1. Introduction
Selective oxyfunctionalization, that is, the regio- and enantioselective insertion of an oxygen atom into (nonactivated) C–H bonds still represents one of the major challenges for organic synthesis. Among the biocatalytic methods (striking by their high selectivity), peroxygenase-catalyzed oxyfunctionalization reactions have been receiving particular interest [1, 2, 3]. Particularly, heme-containing enzymes catalyze a broad range of synthetically relevant oxyfunctionalization reactions. The catalytically active species in these enzymes is the so-called Compound I (CpdI, a highly reactive oxyferryl heme species). Compared to P450 monooxygenase-catalyzed oxyfunctionalization reactions [4], peroxygenases are marked by their simplicity owing to the following reason. Instead of relying on complex and weak electron transport chains to generate CpdI via reductive activation of O2 as in the case of P450 monooxygenases, unspecific peroxygenases (UPOs) form CpdI directly from H2O2 (Scheme 1).
Comparison of Compound I (CpdI) formation in the case of P450 monooxygenases and peroxygenases (UPOs).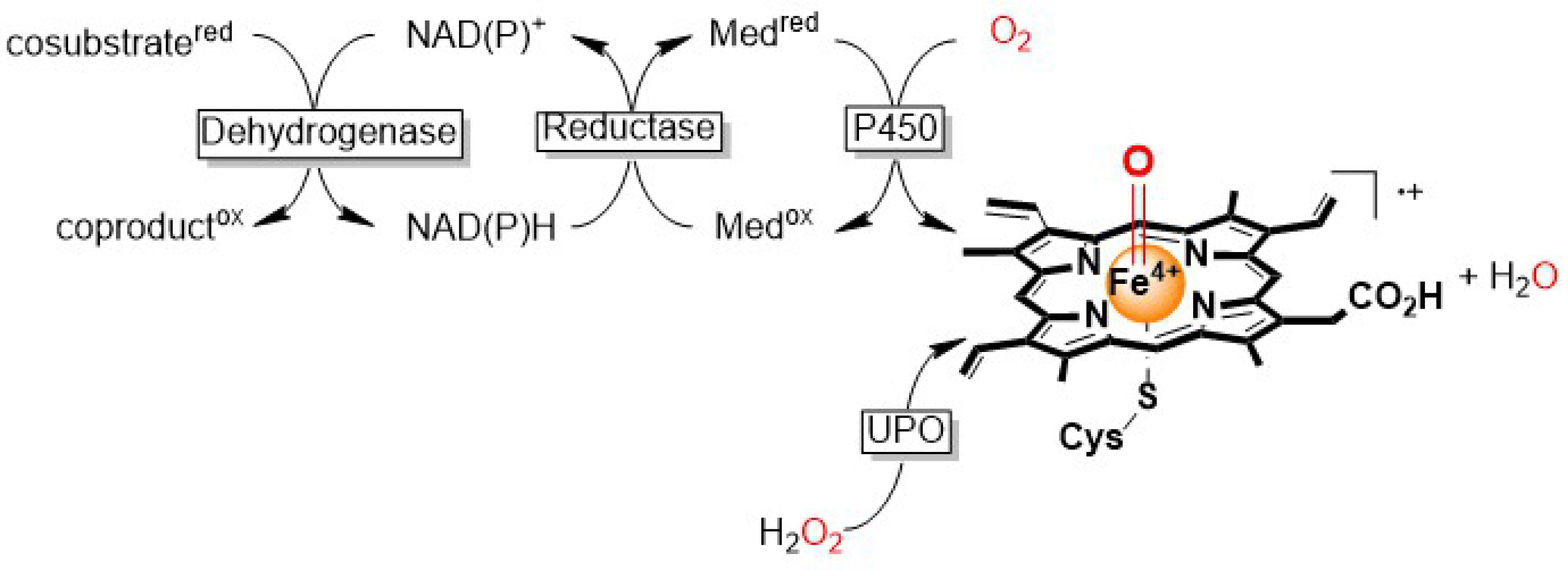
The enormous synthetic potential of UPOs is exemplified by their outstanding performance in terms of total turnover numbers [5, 6] and productivity achievable [7]. Frequently, UPOs outperform their P450 monooxygenase counterparts [8].
However, one current drawback of UPOs is the rather narrow substrate scope, which can be assigned to the yet low number of practical UPOs and UPO-mutants reported so far [9, 10, 11, 12, 13, 14, 15]. Hence, broadening the scope of UPOs available is mandatory to address this limitation.
Recently, two new UPOs from the ascomycetes Collariella virescens (CviUPO) and Daldinia caldariorum (DcaUPO) have been reported for the oxyfunctionalization of fatty acids [16, 17, 18, 19, 20, 21, 22]. Both enzymes have been expressed in Escherichia coli and preliminarily evaluated for alkane hydroxylation. Though alkane hydroxylation (e.g., on renewable fatty acids) is of tremendous interest for the synthesis of polyester precursors, it represents only a fraction of chemically relevant oxyfunctionalization reactions.
We therefore set out to further explore the scope of CviUPO and DcaUPO for more oxyfunctionalization reactions.
2. Materials and methods
2.1. Expression of UPO genes in E. coli
The pET-28a plasmids containing gene sequences for the UPOs from C. virescens or D. caldariorum (SI 1) were transformed into chemically competent E. coli BL21 (DE3). Recombinant E. coli strains were grown in autoinduction media (12 g⋅L−1 peptone, 24 g⋅L−1 yeast extract, 15 mg⋅L−1 glucose, 220 mg⋅L−1 lactose, 6.3 g⋅L−1 glycerol, 90 mM KPi buffer, pH 7.0) for 4 days at 16 °C and 140 rpm [23]. Production cultures were inoculated to an optical density at 600 nm (OD600) of 0.05 from overnight precultures. When the cells reached an OD600 of 0.5, 5-aminolevulinic acid (5-Ala) and FeSO4 were added to final concentrations of 500 μM and 0.1 M, respectively.
2.2. Preparation of crude cell extract
After cultivation, cells were harvested by centrifuging at 17,500×g for 30 min at 4 °C. The supernatant was discarded, and the remaining cell pellets were washed with 50 mM phosphate buffer (pH 7.0). The remaining cells were resuspended in the same buffer at a concentration of 50 g/L after which the cells were disrupted during three consecutive cycles using a CF1 Cell Disruptor from Constant Systems at 1.5 kbar. The remaining solution after cell disruption was spun down for 30 min at 36,635×g and 4 °C. The supernatant containing the enzymes was stored at −20 °C until further use.
2.3. ABTS activity assays
To determine the peroxidase activity of the UPOs, a photometric assay using 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as the substrate was performed. Citric acid buffer of 850 μL 100 mM (pH 4.4) was mixed with 50 μL 10 mM ABTS and 50 μL CFE (diluted if necessary). The reaction was started with the addition of 2 mM H2O2 (final concentration). The increase in absorption at 420 nm was tracked for 90 s in a Cary 60 UV–Vis Spectrophotometer (Agilent Technologies). The linear slope between 20 and 80 s was used to calculate the volumetric activity (U⋅ml−1) using the extinction coefficient for ABTS (ε420 = 36.0 mM−1⋅cm−1). 1 U is defined as the amount of enzyme required to convert 1 μmol substrate in 1 min under assay conditions.
2.4. CO-difference spectra
The amount of UPO present in the CFE was determined using CO-difference spectra. 950 μL of the enzyme was diluted in 50 mM phosphate buffer (pH 7). A baseline spectrum was recorded between 400 and 500 nm, after which the sample was shortly exposed to CO. Next, 50 μL of 1 M Na2S2O4 was added and the difference spectrum between 400 and 500 nm was recorded. Per sample, 10 spectra with 30 s intervals were recorded to obtain the maximum difference spectra. Measurements were carried out in duplicate. The UPO concentration was calculated using the difference in absorption at 490 nm and the maximum absorption value at 446 nm (DcaUPO) and 442 nm (CviUPO). Because the extinction coefficients for these enzymes are so far unknown, the general P450 extinction coefficient in CO-difference spectra of 91 mM−1⋅cm−1 was used [24].
2.5. Substrate screening assays
Substrate screening was performed under the same reaction conditions with a total reaction volume of 500 μL. Reactions were performed in 50 mM phosphate buffer (pH 7) with 1 μM UPO and 5 mM of the substrate in 10 vol% ACN using a feeding rate of 2 mM H2O2 per hour. As the negative control, CFE of E. coli transformed with an empty vector containing no peroxygenase gene was used. Reactions were continued for 3 h at 25 °C and 600 rpm in an Eppendorf Thermomixer. Reaction mixtures were analyzed using gas chromatography (GC); detailed GC protocols can be found in supplementary information (SI 2).
3. Results and discussion
3.1. Cloning and expression of CviUPO and DcaUPO
DcaUPO and CviUPO were produced in recombinant E. coli BL21 (DE3) containing the respective pET28a-expression plasmids. Typically cells were cultivated in Studier’s autoinduction medium [23] for 4 days at 16 °C and 140 rpm. After cultivation, E. coli cells were harvested and disrupted resulting in crude cell extract containing the UPOs of interest. An SDS-PAGE analysis revealed that both genes were overexpressed upon induction but mostly yielded the desired UPOs as an insoluble fraction (Figure 1).
SDS-PAGE analysis of the E. coli production of DcaUPO and CviUPO. Lanes marked with B.I. and A.I. are whole-cell SDS samples before and after induction of protein expression, respectively. CFE samples show proteins in the supernatant after cell disruption. CFE SDS samples have been normalized to a protein concentration of 1 mg/mL based on BC assay. The Bio-Rad Precision Plus Protein™ Unstained Protein Standards ladder was used for size reference.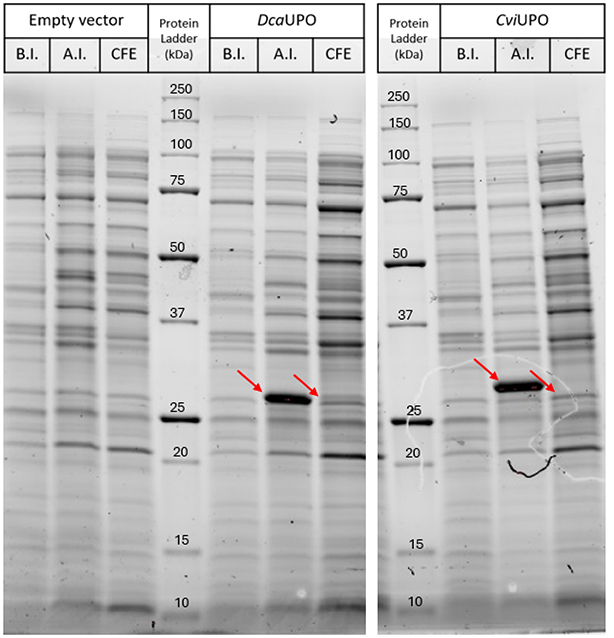
Nevertheless, correct folding of the UPOs in the soluble lysate was confirmed though recording of CO-difference spectra, showing the characteristic shift of the Soret peak from 420 to 442 (CviUPO) and 446 nm (DcaUPO) when CO is bound to the reduced heme cofactor (Figure 2). Based on the abundance of the 450 nm peak, we estimated the UPO titers to be 8.4 mg⋅L−1 and 5.8 mg⋅L−1 for CviUPO and DcaUPO, respectively.
CO-difference spectra of E. coli cell crude extracts expressing CviUPO (—, red) and DcaUPO (—, green) compared to the negative control (empty vector, ⋯ ).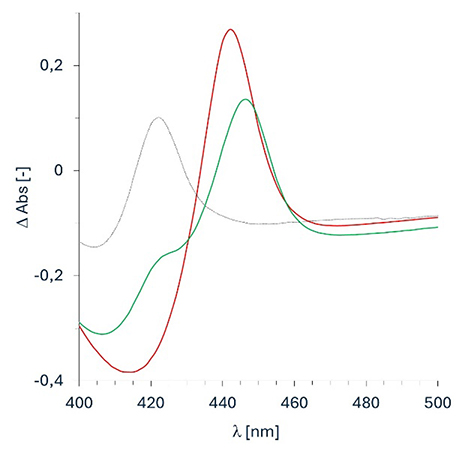
Soluble production of the UPOs was further confirmed by measuring the peroxidase activity. The peroxidase activity of the produced UPOs was tested with an ABTS assay using crude cell extract (Table 1).
ABTS activity determination of E. coli cell extracts
| Enzyme | E. coli BL21 empty vector | DcaUPO | CviUPO |
|---|---|---|---|
| Activity (U⋅mg−1) | 0.006 ± 0.0004 | 0.034 ± 0.002 | 0.662 ± 0.167 |
3.2. Oxidation reactions
Having CviUPO and DcaUPO in hand, we investigated their applicability for several chemical transformations of interest. Among them, we investigated C–H bond hydroxylation reactions, alcohol oxidations, epoxidation reactions, and sulfoxidation reactions.
3.2.1. Oxidation of alcohols
A selection of primary and secondary alcohols was subjected to CviUPO- and DcaUPO-catalyzed conversion. Already, the semi-quantitative screening revealed some interesting observations (Figure 3). First, DcaUPO generally was more active than CviUPO. Second, both enzymes exhibited a clear preference for secondary alcohols over primary alcohols. The latter observation is in line with the lower C–H bond dissociation energy of secondary alcohols compared to primary alcohols. Rhododendrol was not converted by any of the UPOs, possibly due to steric hindrance.
Substrate screening of selected alcohols using DcaUPO (red) and CviUPO (black). Conditions: c(UPO) = 1 μM, c(substrate) = 5 mM, buffer: 50 mM NaPi buffer, pH = 7.0, containing 10% (v/v) of acetonitrile. H2O2 addition: 2 mM per hour (from 100 mM stock). T = 25 °C, orbital shaking at 600 rpm, reaction time = 3 h. Red/black: estimated conversion into the envisaged carbonyl product (aldehyde or ketone), light red/gray: yet undefined side product.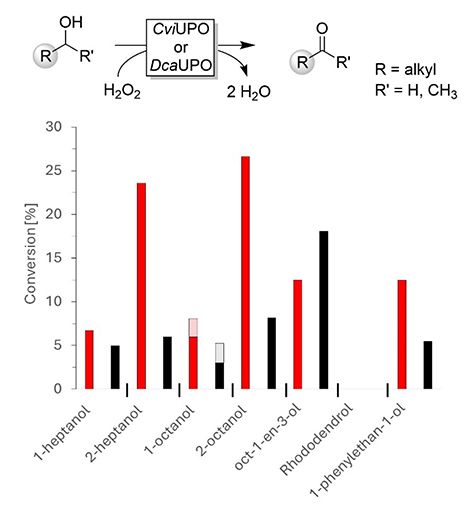
The transformations of 2-heptanol, 2-octanol, oct-1-en-3-ol, and 1-phenyl ethanol were analyzed in more detail based on calibration curves with authentic standards (Table 2). Chiral GC analysis of the DcaUPO-catalyzed oxidation reactions showed that both enantiomers of, for example, 2-octanol or 1-phenyl ethanol were converted at roughly the same rate with a slight preference for the (R)-enantiomers (SI 3).
Quantitative analysis of some alcohol oxidations catalyzed by DcaUPO and CviUPO
| Product[a] | UPO | Concentration [mM][a] | TN (UPO)[b] |
|---|---|---|---|
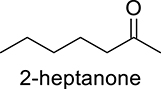 |
DcaUPO | 1.42 | 1417 |
| CviUPO | 0.36 | 358 | |
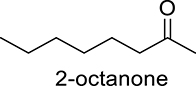 |
DcaUPO | 1.43 | 1425 |
| CviUPO | 0.44 | 437 | |
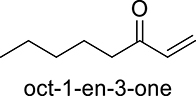 |
DcaUPO | 0.9 | 899 |
 |
DcaUPO | 0.62 | 618 |
| CviUPO | 0.24 | 243 |
[a]Based on authentic standards; [b]TN = c(product) × c(UPO)−1.
3.2.2. Oxidation of arenes
Next, a range of substituted arenes were evaluated (Figure 4). Like the oxidation of alcohols, DcaUPO generally exhibited a significantly higher activity as compared to CviUPO. Toluene was not converted by either UPO whereas ethyl benzene, 4-phenyl-2-butanone (containing elongated alkyl substituents), and pseudocumene (1,2,4-trimethyl benzene) as well as p-cymene (4-isopropyl toluene) and thymol (2-isopropyl-5-methylphenol or 3-hydroxy p-cymene) were converted at least by DcaUPO.
Substrate screening of some substituted arenes using DcaUPO (top) and CviUPO (bottom). Conditions: c(UPO) = 1 μM, c(substrate) = 5 mM, buffer: 50 mM NaPi buffer, pH = 7.0, containing 10% (v/v) of acetonitrile. H2O2 addition: 2 mM per hour (from 100 mM stock). T = 25 °C, orbital shaking at 600 rpm, reaction time = 3 h. Blue: estimated conversion into the envisaged carbonyl product (aldehyde or ketone), red: yet undefined side product.
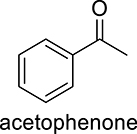
DcaUPO-catalyzed conversion of ethyl benzene yielded (R)-1-phenyl ethanol in very modest enantiomeric excess (ee) of 55%. Furthermore, some overoxidation to acetophenone was observed. With toluene, no apparent transformation was observed, which may be explained by the higher C–H bond strength of benzylic CH3 group compared to the benzylic CH2 group in the case of ethyl benzene. Interestingly, however, pseudocumene (1,3,4-trimethyl benzene) was converted comparably well. Possibly the electron-donating effect of the additional CH3 groups already sufficed to activate the CH3 group converted. Preliminary GC- and GC/MS data suggest selective CH3 hydroxylation (SI). Future, preparative-scale transformations will yield sufficient amounts for more detailed structure elucidation. p-Cymene, containing a highly activated tertiary C–H group, however, was almost not converted. Possibly steric effects impeding the interaction of the bulky CH(CH3)2 with CpdI dominated the electronic activation.
Thymol was accepted by both UPOs with a notable difference with respect to selectivity; while the DcaUPO-catalyzed transformation was apparently highly selective, with CviUPO at least four different products were observed. The occurrence of multiple products may be attributed to the peroxidase activity of UPOs with activated arenes such as phenols resulting in radical-coupling products [19, 20, 21]. We therefore compared the DcaUPO-catalyzed conversion of thymol in the presence and absence of ascorbic acid, a well-established radical scavenger (Figure 5). Indeed, the product spectrum completely changed; GC-MS analysis suggested thymoquinone being the main product in the absence of ascorbic acid and the corresponding thymohydroquinone.
(A) GC chromatograms of thymol without the addition of ascorbic acid (black) and with the addition of 20 mM ascorbic acid (pink). (B) Suggested reaction pathway.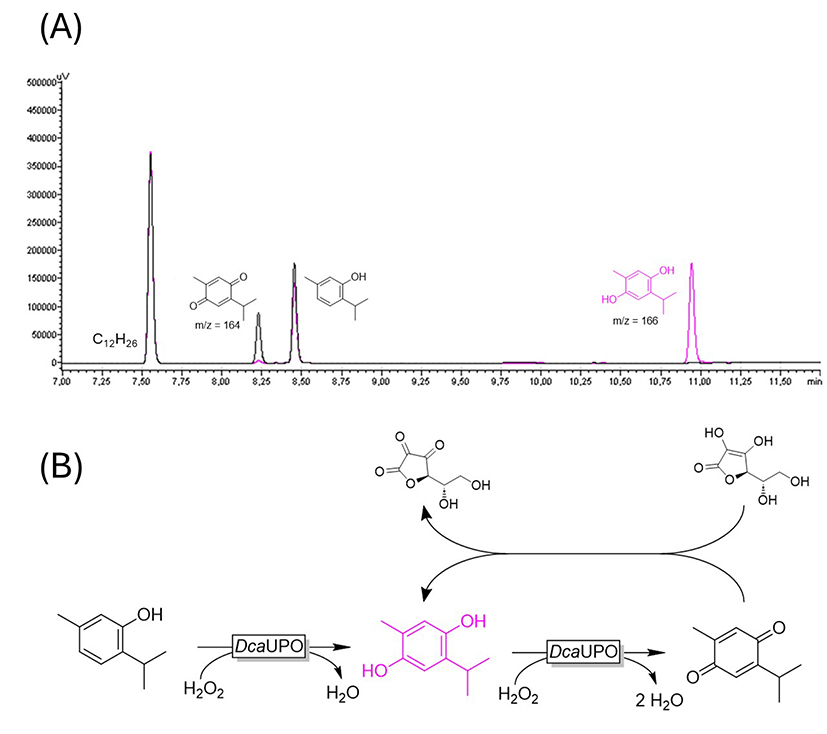
Interestingly, 4-phenyl-2-butanone was converted by DcaUPO while in the case of structurally related rhododendrol, no conversion was detectable. It is also interesting to note that three different products were observed exhibiting M+ peaks of 164, 146, and 106. This may be rationalized by assuming that DcaUPO catalyzed the benzylic hydroxylation of the starting material (resulting in the main product with a putative molar mass of 164 g⋅mol−1). The latter may spontaneously undergo either dehydration (yielding the product with a putative molar mass of 146 g⋅mol−1) or a retro-aldol reaction resulting in benzaldehyde (MW = 106 g⋅mol−1 and acetone, which under current analytical conditions was not detectable) (Scheme 2).
Hypothesized benzylic hydroxylation of 4-phenyl-2-butanone followed by (spontaneous) dehydration and/or retro-aldol reaction.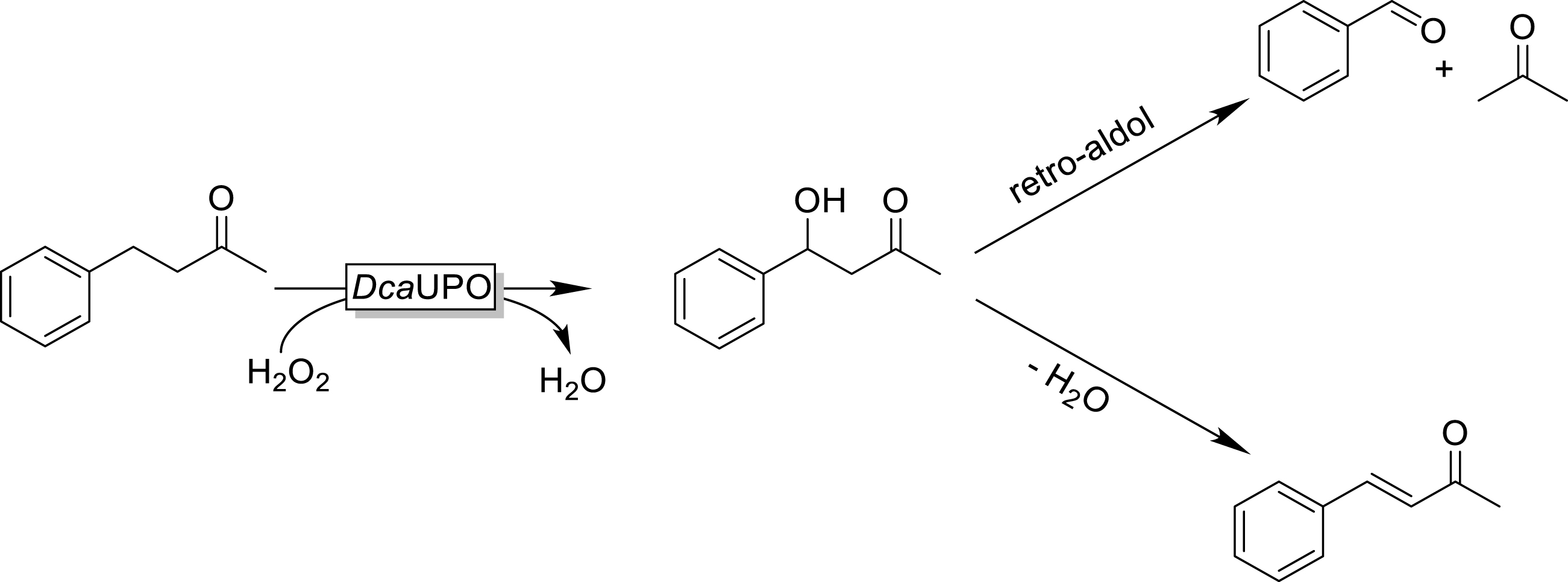
3.2.3. Epoxidation reactions
Next to hydroxylation reactions, epoxidations are of significant interest for organic synthesis. Therefore, we evaluated the activity of DcaUPO and CviUPO with some styrene derivatives (Figure 6).
Product distribution of the DcaUPO- and CviUPO-catalyzed epoxidation of some styrene derivatives. Conditions: [UPO] = 1 μM, [substrate] = 5 mM, buffer: 50 mM NaPi buffer, pH = 7, containing 10% (v/v) of acetonitrile. H2O2 addition: 2 mM per hour (from 100 mM stock). T = 25 °C, orbital shaking at 600 rpm, reaction time = 3 h.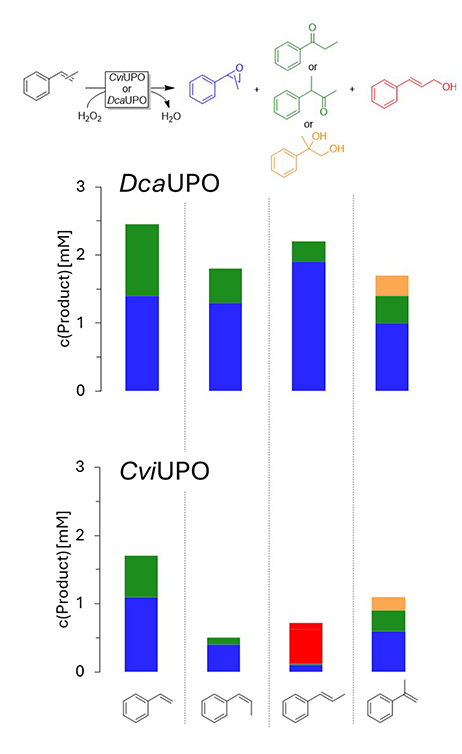
First, the activity difference between DcaUPO and CviUPO was not as pronounced as for the transformations discussed before. The enantioselectivity of the epoxidation reactions was highly dependent on the alkene substitution pattern. Styrene oxide was obtained with rather low (S)-selectivity (24 and 34% ee) with DcaUPO and CviUPO, respectively. In contrast, epoxides obtained from α-methyl styrene were essentially optically pure. Interestingly, DcaUPO yielded the (R)-enantiomer whereas CviUPO produced the (S)-enantiomer exclusively. Moreover, in the case of cis-β-methyl styrene both enzymes were enantiocomplementary albeit again with rather low enantioselectivities. Expectedly, the epoxides obtained from trans-β-methyl styrene were enantiomeric to those obtained from the cis-starting material, again with poor enantioselectivity. It is worth noting that with trans-β-methyl styrene, the main products observed with CviUPO were allylic hydroxylation products (alcohol and aldehyde).
Terpenes such as limonene, α-pinene, and β-ionone were converted at low selectivity (Figure 7). One notable exception was the rather selective hydroxylation of β-ionone to the 4-hydroxy product.
Selection of terpene hydroxylation reactions catalyzed by DcaUPO and CviUPO. Conditions: [UPO] = 1 μM, [substrate] = 5 mM, H2O2 feeding rate = 2 mM⋅h−1, performed in phosphate buffer (50 mM, pH = 7, NaPO4) with 10% (v/v) acetonitrile for 3 h (25 °C, 600 rpm).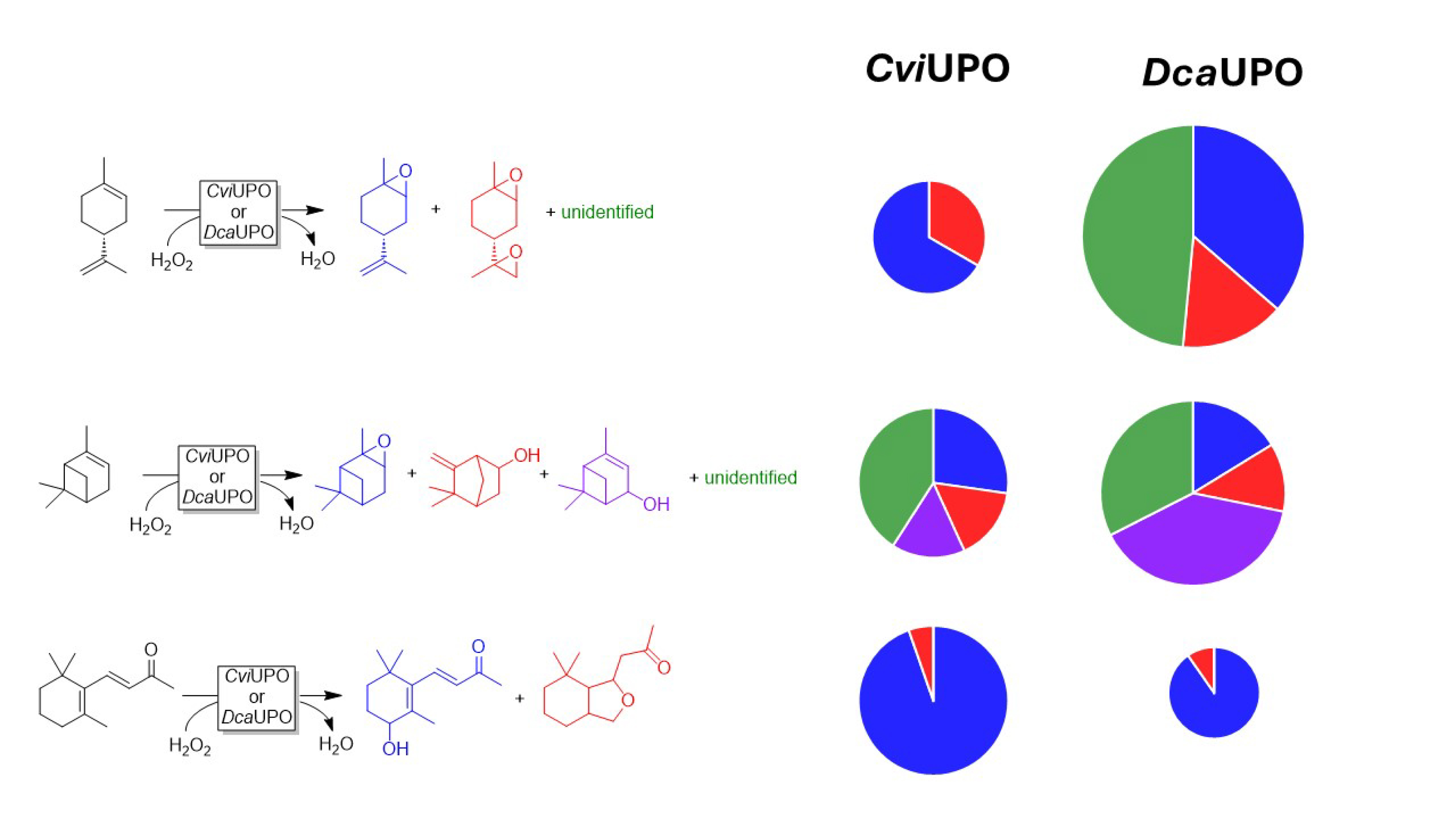
3.2.4. Sulfoxidation reactions
Finally, we evaluated (p-Me-)thioanisole as the substrate for sulfoxidation (Figure 8). Both UPOs exhibited significant activity but were not completely selective for the sulfoxide products as in both cases sulfone formation was also observable.
Sulfoxidation of p-methylthioanisole. Reaction conditions: [UPO] = 1 μM, [substrate] = 5 mM, H2O2 feeding rate of 2 mM⋅h−1, performed in phosphate buffer (50 mM, pH = 7, NaPO4) with 10% (v/v) acetonitrile for 3 h (25 °C, 600 rpm). Unfortunately, our current analytical setup does not permit baseline separation of sulfoxide enantiomers, which is why no information about the optical purity of the sulfoxide can be given.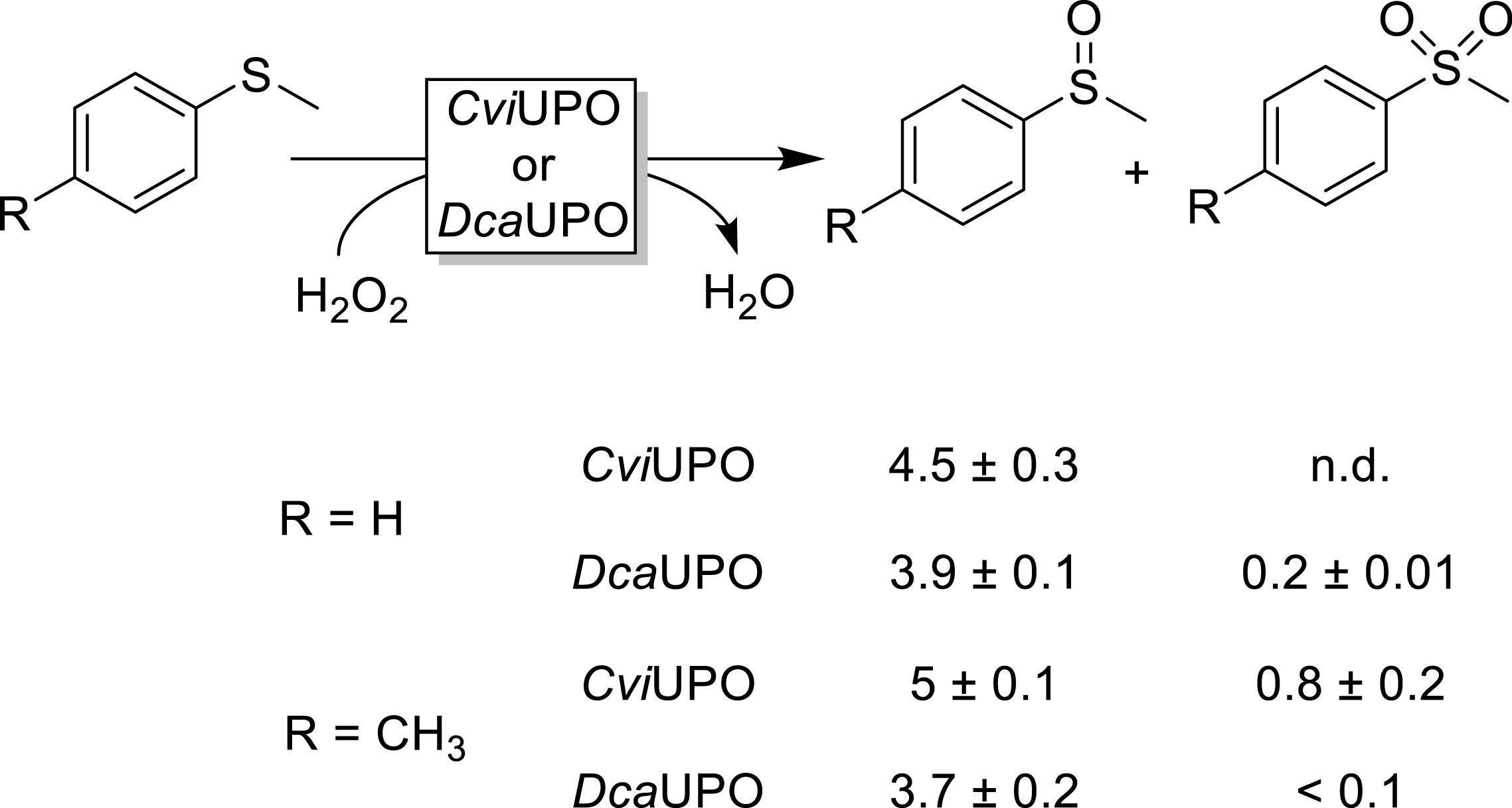
4. Conclusions
In conclusion, with this contribution we have broadened the known substrate scope of CviUPO and DcaUPO. In addition to the previously reported ω-1 hydroxylation activity, both enzymes are also capable of other typical peroxygenase reactions. Some reactivities such as the formation of β-hydroxy ketones and selective allylic hydroxylation, however, stand out from the regular portfolio and certainly deserve further investigation and rationalization. Currently, the poor performance of the E. coli based expression system presents the major hurdle en route to further investigation and upscaling. Our focus in further investigations will be on producing these enzymes via Pichia pastoris based expression systems.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
This work was funded by the European Union (European Research Council [ERC], PeroxyZyme, No. 101054658). The views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or the ERC. Neither the European Union nor the granting authority can be held responsible for them.
Supplementary data
Supporting information for this article is available on the journal’s website under https://doi.org/10.5802/crchim.375 or from the author.





 CC-BY 4.0
CC-BY 4.0