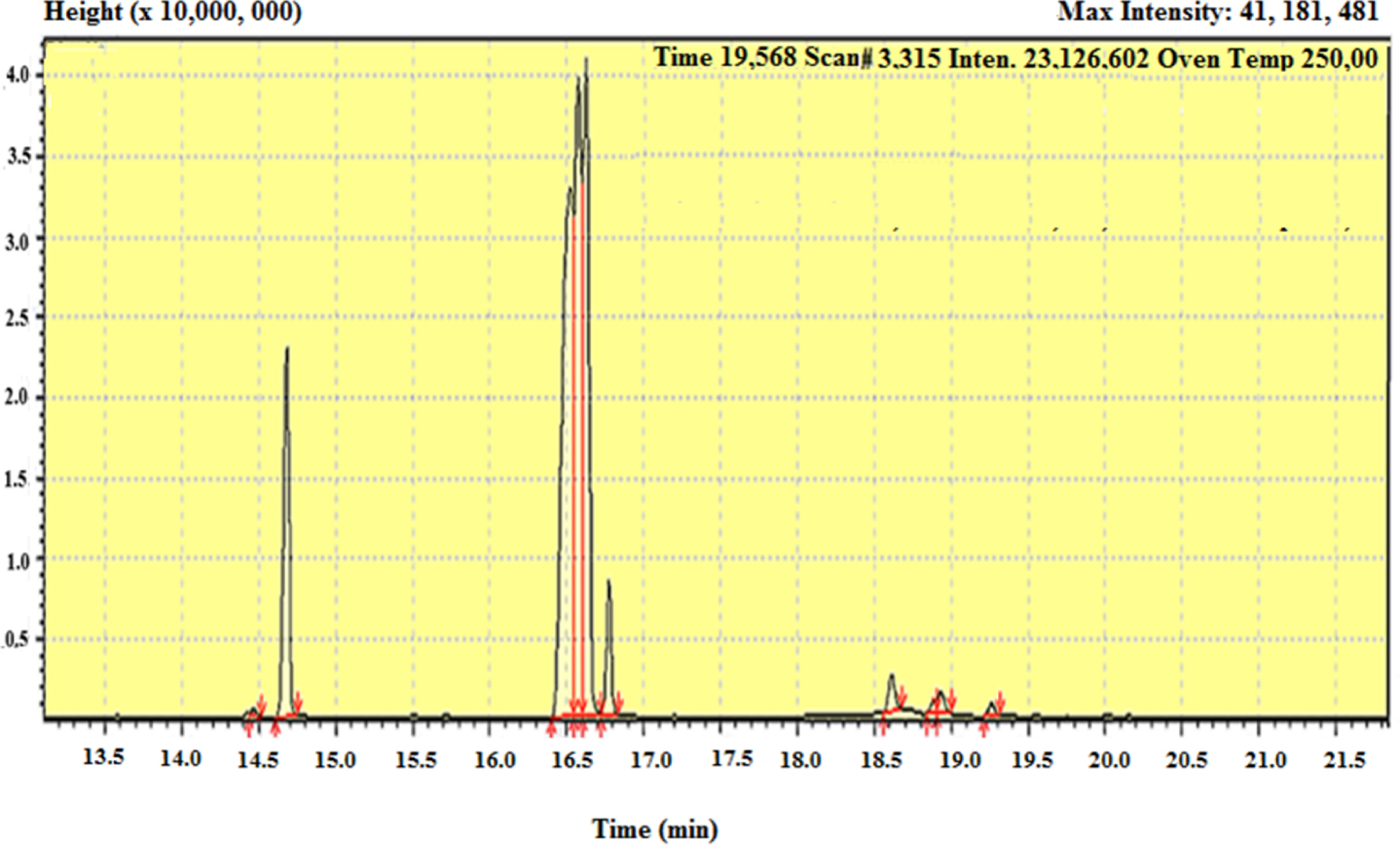
GC/MS chromatogram showing the sequence of peaks of fatty Acid methyl ester compounds from onion seed oils for Sample one (Run 13: 50 °C, 150 bar, 0.25 mm, 75 g/min).
Then the pattern of mass fragmentation of the detected elutants molecule ions can be observed in the spectrum of Figure 6. This could be achieved by comparison of the retention times which depict sequential peaks of separated analyte components and the mass spectra with those of known compounds appearing in a reference library or database [21]. Table 5 shows the analyses results concerning three distinct samples extracted at different operating conditions. In total ten major components were identified.
Fatty acid profile of onion seeds analyzed by GC-MS
| Component | Molecular formula | Mol weight | 1Sample 1 (peak area%) | 2Sample 2 (peak area%) | 3Sample 3 (peak area%) |
|---|---|---|---|---|---|
| 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | 294 | 24.78 | 37.98 | 21.68 |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | C19H34O2 | 294 | 33.68 | 21.86 | 27.76 |
| 9-Octadecadienoic acid (Z)-, methyl ester | C19H36O2 | 296 | 22.89 | 21.37 | 3.62 |
| Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 11.79 | 11.99 | 31.61 |
| Methyl stearate | C19H38O2 | 298 | 3.83 | 3.64 | 1.12 |
| (Z)-Methyl hexadec-11-enoate | C17H32O2 | 268 | 0.15 | 0.15 | / |
| Cyclopropaneoctanoic acid, 2-2-[(2-ethylcyclopropyl)methyl] cyclopropyl]methyl]-, methyl ester | C22H38O2 | 334 | 1.26 | / | / |
| 11,14-Eicosadienoic acid, methyl ester | C21H38O2 | 322 | 0.39 | / | / |
| 6-Octadecenoic acid, methyl ester,(Z)- | C19H36O2 | 296 | 0.77 | / | / |
| Eicosanoic acid, methyl ester | C21H42O2 | 326 | 0.46 | 0.40 | / |
| 13-Oxabicyclo[10.1.0]tridecane | C12H22O | 182 | / | 1.46 | / |
| 7-Tetradecenal,(Z)- | C14H26O | 210 | / | 0.80 | / |
| 8,11,14-Docosatrienoic acid, methyl ester | C23H40O2 | 348 | / | 0.35 | 1.21 |
| Methyl tetradecanoate | C15H30O2 | 242 | / | / | 0.13 |
| 8,9,9,10,10,11-Hexafluoro-4,4-dimethyl-3,5-dimethyl-3,5-dioxatetracyclo[5.4.1.0(2,6).0(8,11)]dodecane | C12H12F6 O2 | 302 | / | / | 0.20 |
| 4-(3-Oxocyclohexyl)butyric acid, methyl ester | C11H18O3 | 198 | / | / | 12.22 |
| 9-Tetradecenal,(Z)- | C14H26O | 210 | / | / | 0.45 |
| Total | / | / | 100% | 100% | 100% |
| Component | Molecular formula | Mol weight | 4Sample 4 (Peak area%) | 5Sample 5 (Peak area%) | 6Sample 6 (Peak area%) |
| 9-Octadecadienoic acid (Z)-, methyl ester | C19H36O2 | 296 | 30.85 | 32.19 | 29.17 |
| 9,12-octadecadienoic acid, methyl ester | C19H34O2 | 294 | 0.72 | 53.91 | 52.6 |
| hexadecanoic acid, methyl ester | C17H34O2 | 270 | 12.45 | 9.23 | 8.88 |
| Methyl stearate | C19H38O2 | 298 | 3.51 | 3.23 | 3.34 |
| 9,12-octadecadienoic acid (Z,Z)-, methyl ester | C19H34O2 | 294 | 51.01 | / | / |
| 11,14-Eicosadienoic acid, methyl ester | C21H38O2 | 322 | 0.61 | / | / |
| 6-octadecenoic acid, methyl ester, (Z)- | C19H36O2 | 296 | 0.44 | / | / |
| Myristate <methyl- >; Tetradecanoic acid <methyl- >ester | C15H30O2 | 242 | 0.27 | / | / |
| 8,9,9,10,10,11-Hexafluoro-4,4-dimethyl-3,5-dimethyl-3,5-dioxatetracyclo[5.4.1.0(2,6).0(8,11)]dodecane | C12H12F6O2 | 302 | 0.15 | / | / |
| 9-Octadecenal | C18H34O | 266 | / | 0.65 | / |
| Tridecanoic acid, methyl ester | C14H28O2 | 228 | / | 0.25 | / |
| 9-Tetradecenal, (Z)- | C14H26O | 210 | / | 0.54 | 0.6 |
| Eicosane | C20H42 | 282 | / | / | 1.31 |
| Docosane,11-decyl- | C32H66 | 450 | / | / | 1.92 |
| Tetrapentacontane,1,54-dibromo- | C54H108Br2 | 914 | / | / | 0.57 |
| (Z)-Methyl hexadec-11-enoate | C17H32O2 | 268 | / | / | / |
| Cyclopropaneoctanoicacid, 2-[[2-[(2-ethylcyclopropyl)methyl] cyclopropyl]methyl]-, methyl ester | C22H38O2 | 334 | / | / | / |
| Eicosanoic acid, methyl ester | C21H42O2 | 326 | / | / | / |
| 13-Oxabicyclo[10.1.0]tridecane | C12H22O | 182 | / | / | / |
| 7-Tetradecenal,(Z)- | C14H26O | 210 | / | / | / |
| 8,11,14-Docosatrienoic acid, methyl ester | C23H40O2 | 348 | / | / | / |
| Methyl tetradecanoate | C15H30O2 | 242 | / | / | / |
| 4-(3-Oxocyclohexyl)butyric acid, methyl ester | C11H18O3 | 198 | / | / | / |
| Total | / | / | 100% | 100% | 100% |
1Sample one (Run 13: 50 °C, 150 bar, 0.25 mm, and 75 g/min); 2Sample two (Run 14: 50 °C, 250 bar, 0.25 mm, and 75 g/min); 3Sample three (Run optimum: 57 °C, 250 bar, 0.25 mm, and 50 g/min); 4Sample four (Run 1: 35 °C, 150 bar, 0.55 mm, and 75 g/min); 5Sample five (Run 2: 65 °C,150 bar, 0.55 mm, and 75 g/min); 6Sample six (Run 17: 35 °C, 200 bar, 0.25 mm, and 75 g/min).
Based on the GCMS analysis results, it has been discovered that among the 10 components obtained, the following four primary components were present in significant percentages in all extracts obtained under varying conditions:
- 9,12-octadecadienoic acid, methyl ester.
- 9,12-octadecadienoic acid (Z, Z)-, methyl ester.
- 9-octadecadienoic acid (Z)-, methyl ester.
- Hexadecanoic acid, methyl ester (Palmitic acid Methyl ester).
By changing the pressure applied during the extraction process, both chemical and physical characteristics of the oil could be modified. For instance, when the pressure was raised from 150 to 250 bar, the primary compounds remained present at both pressures, but at different percentages. For example, 9, 12-Octadecadienoic acid, methyl ester was present at 24.78% at 150 bar, but increased to 37.98% at 250 bar. This was also valid for the compositions of the secondary compounds.
By increasing the flow rate from 50 to 75 g/min, the quality of the extract was impacted. This was evident from the increase in the mass percentage of compound 9-octadecadienoic acid (Z)-, methyl ester from 3.62 to 21.37%. Therefore CO2 flow rate had a more significant impact on the quality of the extract than the on the quantity.
Fatty acids are critical molecules of essential components for the human body. They not only do serve as a primary source of energy, but they also have crucial roles in the building and operation of cell membranes, and in the production of hormones and other signal molecules. Saturated fats are typically solid at room temperature and commonly found in animal-based products whereas unsaturated fatty acids are usually as liquid at room temperature and are present in plant-based foods like nuts, seeds, and vegetable oils. They are widely acknowledged to be more beneficial for health than saturated fats, as studies have linked them with a reduced risk of heart disease and other chronic conditions [25]. According to the result obtained in the present study, it is important to note that the onion seed extracts contain a remarkably high proportion of these unsaturated fatty acids. A literature search did not show any study dealing particularly with the quality of FFA obtained by different extraction methods, for comparison purposes. However the results reported in the literature concerning the extraction of walnut oil by cold pressing, Soxhlet extraction, and ultrasonic extraction, confirmed the effect of the selected extraction technique on the quality of the extracted oil [26]. Knowing that 9, 12-Octadecadienoic acid (Z, Z), also known as Omega 6, is considered to be the most significant among the various fatty acids due to its numerous biological functions which induce its impact on the inflammatory cascade, its ability to reduce oxidative stress, as well as its role in providing neuro and cardio-vascular protections [27].
4. Conclusion
The present study had clearly shown that many natural plants, vegetables, fruits, etc., may be great sources of valuable substances with important applications in sensitive fields like pharmaceutical, cosmetic and food industries. However in order to obtain the valuable substances from these natural sources, performing fluid based extraction techniques are required. One of these non conventional techniques is the supercritical CO2 extraction which was used in the present work to extract oil from onion seed as a model system.
The results did put into evidence one of the important features of the supercritical CO2 extraction consisting of manipulating the operating parameters in order to achieve extracts with a high purity and a targeted composition, confirming the merit of fluids at supercritical state. The “green” aspect of the supercritical CO2 extraction had also been confirmed, excluding the use of organic solvents, preserving the environment by a valorization of process residues, avoiding the release of CO2 into the atmosphere, etc. All these factors can be exploited to promote sustainability.
Finally through this study it can be seen that sustainability may be induced by means of non conventional techniques like the supercritical fluid extraction which is characterized by the possibility to manipulate the composition of the extracts according to preset goals and also to valorize the extraction residues as biosorbents, biofertilizers, etc. promoting a circular economy.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.





 CC-BY 4.0
CC-BY 4.0