1. Introduction
Phthalocyanines (Pcs) and their derivatives represent aromatic macrocycles with an 18-π-electron delocalization system characterized by high chemical and thermal stability. These compounds possess diverse coordination properties, exhibit low toxicity, and have the ability to absorb within the visible region (600–800 nm). The versatility of Pcs is featured in a wide range of applications [1, 2, 3, 4, 5], such as semiconductor devices [6], liquid crystals [7, 8, 9], sensors [10, 11, 12], catalysts [13], and dye-sensitized solar cells [14, 15]. Furthermore, Pcs have emerged as highly promising photosensitizers in the context of photodynamic therapy (PDT) [16, 17, 18, 19, 20, 21, 22].
The photophysical characteristics of Pcs are notably influenced by the central metal ion, with zinc (Zn) proving particularly valuable due to its ability to confer Pcs with enhanced fluorescence and singlet oxygen production properties. These attributes make them suitable for applications in tumor detection and treatment [23].
The solubility of Pcs is a pivotal factor for diverse applications. Introducing functional groups such as alkyl, tertiary butyl groups, crown ethers, alkoxy, alkylthio, and amide groups into peripheral benzene rings of the Pc structure enhances solubility in both protic and non-protic solvents [24, 25, 26, 27, 28, 29, 30, 31, 32]. Notably, water solubility assumes a crucial role in PDT applications [33, 34, 35]. While sulfonates [36], carboxylates [37, 38], and quaternized amino groups [39, 40, 41] are commonly employed to achieve water solubility in dyes, tert-butyl groups confer Pcs with hydrophobic properties [42].
An important consideration in the study of Pcs is their tendency to form aggregates, especially in aqueous solutions [43]. This property proves unfavorable as it reduces solubility and adversely impacts photophysical characteristics, such as shortening the triplet state lifetime and hindering the efficient generation of singlet oxygen. In the context of in vivo activity as photosensitizers, aggregation not only diminishes photoactivity but also restricts access to neoplastic cells [44]. The degree of this effect is significantly influenced by the molecular structure, particularly the nature of the central metal ion and functional groups present in the peripheral substituents [45, 46]. Since photochemical activity is important only for Pc monomers, assessing aggregation parameters becomes essential for accurately determining the photophysical properties of these compounds. The formation of aggregates is primarily driven by π–π stacking between the nearly planar Pc macrocycles and van der Waals forces. Depending on their geometry, H-type (sandwich) and J-type (head-to-tail) aggregates can be distinguished, exhibiting significant differences in optical properties according to Kasha’s exciton model [47, 48].
Considering the cost-effectiveness and commercial availability of tert-ZnPc(II), it emerges as an intriguing compound with a vast field of applications. In this study, we aim to explore the impact of solvent polarity on the photophysical properties (absorption and fluorescence emission spectroscopies) of tert-ZnPc(II). This work explores its photophysical properties, with special attention given to the effect of different concentrations of water on absorption behavior and aggregation in homogeneous solutions.
2. Results and discussion
The most important properties of Pcs as materials lie in their exceptional thermal, chemical, and photochemical stabilities. Typically, unsubstituted Pc complexes exhibit low solubility across a wide range of solvents. Solubility in organic solvents is crucial for exploring the diverse applications of these molecules. Enhancing solubility in water or common organic solvents can be effectively achieved through peripheral modifications of Pcs, particularly by introducing the right substitutions on benzene rings [49, 50, 51].
In contrast to unsubstituted Pc metal complexes, tert-ZnPc(II), despite existing as a mixture of four positional isomers [52, 53, 54, 55, 56, 57], stands out due to the incorporation of bulky tert-butyl substituents. This modification mitigates the aggregation tendencies of Pcs and enables an increase in their solubility across various common organic solvents without causing a significant shift in their ultraviolet–visible (UV–vis) spectra [58]. The chemical structure of tert-ZnPc(II) is shown in Figure 1.
Chemical structure of tert-ZnPc(II).
2.1. Effect of the solvent
2.1.1. Ground-state electronic absorption spectra
The photophysical characteristics of Pc macrocycles are primarily influenced by their molecular structure and their interaction with the surrounding environment [59]. The UV–vis absorption spectra of tert-ZnPc(II), as illustrated in Figure 2, were measured in various solvents. Solvent polarity is quantified by the ET(30) parameter, with higher ET(30) values indicating increased solvent polarity (ET(30) water = 63.1, ET(30) glycerol = 57, ET(30) methanol = 55.4, ET(30) ethanol = 51.9, ET(30) acetonitrile = 45.6, ET(30) DMF = 43.8, ET(30) chloroform = 39.1, ET(30) ethyl acetate = 38.1, ET(30) toluene = 33, ET(30) FBS = unknown) [60, 61].
Absorption spectra of tert-ZnPc(II) in 10 different solvents (7.5 × 10−6 mol⋅L−1).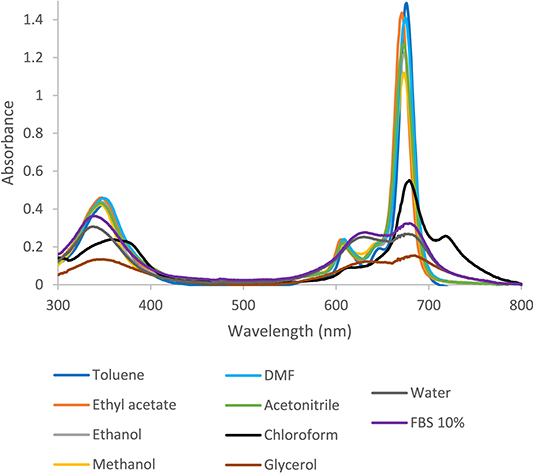
A DMSO stock solution (mtert-ZnPc(II) = 4.75 mg, VDMSO = 4.75 mL) was prepared, ensuring compound solubility. This stock solution was further diluted in 10 different solvents to investigate the specific impact of solvent polarity on photophysical properties. Concentration standardization was achieved in toluene by adjusting the peak absorbance at 610 nm to approximately 0.2, equivalent to 6 × 10−3 mg⋅mL−1. The solvents include toluene, ethyl acetate, ethanol, methanol, DMF, acetonitrile, chloroform, glycerol, water, and a 10% dilution of fetal bovine serum (FBS) in a biological medium. The absorption spectra are presented in Figure 2.
The pronounced tendency of Pcs to form dimers and trimers in solution leads to a reduction in its photoactivity as a photosensitizer. This is attributed to the fact that the monomer structure is responsible for optimal photochemical activity [44, 45, 46]. In Figure 2, the absorption spectra of tert-ZnPc(II) in toluene, ethyl acetate, ethanol, methanol, DMF, and acetonitrile are depicted. The Soret and Q bands exhibit consistent shapes with minor shifts, aligning with the characteristic UV–vis spectra of Pcs.
On the other hand, absorption spectra of tert-ZnPc(II) in chloroform, glycerol, water, and FBS show a significant alteration in the shape, intensity, and split absorption peaks in comparison to the spectra in toluene, indicating an aggregation effect. tert-ZnPc(II) exhibits two main absorption bands at 350 nm and 610 nm. These bands exhibit diminished intensity in aqueous medium, glycerol, and chloroform, coupled with a red shift. Conversely, the spectra appear quite similar in the other solvents.
The molar extinction coefficient (𝜀) is an intrinsic property of molecules depending mostly on their structure and composition. It assesses how chemical species absorb photons at specific wavelengths. The molar extinction coefficients of tert-ZnPc(II) are calculated and depicted in Table 1.
Absorbance B and Q bands and molar extinction coefficient of tert-ZnPc(II) in different solvents
| Solvents | B band (nm) | Q band 1 (nm) | Q band 2 (nm) | 𝜀350 | 𝜀610 |
|---|---|---|---|---|---|
| Toluene | 350 | 610 | 676 | 56,802 | 29,403 |
| Ethyl acetate | 347 | 606 | 671 | 60,277 | 28,067 |
| Ethanol | 347 | 608 | 674 | 57,738 | 28,067 |
| Methanol | 346 | 608 | 673 | 55,064 | 27,933 |
| DMF | 349 | 609 | 675 | 61,079 | 31,675 |
| Acetonitrile | 346 | 606 | 672 | 56,535 | 27,933 |
| Chloroform | 357 | 679 | 718 | 30,740 | 11,494 |
| Glycerol | 349 | 633 | 685 | 17,776 | 12,162 |
| Water | 338 | 630 | 678 | 37,422 | 25,527 |
| FBS | 339 | 630 | 679 | 45,174 | 26,998 |
2.1.2. Fluorescence quantum yields
The fluorescence spectra in different solvents after excitation at 610 nm are shown in Figure 3.
Fluorescence spectrum of tert-ZnPc(II) in 10 solvents excited at 610 nm (7.5 × 10−6 mol⋅L−1).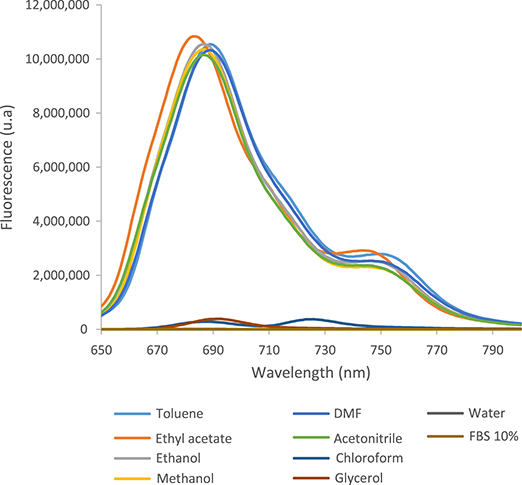
The fluorescence spectra of tert-ZnPc(II) reveal two distinct groups of solvents (Figure 3). In FBS, chloroform, and glycerol, the spectra exhibit two peaks of very low intensity at 690 nm and 725 nm while no fluorescence is observed in water. Conversely, in the remaining solvents, the emission spectra show the characteristic patterns of Pc macrocycles. The first maxima spectra are situated between 680 nm and 690 nm and the second between 740 nm and 750 nm, with both peaks exhibiting higher intensity in alignment with the absorption spectra of Pcs.
The fluorescence quantum yields in toluene, ethyl acetate, ethanol, methanol, DMF, and acetonitrile exhibit close values, ranging from 0.35 to 0.27 (Table 2). In contrast, chloroform, glycerol, and FBS show fluorescence quantum yields less than 0.1 while as expected, the fluorescence quantum yield is zero for water. Notably, the fluorescence quantum yields at 350 nm closely mirror those at 610 nm, indicating that the quantum yield remains consistent irrespective of the wavelength at which it is measured. This emphasizes the wavelength-independent nature of fluorescence quantum yield.
Emission band, fluorescence quantum yield of tert-ZnPc(II) in different solvents
| Solvents | Λext (nm) | Λem Band 1 (nm) | Λem Band 2 (nm) | Λem Band 3 (nm) | Φf 610 |
|---|---|---|---|---|---|
| Toluene | 610 | 689 | 749 | - | 0.35 |
| Ethyl acetate | 610 | 683 | 743 | - | 0.31 |
| Ethanol | 610 | 687 | 746 | - | 0.29 |
| Methanol | 610 | 686 | 745 | - | 0.27 |
| DMF | 610 | 689 | 746 | - | 0.29 |
| Acetonitrile | 610 | 687 | 741 | - | 0.28 |
| Chloroform | 610 | 687 | - | - | 0.03 |
| Glycerol | 610 | 692 | - | - | 0.02 |
| Water | 610 | 700 | 720 | 738 | 0 |
| FBS | 610 | 686 | - | - | <0.1 |
2.2. Effect of pH medium
The UV–vis spectrum of tert-ZnPc(II) in water reveals a broader absorption profile with split peaks, exhibiting an extensive broadening of the Q band (Figure 4). This is accompanied by a red shift in the absorption maximum, compared to toluene, and a blue shift in the Soret band from 350 nm to 336 nm (Table 3), indicative of H-aggregate formation. The H-aggregate, characterized by a face-to-face arrangement, features a large contact area and strong π–π forces. Previous research has shown that nonaxial modified phthalocyanines, such as tetra(4-tert-butyl)phthalocyanine (abbreviated as TbPc), tend to form H-aggregates during self-assembly [15].
UV–vis absorption spectra of tert-ZnPc(II) in water at different pH (7.5 × 10−6 mol⋅L−1).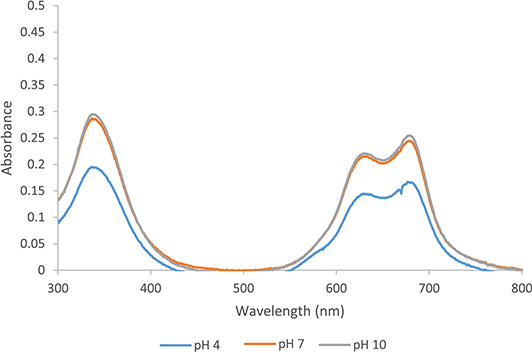
Absorbance B and Q bands of tert-ZnPc(II) in water at different pH (7.5 × 10−6 mol⋅L−1)
| Water | B band (nm) | Q band 1 (nm) | Q band 2 (nm) |
|---|---|---|---|
| pH4 | 336 | 630 | 677 |
| pH7 | 337 | 630 | 677 |
| pH10 | 337 | 630 | 677 |
Moreover, pH is identified as a factor that could influence photophysical properties. UV–vis absorption and fluorescence emission of tert-ZnPc(II) in water at a concentration of 7.5 × 10−6 mol⋅L−1 were measured under different pH conditions (pH 4, 7, and 10). The absorption spectra are relatively similar for pH 7 and 10, whereas a significant decrease in intensity is observed for pH 4. In water, 7.5 × 10−6 mol⋅L−1 exhibits no fluorescence across all pH ranges, indicating the presence of aggregation (Figure 5).
Fluorescence spectrum of tert-ZnPc(II) in water excited at 610 nm (7.5 × 10−6 mol⋅L−1) at different pH.
2.3. Effect of water concentration on UV–vis absorption properties of tert-ZnPc(II)
The observation of a notable change in the Q band shape within the UV–vis absorption spectrum of tert-ZnPc(II) in a water environment motivated us to examine the impact of water on the absorption intensity for detecting trace amounts of water in diverse organic solvents, including ethanol, methanol, and acetonitrile.
Indeed, water stands as an important and indispensable substance, holding significance not only in the realm of everyday human existence but also within the domain of chemistry. It serves as a critical impurity requiring elimination from numerous chemical and industrial production processes.
Water possesses distinctive characteristics that engender specific interactions with excited molecules owing to its substantial dielectric constant and ability to donate and accept protons. This makes it a potential sensor for detecting moisture in organic solvents. Another important factor that needs to be considered is the strong tendency of tert-ZnPc(II) to form aggregates.
The probe solution of tert-ZnPc(II) (7.5 × 10−6 mol⋅L−1) is dissolved in each of the dry organic solvents ethanol, methanol, and acetonitrile, and various concentrations of water (0–70%, v/v) were added. All spectra were obtained immediately after the addition of water to each solvent at 25 °C.
Various concentrations of water in ethanol, methanol, and acetonitrile solutions containing tert-ZnPc(II) did not lead to significant wavelength shifts or notable changes in spectral shape; the only difference lay in the absorption coefficient magnitudes. Dicelio and colleagues conducted a study on the aggregation of tetra, tert-butyl phthalocyanine, revealing that both the monomer and dimer of tetra, tert-butyl phthalocyanine exhibited nearly identical UV–vis absorption spectra [62]. Consequently, the addition of water to tert-ZnPc(II) induced the formation of tert-ZnPc(II) aggregates without entirely altering the UV–vis absorption spectra. Explaining the shapes and intensities of the dimer spectra accurately would necessitate a comprehensive theoretical investigation, extending Kasha’s exciton theory to degenerate excited states and incorporating vibronic coupling into the model as proposed by previous research [47, 63].
The UV–vis absorption spectra of tert-ZnPc(II) in ethanol, methanol, and acetonitrile (Figures 6–8) exhibited a nearly identical absorption shape even after the addition of varying concentrations of water. However, the notable alteration was observed in the absorption intensity. Specifically, the introduction of small aliquots of water (0.1%) into ethanol, methanol, and acetonitrile resulted in a decrease in absorption intensity from 1.228 to 0.593, 1.115 to 0.415, and 1.270 to 0.632, respectively. These values were 2, 2.60, and 2 times lower than those observed in pure ethanol, methanol, and acetonitrile, respectively (Tables 4–6 in Supplementary Material).
UV–vis absorption spectra as a function of water content of tert-ZnPc(II) in ethanol at 7.5 × 10−6 mol⋅L−1.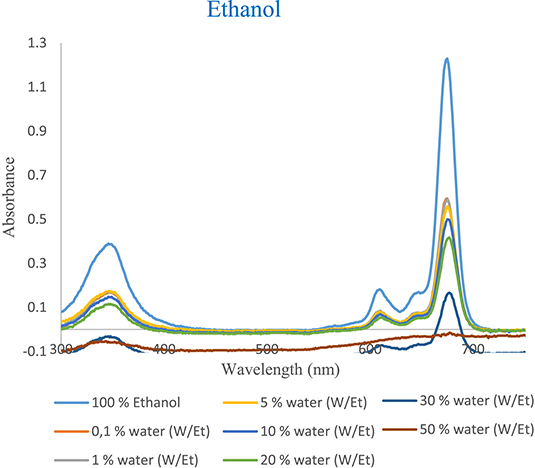
UV–vis absorption spectra as a function of water content of tert-ZnPc(II) in methanol at 7.5 × 10−6 mol⋅L−1.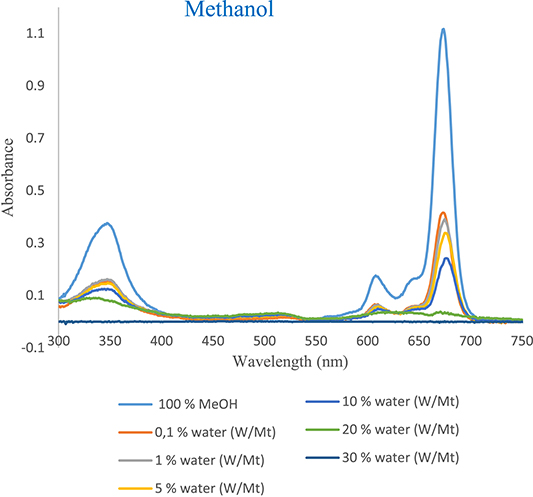
UV–vis absorption spectra as a function of water content of tert-ZnPc(II) in acetonitrile at 7.5 × 10−6 mol⋅L−1.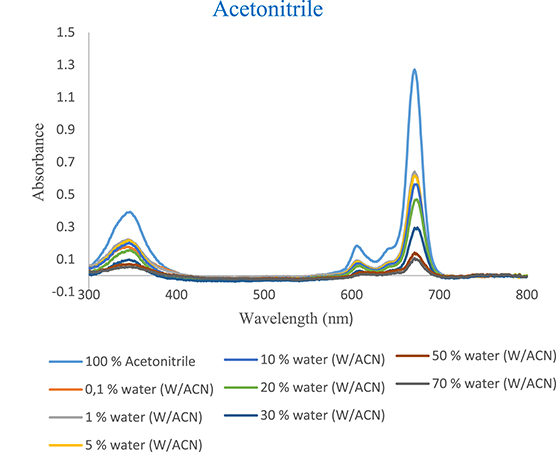
This diminished intensity, attributed to the addition of a minimal quantity of water, emphasizes the high sensitivity of tert-ZnPc(II) to water content. The absorption intensity at 674 nm, 673 nm, and 672 nm in ethanol, methanol, and acetonitrile progressively decreased with increasing water content from 0.1% to (30%, 10%, and 70%), reaching a plateau at each respective concentration. At the 30%, 10%, and 70% water content levels, the absorption intensity at 674 nm, 673 nm, and 672 nm were approximately 7-, 4.62-, and 12-fold lower than in pure ethanol, methanol, and acetonitrile, respectively (Figures 6–8). Evidently, a linear decrease in absorption intensity was observed across all wavelengths with increasing percentage of water content.
It is noteworthy that despite the likely occurrence of aggregation, the spectra maintained their consistent absorption shapes even when incorporating 30%, 10%, and 70% water into homogeneous solvents of ethanol, methanol, and acetonitrile, respectively.
3. Conclusion
This study focuses on the exploration of tert-ZnPc(II) photophysical properties. The investigation highlights the influence of solvent polarity on ground-state electronic absorption spectra and fluorescence capability. It is noteworthy that tert-ZnPc(II) exhibits typical UV–vis spectra in all organic solvents examined except chloroform, water, glycerol, and FBS. In these specific solvents, a significant alteration in the shape of absorption spectra is observed coupled with a notable absence of fluorescence, likely attributed to well-known aggregation phenomena inherent in phthalocyanines. Additionally, the UV–vis spectra of tert-ZnPc(II) in water demonstrate high sensitivity to water content, maintaining consistent absorption shapes even with the incorporation of a substantial amount of water into the solvents.
4. Materials and methods
All solvents and reagents were of reagent grade quality and obtained commercially from Aldrich, TCI, Fluka, and Merck.
4.1. Photophysical properties
Absorption spectra were recorded on a UV-3600 UV–visible double beam spectrophotometer (Shimadzu, Marne la Vallée, France). Fluorescence spectra were recorded on a Fluorolog FL3-222 spectrofluorometer (HORIBA Jobin Yvon, Longjumeau, France) equipped with a 450 W xenon lamp, a thermostated cell compartment (25 °C), a UV–visible photomultiplier R928 (HAMAMATSU, Japan), and an InGaAs infrared detector (DSS-16A020L, Electro-Optical Systems Inc, PA, USA).
The excitation beam and the emission beam were diffracted by a double-ruled grating SPEX monochromator with 1200 grooves/mm blazed at 330 nm and 1200 grooves/mm blazed at 500 nm, respectively. All spectra were measured in four-face quartz cuvettes. All the emission spectra have been displayed with the same absorbance (less than 0.2) with the lamp and photomultiplier correction.
4.2. Parameters for fluorescence quantum yields
The fluorescence quantum yields of tert-ZnPc(II) (𝛷f) were calculated in different solvents by Equation (1) [64]:
| (1) |
Funding
The authors thank the French Institute of Tunisia for providing financial support.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Supplementary data
Supporting information for this article is available on the journal’s website under https://doi.org/10.5802/crchim.364 or from the author.





 CC-BY 4.0
CC-BY 4.0