1. Introduction
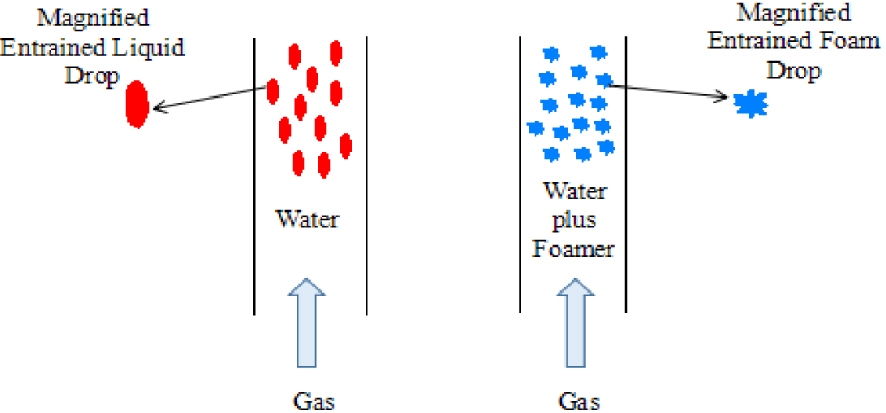
The working principle of a foam drainage agent.
Natural gas is an important energy source in China to deal with growing demand [1, 2]. However, as many gas fields gradually enter the middle and late stages of development, the reservoir energy tends to become insufficient. The gas is unable to bring the produced water from the wellbore to the ground, and the water is gradually accumulated in the wellbore, which severely affects the production of the gas well [3, 4, 5, 6]. The application of foam drainage and gas production technology can solve this problem. Foam drainage consists in injecting surfactants into the wellbore, mixing surfactants and wellbore fluid through the stirring of airflow to produce a large volume of low-density water-bearing foam which is more easily entrained, thus reducing the wellbore fluid accumulation (Figure 1) [7, 8]. Excellent surfactants have higher foaming ability, longer precipitation half-life and foam half-life. In addition, they have higher foaming ability and longer foam half-life under harsh conditions such as high oil saturation, high temperature, high salt content, and so on [9, 10, 11]. There are many kinds of foaming agents used in foam drainage gas production technology, and their foam performance, liquid-carrying capacity, and foam stability are different [12, 13]. Therefore, to make better use of the foam effect of surfactants, it is necessary to fully understand the characteristics and mechanism of foam properties. At present, there are two main directions in the research and development of surfactants. One aims to study the relationship between the structure and properties of surfactants and then synthesize new surfactants. The other aims to obtain new products with superior properties through the combination of commercial surfactants to expand their application range [14, 15]. Betaine surfactants are used widely in oil field chemistry as well as other industrial applications, but their foaming ability is very poor so that it cannot be used in foaming. In this paper, the effect of octadecyl ammonium oxide on the foam properties of oleate amide propyl betaine is studied based on foam performance. Then, the optimum proportion of the compound is determined. Moreover, the foam properties of the compound surfactant are evaluated in detail.
2. Experimental section
2.1. Materials
Oleate amide propyl betaine (40%) was purchased from Huainan Huajun new materials Technology Co., Ltd. Octadecyl ammonium oxide (20%) was purchased from Yongrun Chemical Co., Ltd. Sal communis, anhydrous calcium chloride, and muriate of potash were purchased from Tianjin Shengao Chemical Reagent Co., Ltd. Methanol was purchased from Tianjin Fuyu Fine Chemical Co., Ltd. Crude oil was obtained from Yanchang Oilfield, China.
2.2. Foam properties
There are many methods for generating foam such as the sparge tube technique or gas flow and whipping [16]. In the present study, the high-speed stirring method is used to evaluate the foam properties of surfactant solutions. The experimental temperature is room temperature, the volume of the solution is 100 mL, the stirring rate is 7000 r/min, and the stirring time is 3 min. When the stirring stops, the foam is poured into a 500 mL measuring tube and a stopwatch is turned on to record the foam volume and foam half-life time. Each experiment is repeated at least thrice. Foam stability is determined by the foam half-life time, which is markedly affected by the intensity of the liquid membrane and the Brownian motion and viscosity [17]. To simulate the condition of foam drainage, in this study, a Roche foam tester is used to measure the temperature resistance of surfactants. A 300 mL solution is prepared, and the solution is injected according to the operation steps. The foam height is read immediately; it is read every 5 min thereafter, and the data are recorded.
2.3. Surface tension measurement
All surface tension measurements were carried out using the platinum sheet method with a surface tension meter, which was purchased from Shanghai Fangrui Instrument Co., Ltd. Distilled water was used to prepare 0.0001%, 0.0005%, 0.001%, 0.005%, 0.01%, 0.05%, 0.1%, 0.2%, and 0.3% (mass fraction) oleate amide propyl betaine solution. In addition, 0.5% oleate amide propyl betaine and 0.001%, 0.005%, 0.01%, 0.05%, 0.1%, 0.2%, 0.3%, 0.4%, and 0.5% octadecyl ammonium oxide mixed solutions were prepared. The surface tension of each surfactant solution was determined, and the critical micelle concentration (cmc) was estimated. Each test run was repeated thrice to check repeatability. The average data were recorded.
2.4. Microstructure of foam
100 mL of surfactant solutions were prepared and blended at 7000 r/min for 3 min. Foam was first generated by using a high-speed agitator. Then, the microstructure of foam was measured by an optical microscope, whose light source was polarized light (DM4500P LFD, Germany) [18].
3. Results and discussion
3.1. Foam properties
For foam, the foaming ability of the foaming agent and the stability of foaming are two important static performance indexes. Among them, the stability of foam is the most important factor for evaluating foam performance [19]. As can be seen from Figure 2(a), the initial foam volume increases first and then decreases with increase in concentration. The initial foam volume is the largest at a concentration of 0.5%, which indicates that 0.5% is the optimum foaming concentration. As can be seen from Figure 2(b), the half-life time of foam increases, but the gradient decreases, indicating that the stability of foam increases with increase in concentration. This is because with increase in foaming agent concentration, more surfactant molecules are adsorbed on the gas–liquid interface, which reduces the tension of the gas–liquid interface; hence, the foam volume increases [20, 21]. At the same time, a dense protective film is formed on the interface, and the elasticity of the film is enhanced. This makes it difficult for the gas to diffuse outward through the liquid film, and the foam half-life time increases. When the amount of foaming agent is more than 0.5%, the adsorption of surfactant molecules on the gas–liquid interface reaches saturation, the foam volume no longer increases, and the excess surfactant molecules exist in the solution in the form of micelles, which deform the foam by external force. Moreover, the Marangoni effect is weakened, the foam bursts more easily, the half-life time becomes shorter, and the comprehensive foaming ability is reduced [22]. Considering the initial foam volume and the half-life time, the optimum foaming concentration of oleate amide propyl betaine surfactants is chosen as 0.5% in the following work. At this concentration, the initial foam volume is the largest, and the difference between half-lives at higher concentrations is not significant.
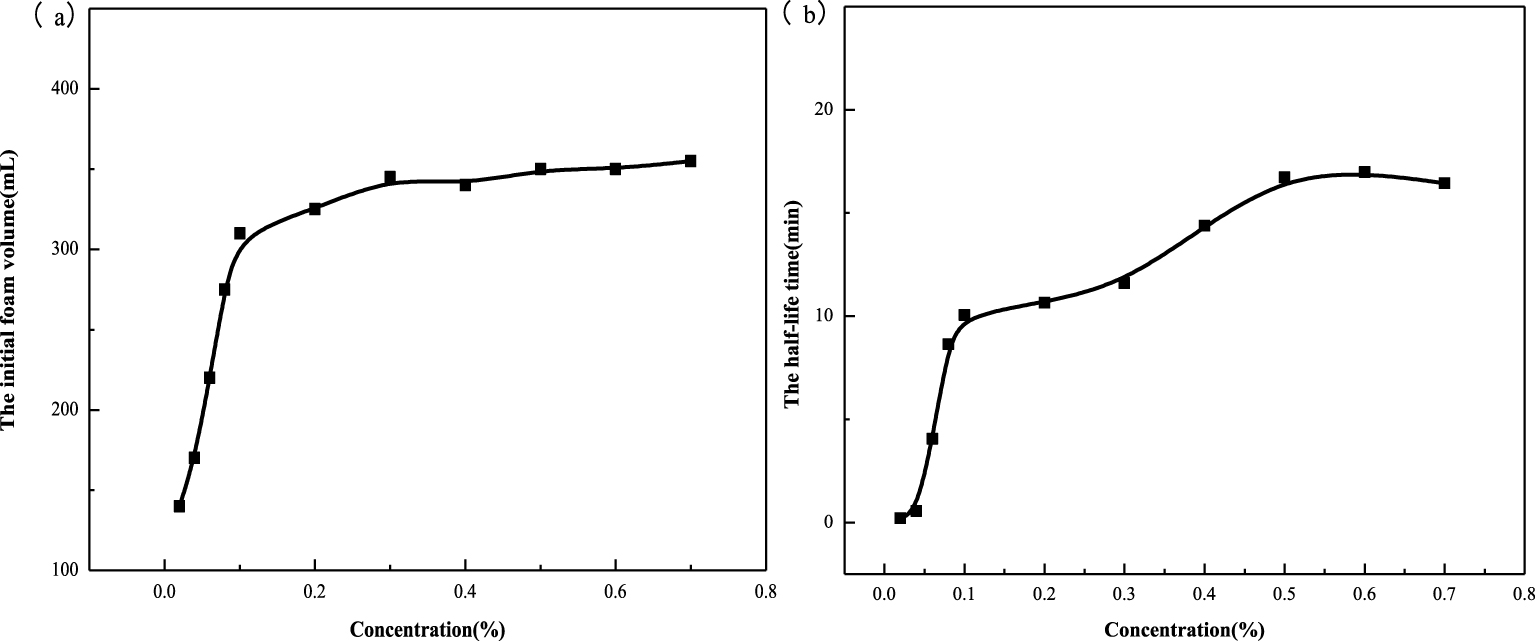
Foaming ability (a) and foam half-life time (b) of oleate amide propyl betaine.
The properties of the compound foaming reagent are studied by adding different concentrations of octadecyl ammonium oxide to oleate amide propyl betaine at a concentration of 0.5%. It can be seen from Figure 3(a) that the initial foam volume increases first and then decreases with increase in the concentration of octadecyl ammonium oxide. The maximum initial foam volume is between 0.1% and 0.2%. As can be seen from Figure 3(b), the half-life time also increases first and then decreases. The half-life time is the longest when the concentration is 0.1%, which indicates that the foam stability at this concentration is the best. Therefore, the formula of 0.5% oleate amide propyl betaine and 0.1% octadecyl ammonium oxide was selected for the follow-up experiment. The key factor in determining the stability of the foam is the strength of the liquid film. Furthermore, the strength of the liquid film depends mainly on the firmness of the surface adsorption film. The higher the surface viscosity, the longer the foam life [23, 24].
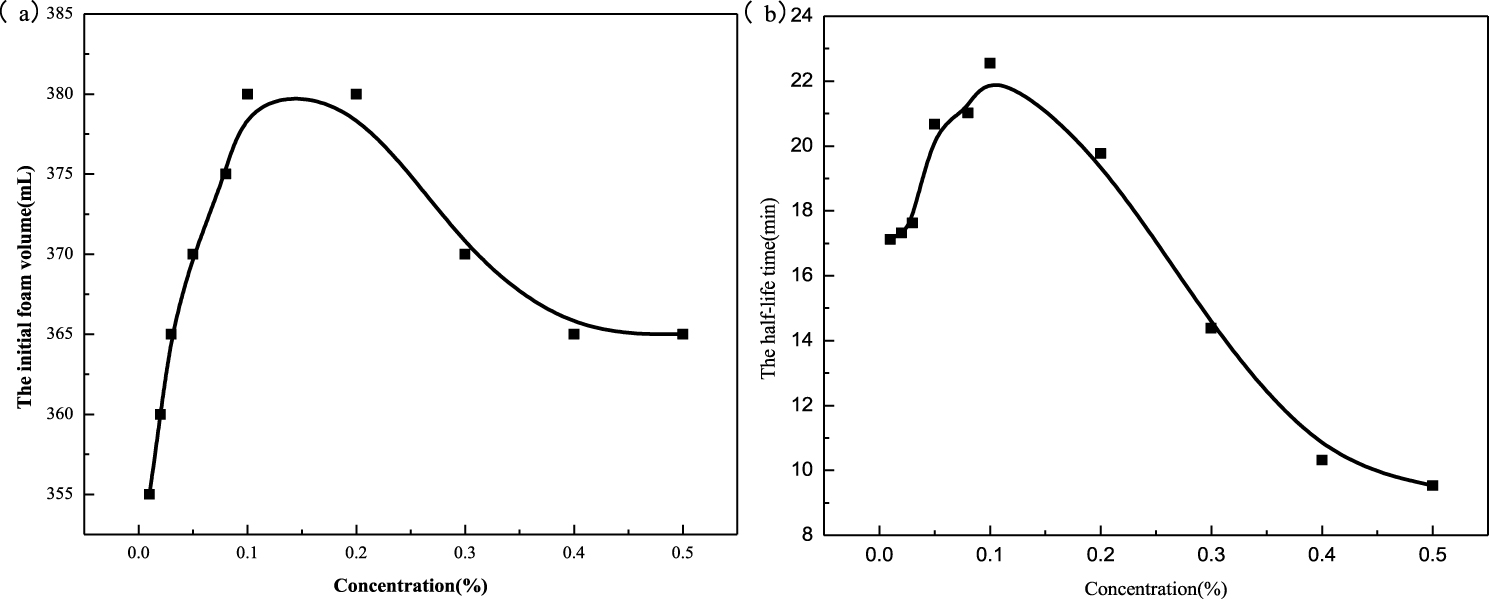
Foaming ability (a) and foam half-life time (b) of compound solution.
3.2. Effect of salinity on foaming ability
A compound surfactant produces a synergistic effect, and the foam performance is often better than that exhibited by a single surfactant. Therefore, a compound surfactant can improve the foam performance and reduce the cost [25]. For high-salt reservoirs, the presence of a large amount of Ca2+ and Na+ in formation water severely restricts the application of foam and requires a more stable foaming reagent. Therefore, it is necessary to study the salt tolerance before the foaming reagent is injected into the gas well [26]. As can be seen from Figure 4 (left), the initial foam volume increases first and then decreases with increase in salt concentration. It turns out that the surfactant solution has high resistance to these salts within the concentration of 10%. Excessive metallic cations weaken the electrostatic repulsion of surfactant molecules and weaken the thinning ability and stability of foam resistant liquid films [27]. It can be seen from Figure 4 (right) that the half-life time of the foam increases gradually with increase in the concentration of NaCl and CaCl2. This shows that the stability of the foam gradually increases, which may be due to the increase in viscosity of the solution after the addition of salt. With increase in the concentration of KCl, the half-life of the foam rises at the beginning and declines later, which may be due to the ionization of a large amount of salt in the solution. This alters the relative amount of surface charge on the foam and weakens the Marangoni effect [28]. Moreover, there is a double-layer structure in the liquid film of foam. The double-layer structure is compressed in the presence of an electrolyte, which forces the foam to discharge liquid faster. Thus, the foam becomes thinner, and the burst is accelerated [29].

Foaming ability (left) and foam half-life time (right) of compound solution at different salinities (room temperature, mNaCl:mCaCl2 = 4:1).
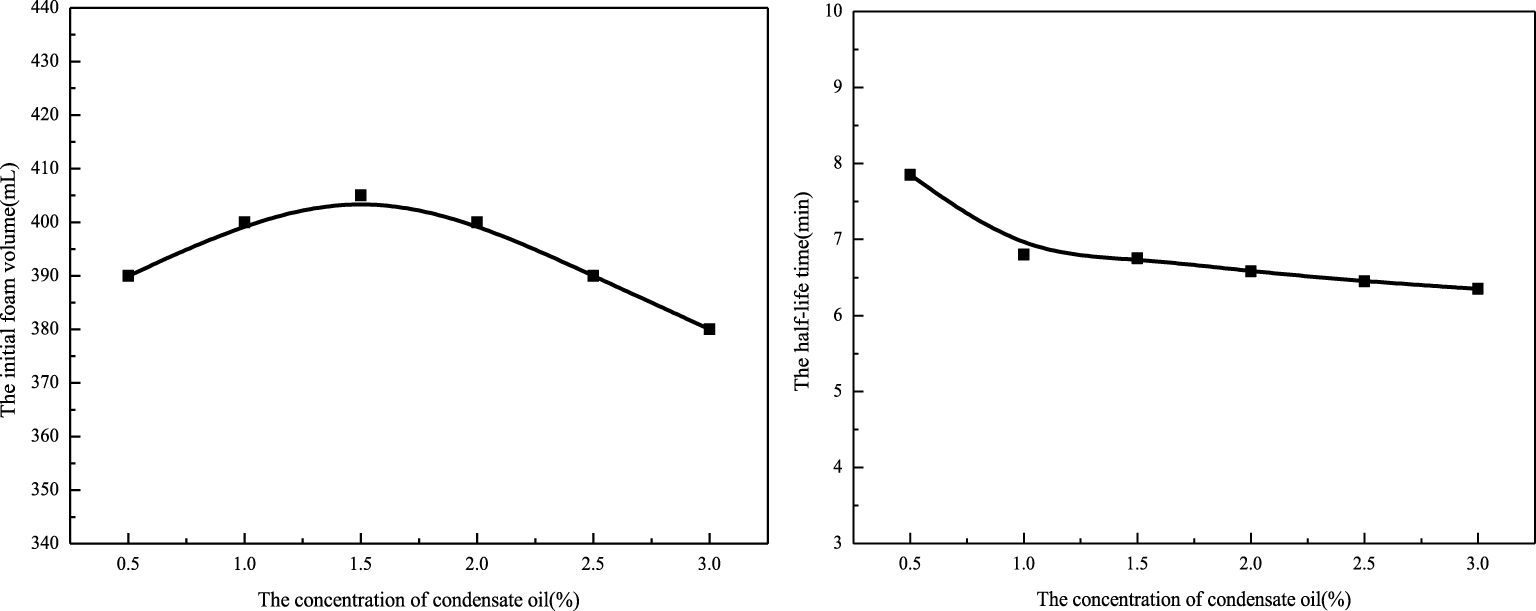
Foaming ability (left) and foam half-life time (right) at different concentrations of condensate oil.
3.3. Effect of condensate oil on foaming ability
Generally, oil is a defoaming agent; so the foam used in drainage gas production should have high condensate oil tolerance. The condensate oil may affect the stability of foam by the process of thinning and merging [30]. As can be seen from Figure 5 (left), with increase in condensate oil concentration, the initial foam volume increases at first and then decreases, indicating that the surfactant solution has a certain resistance to condensate oil. However, there is a certain limit to this resistance. In the range of 1.5%, with increase in condensate oil concentration, the foaming ability of foam increases gradually, but when the concentration is higher than 1.5%, the foaming ability of foam becomes worse. Nevertheless, the initial foam volume is always maintained at a higher level compared with the initial foam volume of the surfactant solution. The results show that condensate oil can improve the foaming ability of the surfactant solution. As can be seen from Figure 5 (right), as the condensate oil concentration increases, the half-life time of the foam is gradually reduced, which shows that the condensate oil can reduce the foam stability. In general, however, the half-life of the foam with different concentrations of condensate oil does not change much, which indicates that the surfactant solution is a good anti-condensate. Condensate oil is a kind of hydrocarbon mixture, which has a specific effect on the water solubility of a surfactant. Because the solubility of aromatics is greater than that of cycloalkanes and methylalkanes, as the content of aromatics in condensate oil is very low, its effect is equivalent to the plasticizing effect. It can strengthen the foam film and reduce the speed at which it is destroyed [31].
To simulate the condition of foam drainage, the foam heights of surfactants produced by adding different concentrations of condensate oil at 50 °C are measured by a Roche foam instrument. The experimental results are shown in Figure 6. As can be seen, the height of the foam produced by the surfactant solution increases with time after the addition of condensate oil. Moreover, with increase in the concentration of condensate oil, the height of the foam increases, and the growth rate and the speed are also obviously increased. However, this does not conflict with previous conclusions. On the one hand, condensate oil can reduce the foam stability and accelerate the decay rate of the foam. On the other hand, the boiling point of condensate oil is low, and at 50 °C, a part of the condensate oil can be volatilized into a gas. Furthermore, new bubbles are formed in the surfactant solution, resulting in an increase in the number of bubbles. Owing to the simultaneous effect of the above two factors, the formation rate of the foam becomes greater than the bursting rate, hence the obvious increase in foam height. At the same time, it is confirmed that the surfactant solution has good anti-condensate properties.
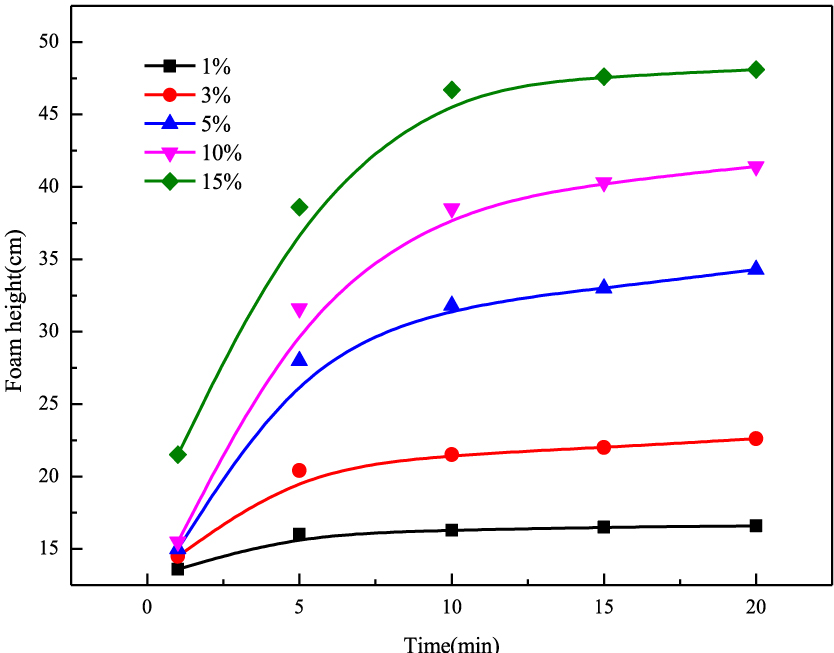
Foaming ability at different concentrations of condensate oil (using Roche foam instrument).
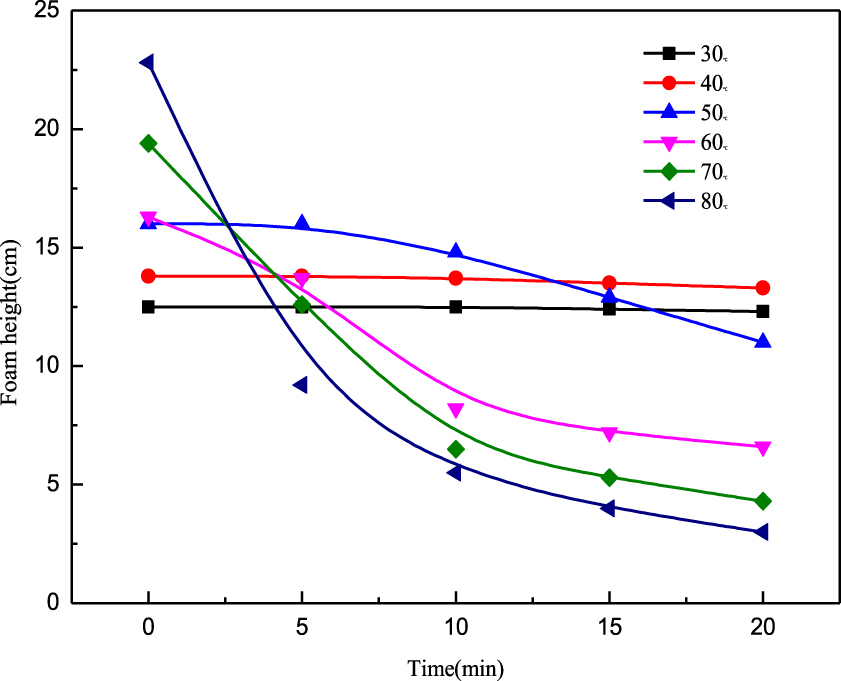
Foaming ability at different temperatures (using Roche foam meter).
The foam height (cm) of surfactant solution at different concentrations of methanol
| Concentration (%) | 0 min | 5 min | 10 min | 15 min | 20 min |
|---|---|---|---|---|---|
| 0.1 | 15.6 | 14.6 | 12.5 | 10.1 | 8.8 |
| 0.5 | 14.1 | 13.8 | 12.0 | 11.0 | 9.6 |
| 1.0 | 13.2 | 12.0 | 11.0 | 10.5 | 10.0 |
| 10.0 | 10.8 | 9.8 | 8.7 | 6.5 | 5.7 |
| 20.0 | 8.7 | 8.2 | 7.5 | 6.3 | 5.2 |
3.4. Effect of temperature on foaming ability
The sensitivity of foam to temperature varies greatly [32]. For example, Brownian motion, cmc, viscosity, and so on are greatly influenced by temperature [33]. In addition, the viscosity of the liquid membrane initially increases and then drops with increasing liquid phase temperature [34]. Thus, the foaming temperature is a significant factor influencing foam properties. As can be seen from Figure 7, the initial foam height increases with increase in temperature. The overall increase in foam height with rise in temperature is assumed to result from Brownian motion, where the kinematic velocity of the molecule increases with increasing temperature and causes more frequent collisions between molecules. Thus, the gradual increase in foam quantity is unsurprising [35]. However, at the same time, the velocity of foam height attenuation also increases due to the effect of temperature on the decay of the foam. At low temperatures, the effect is mainly gas diffusion. At high temperatures, the foam bursts from the top, and the foam volume increases and decreases regularly with time. With increase in temperature, the adsorption capacity decreases, the exclusive area of molecules increases, the surface viscosity decreases, the Marangoni effect weakens, and the surface elasticity decreases, which lead to a reduction in foam stability. Moreover, with increase in temperature, the surface tension of the foam system decreases, which is beneficial to the improvement in foam stability. However, the surface tension is not the dominant factor affecting the stability of foam [36, 37, 38]. In addition, with increase in temperature, the viscosity of the solution decreases, the discharge rate is accelerated, and the foam stability decreases.
3.5. Effect of methanol on foaming ability
When the temperature is low in winter, the generation of natural gas hydrate plugs the wellbore, and the lower tool is damaged. This can even cause dangerous accidents. The addition of an anti-hydrate-formation inhibitor is one of the most widely used anti-hydrate-formation measures. Methanol is one of the main thermodynamic inhibitors used in Chinese gas fields [39, 40]. However, after adding methanol to low-temperature gas wells in winter, the foam performance of some surfactants decreases sharply, which indicates that methanol affects the properties of surfactants. Therefore, it is essential to study the effect of methanol on surfactants. As can be seen from Table 1, the initial foam height decreases with increase in methanol concentration. However, the effect of methanol concentration on the foam height of the surfactant solution is not obvious due to two opposing effects [41, 42]. On the one hand, methanol increases the stability of foam by reducing the surface tension of the surfactant solution. On the other hand, methanol is used as a defoaming agent; it can easily unfold on the foam surface. In this way, the original foam surface agent molecule is replaced by a new liquid membrane, which unbalances the force between molecules. As a result, the molecules are loosely packed on the surface, and the unstable liquid film that is formed ruptures in a short period of time. These two interactions affect each other.
3.6. Microstructure of foams
To study the foam microstructure well, the surface tension of two solutions at different concentrations are first determined. Figure 8(a) shows that with increase in concentration, the surface tension of each surfactant solution decreases rapidly and then slowly. After the inflection point, the change in surface tension decreases and finally becomes flat. It seems that the surfactants begin to automatically assemble and arrange themselves on the surface, reducing the contact area between water and air. Thus, the surface tension of the solution decreases dramatically. As the surface of the solution becomes saturated, the surface tension does not reduce with increase in concentration. The excess surfactant molecules form micelles in the solutions [43, 44, 45]. The cmc values of the two solutions are obtained by mathematically fitting Figure 7. In general, the cmc value depends on how tightly surfactants are aligned for saturation on the surface of a solution [46, 47]. The cmc of the compound solution is 0.12%. It was previously shown that the foam performance of the compound solution with 0.5% oleate amide propyl betaine is best when the concentration of octadecyl ammonium oxide is 0.1%. The latter concentration is close to the cmc, which means that it is likely that the surface tension of the solution at this point is minimal, resulting in good foam stability.
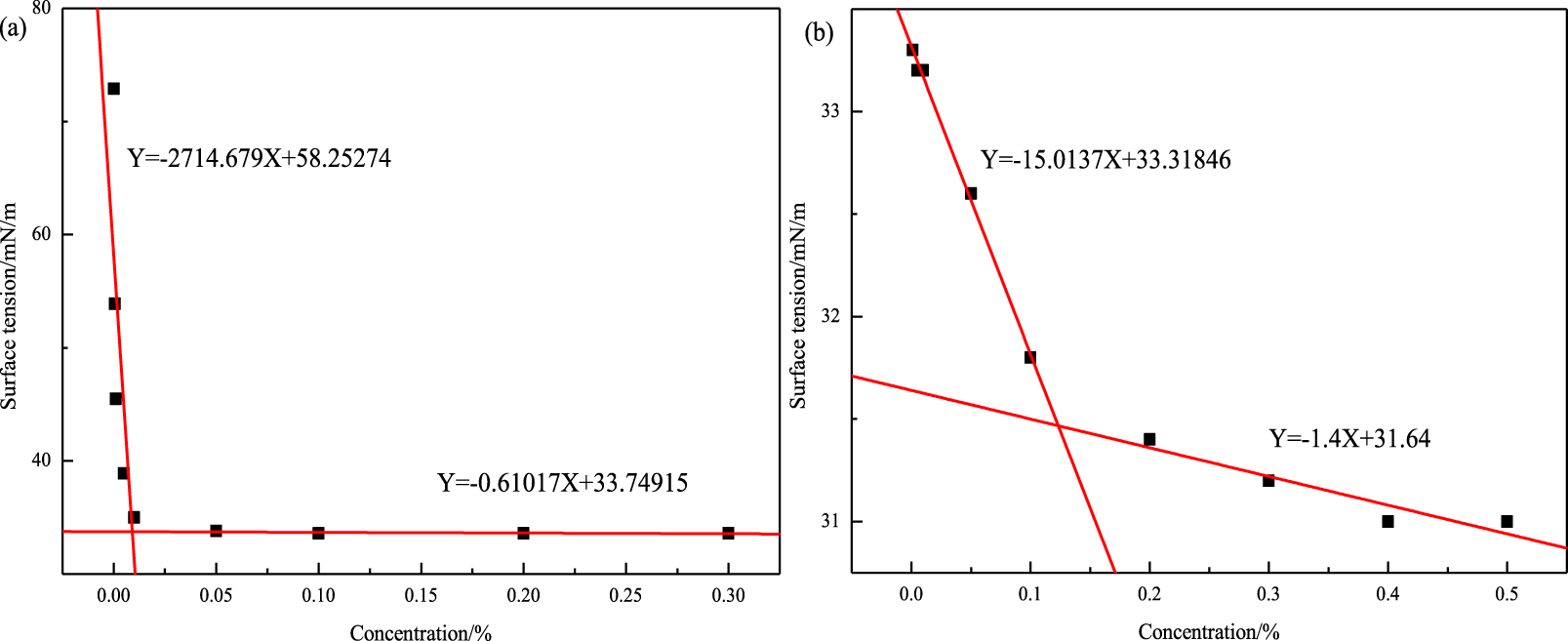
Surface tension at different concentrations of different solutions: oleate amide propyl betaine solution (a) and compound solution (b).
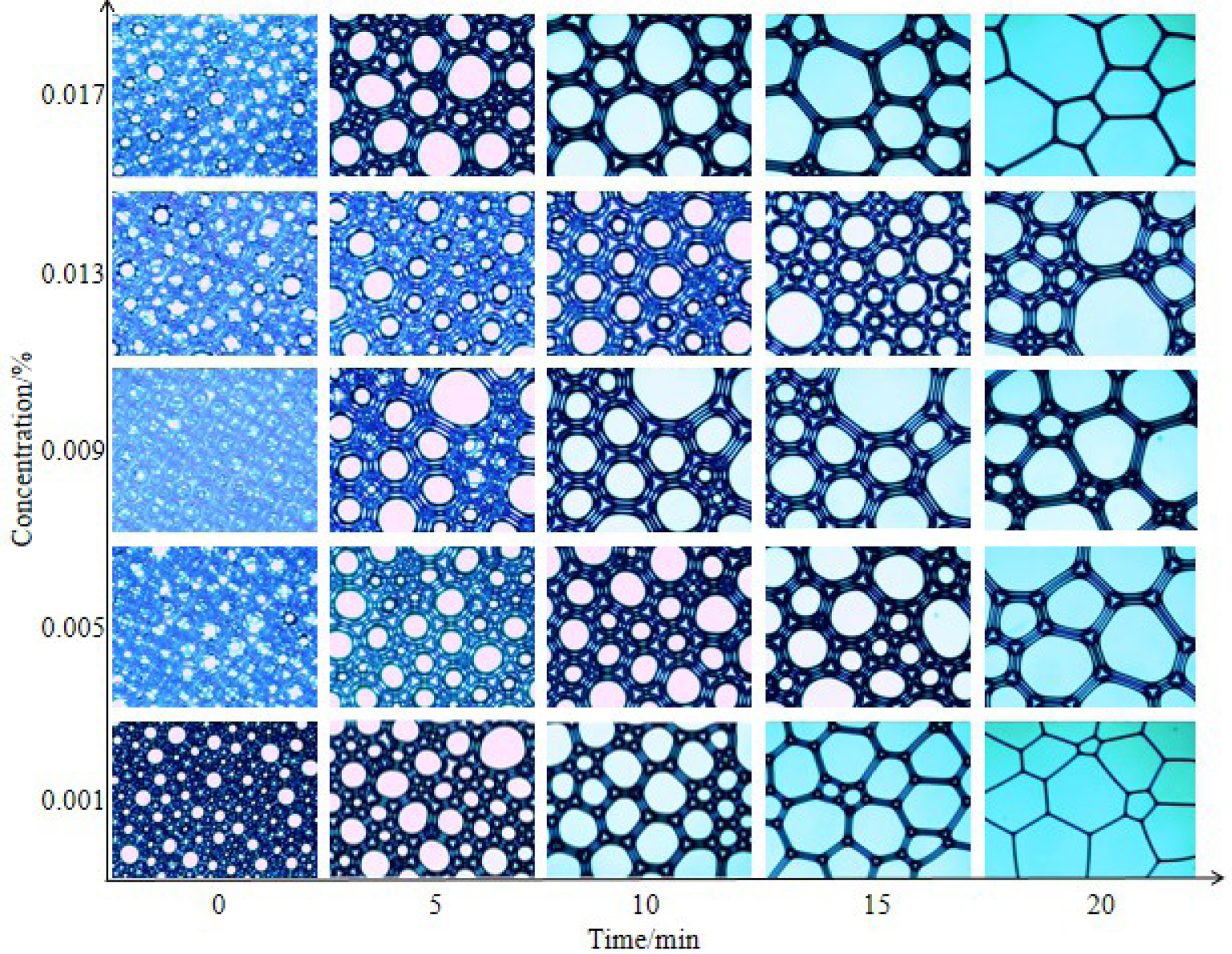
Changes in foam microstructure of oleate amide propyl betaine with time.
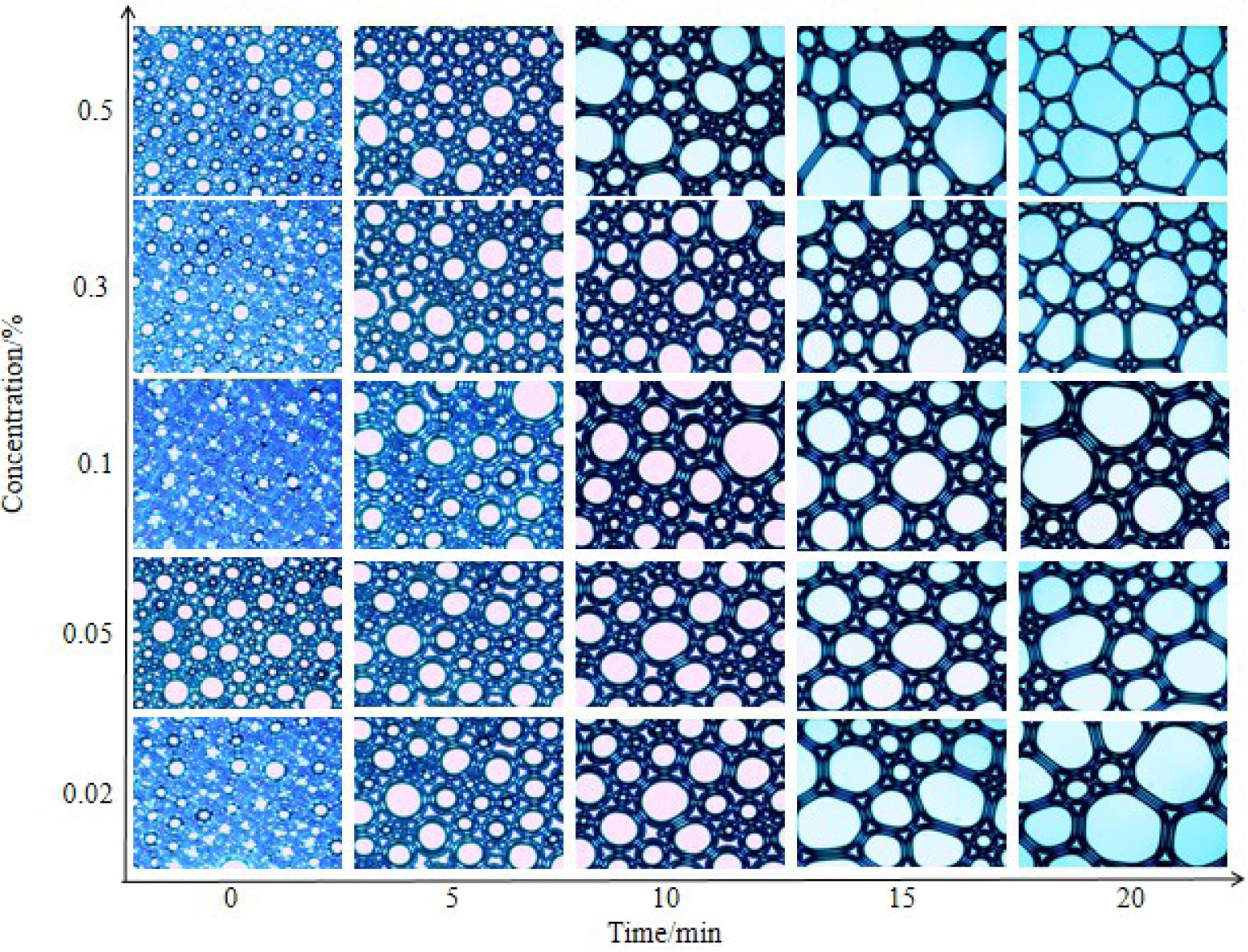
Changes in foam microstructure of compound solution with time.
The changing micromorphology of the foam with time is shown in Figures 9 and 10. Figure 9 shows that the foam walls of the oleate amide propyl betaine solution with a concentration of 0.009% are the thickest. Within 5 min, the foams produced by the solution become very small and uniform, and it is not easy for them to combine. After 10 min, the foam wall obviously becomes thinner. Generally speaking, the thicker the foam wall, the better the mechanical strength [48, 49, 50]. Therefore, the solution of 0.009% oleate amide propyl betaine has the best stability. According to the surface tension test done earlier, it can be seen that 0.009% is the cmc of oleate amide propyl betaine. Therefore, its stability is probably caused by low surface tension, which also proves that lower surface interfacial tension is beneficial to foam [51, 52].
It can be seen from Figure 10 that the foams produced by the compound solution are all spherical in shape at 0 min. When the foam has just formed, the foam has a large moisture content, which is wet foam. The shape of the foam produced by a surfactant solution with a ratio of 0.5% oleate amide propyl betaine/0.02% octadecyl ammonium oxide at 15 min has apparently become polygonal. The foams with the other four octadecyl ammonium oxide concentrations are still spherical or elliptical. Moreover, the surfactant solution with an octadecyl ammonium oxide concentration of 0.1% produces the thickest foam wall. Therefore, when the concentrations of octadecyl ammonium oxide and oleate amide propyl betaine are 0.1% and 0.5%, respectively, the foam stability is the best.
4. Conclusion
In this paper, we study the effect of octadecyl ammonium oxide on the foam properties of oleate amide propyl betaine. We develop a foaming reagent with superior properties through the combination of commercial surfactants to expand their application range. The new compound foaming reagent is developed based on foam performance. The reagent is composed of 0.5 wt% oleate amide propyl betaine and 0.1 wt% octadecyl ammonium oxide. Its salt resistance, methanol resistance, high temperature resistance, anti-condensate oil performance, and emulsification ability are systematically evaluated. Then, the microstructure of the foam is scrutinized. The results show that the compound foaming reagent has good anti-salt and anti-condensate oil properties. In addition, the foam film is thicker compared with that of oleate amide propyl betaine. All results reflect the fact that the compound foaming reagent expands the application range in different environments to various extents, which benefits the design and use of foaming reagents in gas fields.



 CC-BY 4.0
CC-BY 4.0