1. Introduction—membranes as a practical tool to intensify biocatalytic processes
Over the past decades, biocatalysis has emerged as a promising and potentially greener approach to produce value-added molecules [1]. Owing to their unique characteristics, such as their high (enantio)selectivity and stereospecificity, non-toxicity (biodegradable), and their ability to operate under mild conditions (e.g., aqueous media, low temperatures), enzymes have attracted significant interest in organic synthesis and the pharmaceutical industry [2]. Hence, biocatalytic processes have the potential to rapidly become a powerful synthetic tool for the industrial preparation of valuable compounds, such as pharmaceuticals and fine chemicals [3, 4, 5].
However, despite the multiple benefits and advantages they offer, their implementation at the industrial level in the pharmaceutical industry is not straightforward and remains limited [6] due to several challenges. These are generally related to the fact that enzymes are often used in their “free” form, functioning as soluble homogeneous biocatalysts that are difficult to reuse, restricted to batch reactors, and which typically exhibit limited stability [7]. Additionally, enzymes tend to suffer from substrate and/or product inhibition, and—in some cases—unfavorable thermodynamic equilibria for the targeted reactions. The quality of the final product can also be compromised by free enzyme deactivation, resulting in complex purification processes [8].
Some of these challenges can be mitigated by immobilizing enzymes on solid supports [9, 10], which allows for their recovery and reuse and facilitates their implementation in continuous-flow processes [11]. Moreover, immobilization often results in enhancing enzyme stability and tolerance to organic solvents [12, 13]. The transfer from batch to continuous-flow processing is of major industrial interest as it increases the productivity of (bio)catalytic transformations and the efficiency of subsequent/coupled unit operations (e.g., crystallization), thus improving the overall process’s economic viability [1, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. Enzymatic processes arguably pave the way for the development of intensified industrially relevant organic synthesis [24]. Enzyme immobilization can be performed in various ways and on a wide range of functional materials such as polymeric resins, inorganic powders, biopolymers, and membranes [12, 25, 26]. Ready-to-use synthetic resins are traditional ubiquitous carriers, allowing to run biocatalytic reactions in heterogeneous catalysis mode and to easily recover and reuse biocatalysts.
Enzyme immobilization on membranes is a particular case that deserves attention, as it additionally offers the possibility of performing biocatalytic reactions along with membrane separation by a one-pot approach. For example, the removal of a product can favorably shift the biocatalytic reaction equilibrium toward product formation and hence increase the reaction yield. Moreover, membranes can be used to introduce one of the reagents at a controlled rate to avoid enzyme inhibition. The coupling of biocatalytic reaction with membrane operation in so-called “enzyme-membrane reactors” (EMRs) has the potential to intensify biocatalytic processes. Typically, membrane separations require only a limited amount of energy with respect to other unit operations [27, 28, 29]. Furthermore, membrane reactors display relatively easy reactor operation and modulation as well as straightforward scale-up to large systems [8, 30, 31, 32]. Thus, integrated hybrid processes allowing to simultaneously perform flow biocatalytic reactions and product separation (e.g., to drive the equilibrium) or controlled substrate addition are of particular interest for the pharmaceutical industry, as they can help reduce the need for additional downstream steps typically required to obtain pure active pharmaceutical ingredients (APIs) [33, 34, 35].
It must be noted that the concatenation of reaction and membrane separation on catalytically active membranes is already well established, and this has been reviewed extensively. For example, Zhang et al. [36] reviewed the applications of a wide range of polymeric catalytic membranes to intensify chemical processes. Furthermore, conventional enzyme immobilization methods and strategies have already been extensively and thoroughly reviewed [8, 37, 38, 39, 40, 41]. These reports focus on the detailed preparation and/or functionalization of various supports—including membranes—for enzyme immobilization. In addition, some excellent reviews [8, 34, 36, 39, 42, 43, 44] cover the benefits of implementing EMR in organic synthesis by collecting scholarly examples and/or providing useful insights into industrial process considerations. For example, the tutorial reviews by Sitanggang et al. [44] and Dejonghe et al. [34] summarized the advantages of coupling enzymes (free or immobilized) with membrane reactors and exemplified their use in a selection of chemical processes.
In this review, we aim to discuss the recent trends (2010 or later) of EMR processes applied specifically for the production of valuable (chiral) building blocks for the pharmaceutical industry. We cover both ceramic and polymeric membrane applications. Polymeric membranes are most commonly employed as support to develop biocatalytic membrane reactors, and they present an array of advantages with respect to their ceramic counterparts. For example, polymeric membranes tend to be generally cheaper and offer a wider range of manufacturing techniques, which have been developed to enable better control and tailoring of final membrane properties [2, 36]. Yet, their organic nature often hampers their chemical stability, which could be an issue when such membranes are put in contact with organic solvents, and hence limit their applicability in multiphasic membrane reactors [45]. On the other hand, ceramic membranes are able to overcome these drawbacks thanks to their inherent outstanding chemical and thermal stability [45, 46]; hence we consider it also important to cover their applications.
Overall, one of the key advantages of coupling biocatalysis with membrane technology is the ability to run biocatalytic reactions while simultaneously performing product/substrate separation from the enzymes and from the reaction medium. Furthermore, this allows operating the synthesis process in continuous-flow mode, which can enhance productivity and economic feasibility. Using multiple membranes in series with different molecular weight cut-offs (MWCOs) can also enhance product selectivity, and the final step of membrane separation allows the concentration of the non-permeable product. The selected membrane material must be stable under the conditions (temperature, pH, presence of organic solvent) that optimize the enzyme’s catalytic activity. When being coupled with membrane reactors, enzymes can be used either separately from the membrane devices or as membrane-immobilized enzymatic reactors. This defines the two categories of hybrid processes that are discussed in this review. The coupling of enzymes with membrane reactors is exemplified in Section 2 while the use of membrane-immobilized enzymatic reactors is reviewed in detail in Section 3.
2. Main synthetic routes that can benefit from the synergistic use of enzymes and membranes
Chiral compounds are the most important building blocks in the chemical and pharmaceutical industry, as they are widely employed for the production of fine chemicals and drugs [47, 48]. Moreover, it is increasingly important to synthesize enantiopure drugs for the pharmaceutical industry [49, 50]. More precisely, enantiopure alcohols [51] and amines [1, 3, 52] are key examples of prime importance not only in the synthesis of pharmaceuticals but also in the flavor and fragrance industry. Small peptides and short oligosaccharides are other categories of functional molecules that can be accessed via biocatalytic synthesis processes.
The use of transaminases (Figure 1a) and alcohol dehydrogenases (Figure 1b) is a conventional biocatalytic strategy to produce chiral amines and alcohol, respectively [53, 54, 55]. Most industrially relevant transaminations suffer from unfavorable thermodynamics, and transaminases tend to be inhibited by their keto substrate (amino acceptor) and by-products. To achieve high transamination yields while avoiding transaminase inhibition, in situ (co)-product removal and/or controlled substrate addition can be performed using membrane technologies [56, 57, 58]. One possible strategy is the use of pervaporation to remove acetone (the most common co-product of transamination reactions). Pervaporation is especially attractive for temperature-sensitive processes because it can be operated at moderate temperatures. Dejonghe et al. [58] implemented hydrophobic pervaporation at the outlet of a transamination reactor (employing free enzymes) in order to remove the acetone by-product from the biocatalytic system. A polydimethylsiloxane (PDMS) membrane module was used as the pervaporation unit. Such transamination coupling with pervaporation resulted in a 13% increase in product yield after 9 h of reaction compared to the standard transamination process (where no pervaporation was performed). However, it was also observed that the effect of acetone removal by pervaporation is minimal at low acetone concentrations (in the biocatalytic system). This highlights the need to work at high substrate concentrations and possibly to couple pervaporation with another product separation technique (which would allow to primarily push transamination toward high product yields). The other product separation technique should target the amine product, and it may be also performed via membrane technology (e.g., via membrane extraction). Notably, Dejonghe et al. [59] conducted the flow asymmetric synthesis of 1-methyl-3-phenylpropylamine from benzyl acetone using free ATA-v2 mutant enzyme in organic solvent (n-heptane), and employed the polypropylene (PP) membrane contactor for in situ amine product removal. An acidic aqueous solution (pH = 3) was used to efficiently extract the amine product via membrane-assisted extraction. In this study, the use of an organic solvent as the reaction medium was found beneficial in the sense that it allowed increasing the optimal keto-substrate concentration (by 2.5-fold with respect to its aqueous solubility) without triggering inhibition. This approach resulted in a significant increase in terms of the final product yield (99% yield) with respect to the standard transamination process (69% yield when run without membrane-assisted extraction). Additionally, by demonstrating the intensified asymmetric synthesis of the R-sitagliptin drug, Yang et al. [60] successfully expanded the applicability of such synergistic coupling between transamination and membrane-assisted product separation. In this case, transamination was conducted in aqueous medium (pH = 9) and PDMS was used as the membrane contactor for solvent extraction. In all these examples, the use of membrane operations offers avenues to intensify the synthesis process.
Main enzymatic transformations leading to pharmaceuticals and fine chemicals, and susceptible to be coupled with membrane reactors, studied in this work. Products highlighted in red are responsible for enzyme inhibition.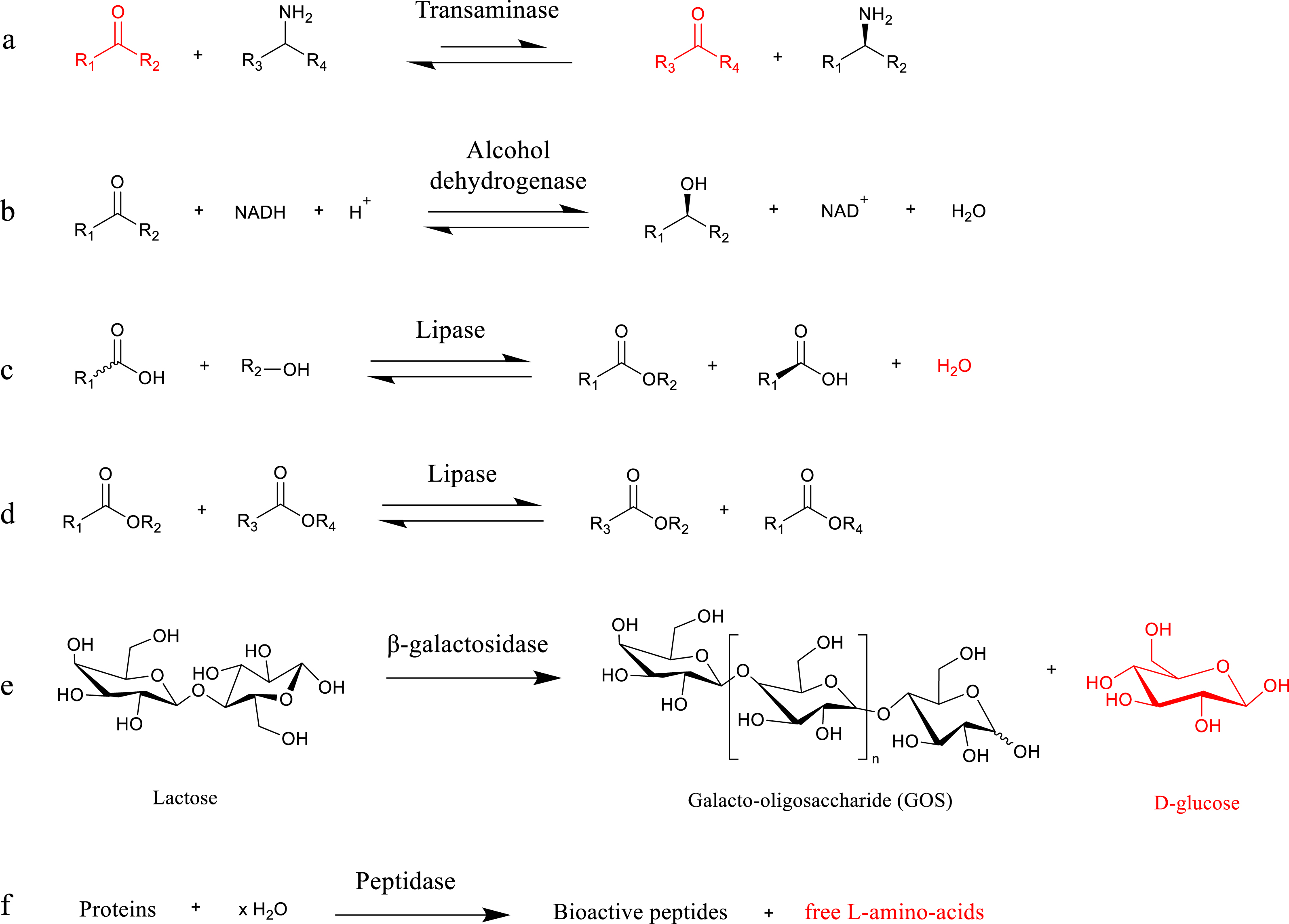
Other (chiral) molecules such as carboxylic acids or esters can lead to the formation of valuable compounds, including chiral intermediates [32, 61, 62, 63]. To this end, lipases have gained much attention lately as they allow for the enantioselective hydrolysis/esterification (Figure 1c) and transesterification (Figure 1d) of poorly water-soluble compounds (i.e., in biphasic media or in organic solvents) [64, 65]. Enzymatic esterification reactions are commonly conducted in non-aqueous solvents, as water accumulation in the reaction medium can promote side reactions (e.g., hydrolysis) and hamper final esterification yields [66, 67]. Additionally, for an enzymatic process, it is well known that excess water should be avoided to preserve high lipase activity [68, 69]. In lipase-catalyzed esterification, for example, the water by-product can be removed in situ from the reactor using membrane technologies. Pervaporation seems particularly suited for such purpose since it requires significantly lower energy consumption and operating costs with respect to distillation processes [70]. Notably, the pervaporation-aided enzymatic production of monoacylglycerols from lauric and caprylic acids with glycerol in solvent-free medium was demonstrated by Satyawali et al. [71] in 2021. Lipases immobilized on polymeric resins in a packed-bed reactor (3 kg scale) operating in recirculation mode were coupled with two zeolite membranes (in series, 56.5 cm2 total area) for pervaporation. Such a coupled esterification–pervaporation process not only allowed pushing the fatty acid conversion toward completion (>95% after 256 h) but also enabled increasing the relative monoacylglycerol content in the final product (with respect to di- and triacylglycerols). Such studies demonstrate the practical applicability of zeolite-based membranes to in situ water removal through hydrophilic pervaporation.
Various functional oligosaccharides, such as lactulose, galacto-oligosaccharides (GOSs) [72], cyclodextrins [73], and oligodextran (5–8 kDa) [74, 75], are known to have potential applications in the fine chemical and pharmaceutical industries. β-galactosidase can catalyze the direct formation of GOS (Figure 1e) and lactulose from lactose via transgalactosylation reactions [72, 76, 77]. Lactose (a disaccharide composed of glucose and galactose) is generated in high content in the dairy by-product of many (bio)chemical processes [78], and it acts as renewable feedstock to produce such building blocks. Cyclodextrin glycosyltransferase and dextranase catalyze the conversion of starch into mixtures of (cyclo)dextrins and the hydrolysis of dextran into oligodextrans of various molecular weights, respectively. A severe limitation in transgalactosylation reactions is that the targeted products tend to spontaneously undergo undesired consecutive reactions (i.e., hydrolysis reactions to monosaccharides glucose and galactose) [79]. Besides, it has been observed that such monosaccharide formation inhibits β-galactosidase (i.e., inhibition of transgalactosylation reaction) [34], which further highlights the need of product separation in these processes. Finally, the selection of producing oligodextran of tailored molecular weight is also a complicated task. Hence, it clearly appears that in all these biocatalytic reactions, coupling with membrane technology is of particular interest since it would enable enhancing the selectivity of the process and the purity of the targeted product (i.e., desired molecular weight) by means of adequate size-exclusion membrane operations.
Peptides are another class of target compounds exhibiting biological activities (e.g., antihypertensive, antioxidative), which find nutritional, cosmetic, and pharmaceutical applications [34, 80]. Peptidases catalyze their production through protein hydrolysis (Figure 1f). However, it is established that the efficiency of peptidases decreases upon accumulation of hydrolysis products (soluble peptides and amino acids) in the medium [34, 81]. Here, conducting protein hydrolysis in continuous membrane reactors (through size-exclusion membrane operations) enables the separation of low-molecular-weight peptides from protein hydrolysates, thereby overcoming the drawbacks of batch reactions, such as product inhibition, low process productivity, and excessive hydrolyses (i.e., prevention of side reactions) [34]. Additionally, the continuous feeding of substrates (e.g., water in this case) to the reactor is advantageous as it allows improving the reaction kinetics and maintaining a constant volume in the reactor by compensating for the permeate flux. Such a beneficial substrate addition was demonstrated by Ma et al. [82], who conducted membrane-assisted enzymatic protein hydrolysis for the tailored production of antihypertensive peptides. First, the membrane ultrafiltration (UF) unit was implemented at the outlet of the reactor tank, which allowed recycling free enzymes (retained in the retentate) and preventing undesired product inhibition (via product separation). When further coupling the enzymatic hydrolysis (performed in recirculating mode) with continuous feeding of water to the reactor, the yield and productivity were respectively enhanced by 62.7% and 22.1% when compared to the standard batch operation (i.e., run without membrane separation and substrate feeding). The continuous addition of the protein substrate (in addition to water feeding) enabled a further boost to peptide productivity.
As mentioned above in the reported examples, when working with free enzymes in solution, size-exclusion membrane operations are often chosen to separate the soluble biocatalyst [42] (retained in the retentate in cross-flow operations; see Figure 2) while isolating the product in the permeate [44]. In such size-exclusion operations, the membrane porosity—which defines its MWCO (in kDa)—has to be carefully chosen based on the molecular size of the enzyme (if present in free form), substrate(s), and product(s). Given that enzyme molecular weights typically range from 10 to 150 kDa, UF membranes are commonly used in membrane reactor designs. The UF membrane pore size must ensure complete enzyme retention while ensuring unobstructed product transport. Nanofiltration (NF) membranes can also be employed in size-exclusion membrane operation design, especially for biocatalysts displaying small molecular weights (typically 0.2–10 kDa, such as, for example, some cysteine proteases [83]) [84, 85]. Additionally, NF membranes are effective in concentrating target compounds as the secondary separation step [86]. As product purification is known to be a major driver of drug manufacturing cost, this is a crucial point.
Schematic representation of biocatalytic processes involving free enzymes in combination with membrane separation units. In case (b), enzymes are recycled (retained) in the retentate through membrane separation. Reproduced from Sitanggang et al. [44] with permission.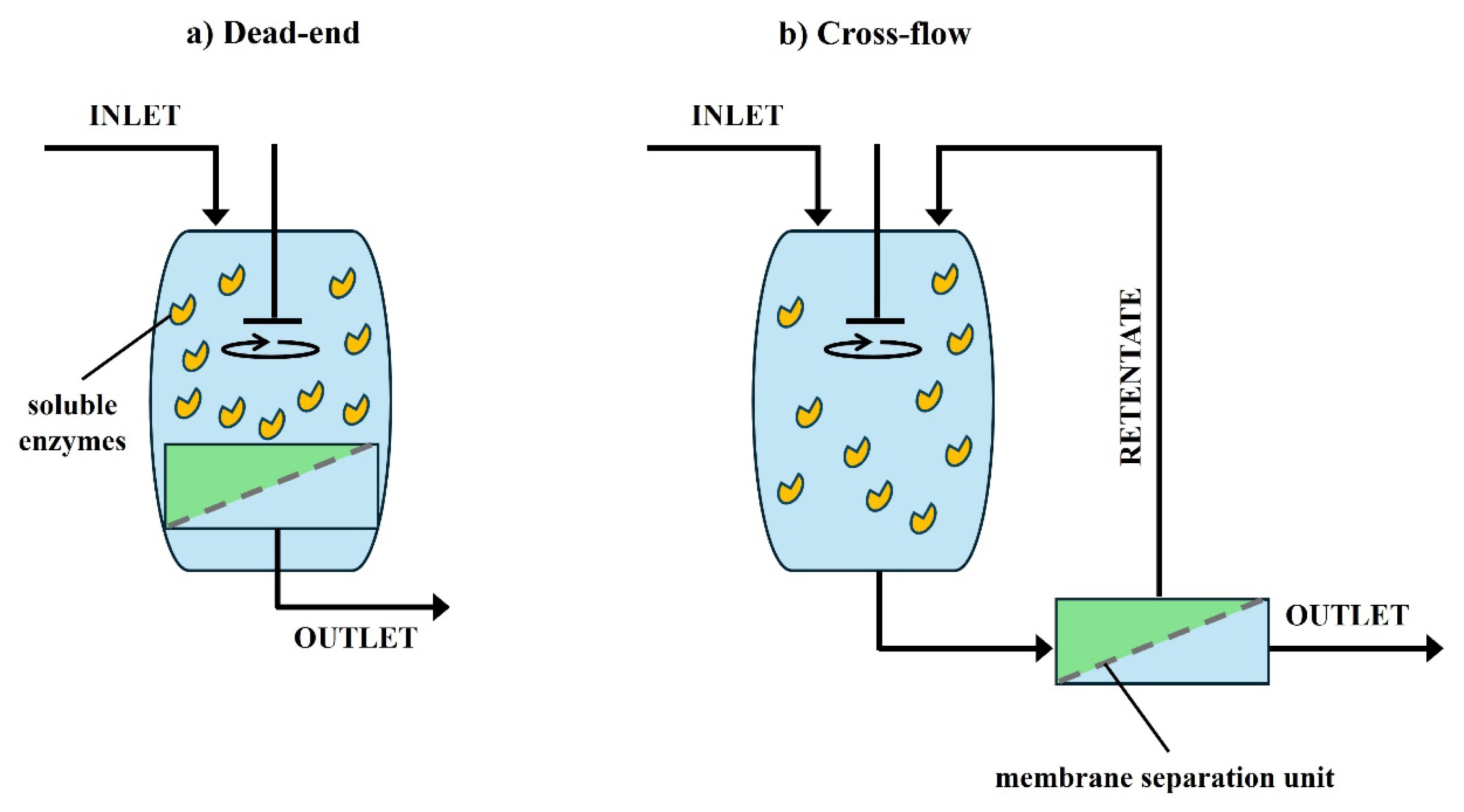
Further than facilitating product separation, membrane-based operations are particularly helpful in displacing thermodynamic equilibrium and improving synthesis yields. Table 1 presents a list of membrane-induced equilibrium-shifting strategies that have already been applied in combination with soluble (or immobilized) enzymes in order to intensify biocatalyzed reactions (among the selected biocatalytic transformations listed in Figure 1). Here, soluble enzymes were typically employed separately from the membrane devices (i.e., membranes were at the boundary or at the effluent side of enzymatic reactors, acting as separation units) as represented in Figure 2 and not in a one-pot manner. Note that other membrane-intensified transaminations employing heterogeneous biocatalysts were also reported [56, 57, 87, 88], yet these studies fall outside the scope of such reports as they involve whole cells (instead of enzymes) as biocatalysts. This list of examples shows the diversity of approaches and applications. We argue that the incorporation of enzyme into/onto membranes represents the next important step in the direction of intensification; as it is both more challenging and emerging, we discuss this in more detail in the following section.
Exhaustive list of recent (2010–2024) examples of enzyme combination with membrane technologies for the considered biocatalytic process intensification
| Reaction(s) | Substrate | Product | Enzyme | Immob. (carrier) | Membrane | Operation | Mode | Refs |
|---|---|---|---|---|---|---|---|---|
| Carboligation | Benzaldehyde + acetaldehyde | (S)-2-hydroxypropiophenone | Benzoylformate decarboxylase | /(free) | Polymeric (N.D., 10 kDa) | UF (dead end) | Flow | [89] |
| Cascade (De)hydrogenation | Octanone + D-glucose | R-octanol + D-gluconolactone | Alcohol dehydrogenase + glucose dehydrogenase | /(free) | N.D. (10 kDa) | UF (dead end) | Flow | [90] |
| Esterification | Fatty acids + glycerol | Monoacylglycerols | Lipase | Immob. (acrylic resin) | Ceramic (zeolite type T, N.D.) | Pervaporation | Recirculation | [71] |
| Esterification | Fatty acids + glycerol | Isopropyl esters | Lipase | Immob. (acrylic resin) | Ceramic (zeolite type T, N.D.) and polymeric (PVA, N.D) | Pervaporation | Recirculation | [91] |
| O-glycosylation | Resveratrol | Resveratrol O-glycosides | β-cyclodextrin–resveratrol | /(free) | Polymeric (N.D., 0.9 or 1.4 kDa) | NF | Recirculation | [92] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | /(free) | Polymeric (cellulose, 50 kDa) | UF + NF | Flow | [93 ] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | /(free) | Polymeric (PES, 10 kDa) | UF | Recirculation | [94] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | /(free) | Ceramic (TiO2, 20 kDa) | UF | Flow | [95] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | /(free) | Ceramic (50 kDa) | UF | Recirculation | [96] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | /(free) | Polymeric (cellulose acetate, N.D.) | UF | Recirculation | [97] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | /(free) | Polymeric (PES, 10 kDa) | UF | Recirculation | [98] |
| Transgalactosylation | Lactose + fructose | Lactulose | β-galactosidase | /(free) | Polymeric (PES, PS, cellulose acetate, fluoropolymers; 10 kDa) | UF (dead end) | Flow | [99, 100, 101] |
| Hydrolysis | Phenylethanol + vinyl butyrate | Phenylethyl butyrate + acetaldehyde | Lipase (in water–oil emulsions) | /(free) | Polymeric (PES, 10 kDa) | UF (dead end) | Flow | [102] |
| Hydrolysis | Starches | Cyclodextrin | Cyclodextrin glycosyltransferase | /(free) | Polymeric (cellulose, 10 kDa) | UF | Flow | [103, 104] |
| Hydrolysis | BSA | Bioactive peptides | Peptidase | /(free) | Polymeric (PES, 3 or 10 kDa) or ceramic (ZrO2/TiO2, 15 kDa) | UF | Recirculation | [105] |
| Hydrolysis | P. yezoensis protein | Bioactive peptides | Peptidase | /(free) | N.D. (3 kDa) | UF | Flow | [82] |
| Hydrolysis | Egg white protein | Bioactive peptides | Peptidase | /(free) | Polymeric (PES, 10 kDa) | UF | Flow | [106] |
| Hydrolysis | Casein | Bioactive peptides | Peptidase | /(free) | Polymeric (PES, 1–10 kDa) | UF | Recirculation | [107] |
| Hydrolysis | Corn gluten meal | Bioactive peptides | Peptidase | /(free) | Polymeric (cellulose, 1–5 kDa) | UF | Flow | [108] |
| Hydrolysis | Casein | Bioactive peptides | Peptidase | /(free) | Polymeric (PES, 10 kDa) and ceramic (Al2O3, 10 kDa) | UF | Flow | [109] |
| Hydrolysis | Wheat gluten | Protein hydrolysate | Peptidase | /(free) | Ceramic (hollow fibers Al2O3, 5 or 10 kDa) | UF | Flow | [110] |
| Hydrolysis | Sesame seeds | Bioactive peptides | Peptidase | /(free) | Polymeric (PES, 5 kDa) | UF | Flow | [111] |
| Hydrolysis | Milk | Bioactive peptides | Peptidase | /(free) | Polymeric (cellulose, 5 kDa) | UF | Flow | [112] |
| Hydrolysis | Whey | Inhibitory peptides | Peptidase | /(free) | Polymeric (PES, 3 kDa) | UF | Flow | [113] |
| Transamination | Pro-sitagliptin ketone + isopropylamine | R-Sitagliptin + acetone | Transaminase | /(free) | Polymeric (PSF, 10 kDa ) + dense PDMS | UF (for enzyme retention) + solvent extraction | Recirculation | [60] |
| Transamination | Benzyl acetone + isopropylamine | R-1-methyl-3-phenylpropylamine + acetone | Transaminase | /(free) | Polymeric (PP, N.D.) | Solvent extraction | Recirculation | [59] |
| Transamination | Acetophenone + isopropylamine | R-MBA + acetone | Transaminase | /(free) | Dense PDMS | Pervaporation | Batch | [58 ] |
N.D.—non-specified; PES—polyethersulfone; PSF—polysulfone; PS—polystyrene; BSA—bovine serum albumin; MBA—methylbenzylamine; PDMS—polydimethylsiloxane; UF—ultrafiltration; NF—nanofiltration; GOS—galacto-oligosaccharide.
3. Membrane-immobilized enzymatic reactors
The incentives for immobilizing enzymes to intensify biocatalytic processes are evident. Enzyme immobilization is a prerequisite to envisaging recovery and reuse. Immobilization usually tends to enhance enzyme stability and tolerance to organic solvents and to allow for the use of different reactor configurations [44], which are key features of industrial processes. It also paves the way for the integration of enzymes within heterogeneous catalysts, forming so-called hybrid chemoenzymatic catalysts that are excellent candidates to run intensified cascade reactions [114, 115, 116, 117, 118, 119]. A potential downside of enzyme immobilization is that enzymatic activity may—in some cases—be decreased through the immobilization process (due to active site blockage, for example). Nevertheless, free enzymes can also experience activity reduction over time due to heat and mechanical stresses during extended biocatalytic processes [120].
Immobilization on membranes is going one step further in the direction of process intensification, as it implies fixing the enzyme on a material that is itself functional in the sense that it is able to perform tailored compound separation. As mentioned in the previous section, the membrane can be used to remove products during reaction, to inject reagents in a controlled way, and so on. In some cases, the membrane itself can also be chemocatalytically active. However, prior to enzyme immobilization on a membrane, the membrane surface often needs to be functionalized or chemically modified. Indeed, appropriate membrane materials that are directly amenable to enzyme immobilization are very rare [8]. A plethora of different surface functionalization techniques (e.g., wet chemical modification, plasma or UV exposure) have been reported in the literature [8, 39, 121, 122], showing a variety of approaches depending on the substrate chemical nature. The implementation of such surface modifications depends on the type of enzyme immobilization (e.g., covalent grafting, electrostatic-assisted adsorption, site-specific immobilization through coordination) that is envisaged as well as the membrane operation that is targeted in the intensified biocatalytic process. Among the different existing strategies, silanization of inorganic membrane surface using (3-aminopropyl)triethoxysilane (APTES) and polydopamine (PDA) coating deposition on polymeric membranes have been employed in recent years [15, 17, 123, 124, 125, 126] to confer amino groups at their surfaces, serving as anchoring points for grafting. In these cases, glutaraldehyde (GA) is most conventionally used as a coupling agent between the enzyme and the functionalized membrane. Another general trend is to try and stabilize the immobilized enzymes via electrostatic interactions; polyelectrolytes such as polyethyleneimine (PEI) are often employed with this aim.
Table 2 presents an exhaustive list of examples of membrane-immobilized enzymes exploited for the production of fine chemicals and pharmaceuticals from among the selected biocatalytic transformations listed in Figure 1. Enzymes are immobilized either in the membrane (i.e., entrapped in the membrane pores) or onto the membrane surface. In these examples, the membrane can either be employed as a mere solid support (i.e., not exploited to perform membrane operations) or as a functional support forming an EMR that acts as a combined reaction–separation unit (Figure 3).
Exhaustive list of recent (2010–2024) examples of membrane-immobilized enzymes acting as simple heterogeneous membrane-immobilized biocatalysts (i.e., not performing membrane operations, operating in batch or in flow mode with or without recirculation) or as enzyme-membrane reactors (i.e., a reaction–separation unit) for the production of fine chemicals and pharmaceutical building blocks
| Reaction(s) | Substrate | Product | Enzyme(s) | Immob. (R. Sp. act) | Comments | Membrane | Separation | Mode | Refs |
|---|---|---|---|---|---|---|---|---|---|
| DKR | Ibuprofen ester | S-ibuprofen acid | Lipase | Entrapment (90%) | Retained 53% of initial sp. activity after 120 h of operation | Polymeric (PAN, 50 kDa) | Solvent extraction (interfacial; OP = isooctane) | Recirc. | [127, 128] |
| Esterification (lipophilization) | Hesperidin + lauric acid | Hesperidin laurate | Lipase | Covalent grafting + cross-linking (≈900%) | / | Polymeric (PDA + GA-modified PES, 30 kDa) | / | Batch | [129] |
| Esterification | Stearic acid + lauryl alcohol | Lauryl stearate | Lipase | CLEAs + entrapment (142%) | / | Polymeric (CLEAs in PVA matrix, on PVA/PES dense layer) | Pervaporation | Recirc. | [68] |
| Esterification | Ethanol + lactic acid | Ethyl lactate | Lipase | Entrapment (≈100%) | Preserved 90% of sp. activity upon 6 cycles (36 h of operation) | Polymeric (sodium alginate network) | Pervaporation | Recirc. | [130] |
| Esterification | Valeric acid + ethanol | Ethyl valerate | Lipase | Covalent grafting (76%) | / | Polymeric (PPC, 150 kDa) | / | Batch | [131] |
| Transesterification | Rac-phenylethanol | S-phenylethanol + R-phenylacetate | Lipase | Covalent grafting (/) | / | Ceramic (ZrO2, 300 kDa) | / | Recirc. | [132] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | Covalent grafting (≈75%) | Retained 50% of its initial sp. activity after 30 days of storage | Polymeric (GA-modified PVDF) | Filtration | Recirc. | [133, 134] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | Covalent grafting + cross-linking (/) | / | Polymeric (PES, 5–50 kDa) and NF (0.4 kDa) | UF or NF | Flow | [135, 136] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | Covalent grafting (104%) | Maintained 54% of its initial sp. activity after 20 cycles | Polymeric (GO + APTES-modified electrospun PS nanofibers) | / | Batch | [137] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | Adsorption (95%) | / | Mixed matrix (PSF + ZrO2, 13.8 kDa) | / | Batch | [138] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | Covalent grafting (/) | Maintained 91% of its sp. activity after 16 h of operation | Magnetic-responsive on polymeric membrane (PES, UF 50 kDa) | UF | Flow | [139] |
| Transgalactosylation | Lactose | GOS | β-galactosidase | Covalent grafting + cross-linking (140%) | / | Polymeric (PEI + GA-modified PSF, 10 kDa) | UF | Recirc. | [140] |
| Hydrolysis | Oleuropein | Oleuropein aglycone | β-glucosidase | Entrapment (≈100%) | Activity was unchanged after 30 h of continuous operation | Polymeric (PSF and cellulose, 30 kDa) | Solvent extraction of product (OP = limonene) | Flow | [141, 142, 143, 144] |
| Hydrolysis | Oleuropein | Oleuropein aglycone | β-glucosidase | Covalent grafting (≈100%) | / | Ceramic (APTES + GA-modified Al2O3 capillary, 30 kDa) | Solvent extraction of product (OP = ethyl acetate) | Flow | [45] |
| Hydrolysis | Dextran | Oligodextran | Dextranase | Covalent grafting (/) | / | Polymeric (tannic Acid + APTES-modified PES (30 kDa) or cellulose (10 kDa)) | UF | Flow | [145] |
| Hydrolysis | Dextran | Oligodextran | Dextranase | Adsorption (/) | / | Polymeric (PES, 20 or 30 kDa) | UF | Flow | [75] |
| Hydrolysis | Wheat gluten | Protein hydrolysate | Peptidase | Entrapment (/) | / | Polymeric (UF) (PES, 10 kDa) | Filtration (dead end) | Flow | [146] |
| Hydrolysis | BSA | Bioactive peptides | Peptidase | Electrostatic interactions | / | Polymeric (PDA + PEI-modified PES, 50 kDa) | UF | Flow | [147] |
| Hydrolysis | BSA | Bioactive peptides | Peptidase | Covalent grafting (/) | 50% initial sp. activity retained after 6 cycles of 1 h | Polymeric (EDC/NHS-modified PVDF, nylon 6,6, chitosan composite) | / | Batch | [148] |
| Hydrolysis | Whey protein | Bioactive peptides | Peptidase | Electrostatic interactions (80%) | / | Polymeric (PDA + PEI-modified PES, 30 kDa) | / | Batch | [149] |
| Oligomerization | Rutin | Oligorutin | Laccase | Entrapment (/) | 90% of initial sp. activity after 3 cycles of 24 h | N.D. (10 kDa) | Filtration (dead end) of product | Batch | [150] |
| Oxidation | Tyrosine | L-DOPA | Tyrosinase | Entrapment (183%) | Unaltered initial sp. activity for 30 h of continuous operation | Polymeric (polyamide, 20 kDa) | UF | Flow | [151] |
| Oxidation | Tyrosine | L-DOPA | Tyrosinase | Covalent grafting (276%) | / | Ceramic (GA-modified NaY zeolite) | / | Recirc. | [152] |
| Oxidation | Tyrosine | L-DOPA | Tyrosinase | Covalent grafting (87%) | Unaltered initial sp. activity after 5 catalytic cycles (≈24 h operation) | Polymeric (DAB + GA-modified PVDF) | / | Batch | [153] |
| Transamination (deamination) | S-MBA + pyruvate | Acetophenone + L-alanine | Transaminase | Coordination (His-tag) (23%) | Retained productivity after 5 days of continuous operation was 81% | Polymeric blend (Cu-functionalized fibers) | / | Flow | [154] |
| Transamination (deamination) | S-MBA + pyruvate | Acetophenone + L-alanine | Transaminase | Covalent grafting + His-tag driving (43.6%) | / | Polymeric blend (Co-derivatized PCADE/PVDF) | / | Batch | [155] |
| Cascade (transamination + (de)hydrogenation) | Cinnamaldehyde | Cinnamylamine | Transaminase + alanine dehydrogenase + formate dehydrogenase | EPC entrapment | / | N.D. (12 kDa dialysis membrane) | / | Batch | [156] |
| Transamination (kinetic resolution) | Rac-BMBA + pyruvate | S-BMBA + BAP + D-alanine | Transaminase | Covalent grafting (85%) | Unaltered initial sp. activity after 8 catalytic cycles (≈16 h operation) | Polymeric (PDA + GDE + PEI-modified PP) | / | Batch | [157] |
R. Sp. act.—recovered specific activity (with respect to free enzymes); Recirc.—recirculation; N.D.—non-determined; DKR—dynamic kinetic resolution; OP—organic phase; PAN—polyacrylonitrile; PDA—polydopamine; GA—glutaraldehyde; PVA—polyvinyl alcohol; PVDF—polyvinylidene fluoride; CLEAs—cross-linked enzyme aggregates; PES—polyethersulfone; PSF—polysulfone; PS—polystyrene; GO—graphene oxide; PPC—polypropylene chloride; APTES—(3-aminopropyl)triethoxysilane; EDC/NHS—(1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide); BSA—bovine serum albumin; PP—polypropylene; PEI—polyethyleneimine; DAB—1,4-diaminobutane; PCADE—polycarvone acrylate di-epoxide; Rac-BMBA—racemic bromo-α-methylbenzylamine; BAP—bromoacetophenone; UF—ultrafiltration; NF—nanofiltration; GOS—galacto-oligosaccharide; EPC—enzyme–polyelectrolyte complex; GDE—glycerol diglycidyl ether.
Schematic representation of membrane-immobilized enzymes acting as simple heterogeneous membrane-immobilized biocatalysts (i.e., not performing membrane operations) operating in (a) batch or (b) recirculating mode or as enzyme-membrane reactors (i.e., reaction–separation unit) in (c) compound separation (e.g., via size-exclusion, ion-exchange, etc.) or (d) solvent extraction operations. Note that when omitting the recirculations, (c) and (d) become continuous-flow operations.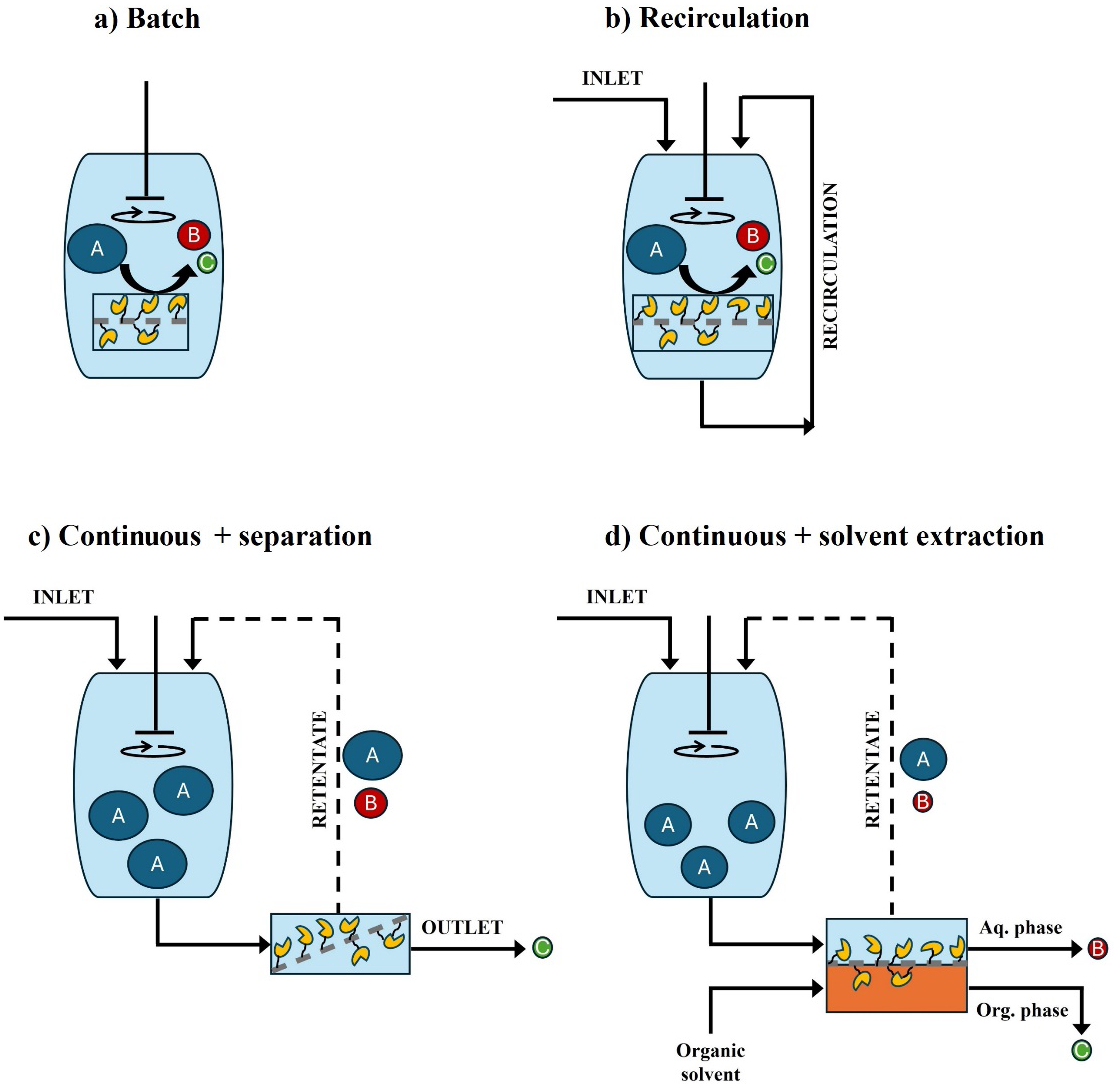
Among the (recirculating) flow operations listed in Table 2, the dynamic kinetic resolution of ibuprofen ester is a prominent example of how the use of a lipase-membrane reactor can intensify a chemoenzymatic process and push such technology to the next level (Figure 4) [127, 128]. In this example, a lipase was entrapped in the porosity of a polyacrylonitrile (PAN) membrane contactor. Such an EMR allowed simultaneously performing the continuous ibuprofen ester hydrolysis and the resulting S-ibuprofen product separation (i.e., membrane-assisted extraction toward an aqueous phase). The unreacted R-ibuprofen ester was then recirculated into a chemocatalytic racemization unit (Amberlyst OH− coated resin) [127, 128]. The resin is an excellent racemization catalyst due to its strong basicity and to its macroreticular network, which provides a high surface-to-volume ratio. Its large pores allow bulky molecules, such as (R)-ibuprofen ester, to diffuse effectively. This feature combined with its strong basic properties enables the rapid racemization of the ester through the ketol–enol tautomerism mechanism [158]. The racemized ibuprofen ester substrate is then recirculated to the organic tank and fed again to the enzyme-membrane module. Compartmentalization of the heterogeneous bio- and chemocatalysts allows protecting the lipase from the basic catalyst and from inhibition that would otherwise happen under the effect of unreacted substrate and by-product (2-ethoxyethanol, which is absorbed by the OH− resin).
Schematic representation of the dynamic kinetic resolution of ibuprofen ester catalyzed by lipase-membrane reactor (Uzir et al., 2011) [127, 128].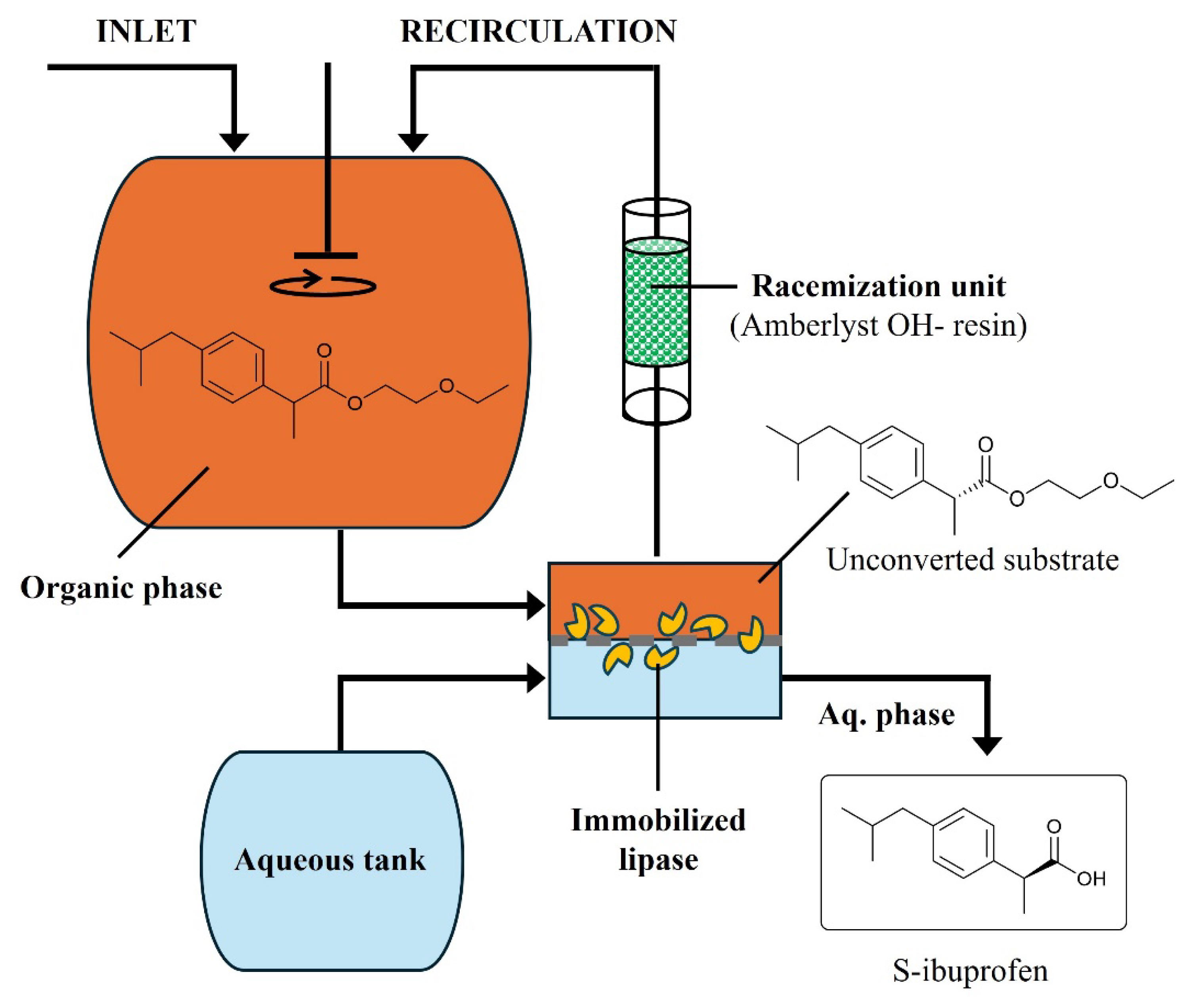
Interestingly, the same kind of approach (membrane-assisted extraction) allowed achieving high product purity in the synthesis of oleuropein aglycone (an important antioxidant) through enzymatic hydrolysis in an EMR. To this end, β-glucosidase was covalently immobilized onto ceramic membrane surfaces, and employed to hydrolyze oleuropein into oleuropein aglycone and glucose (in aqueous medium; see Figure 5) [45]. Given the differences in polarities between oleuropein aglycone and the other compounds involved in the process, membrane-assisted solvent extraction was chosen to intensify the process. The aglycone produced in the membrane contactor was continuously extracted with an organic solvent (ethyl acetate), which allowed its separation and purification from the reaction medium. An identical strategy was implemented with polymeric (polysulfone [PSF]) membranes containing entrapped β-glucosidase in its pores and using limonene as the organic solvent [141, 142, 143]. Yet, the use of such polymeric materials as aqueous–organic contactors for such solvent extraction processes might be less suitable than ceramic membranes given their limited chemical stability.
Schematic representation of oleuropein aglycone production through simultaneous hydrolysis and solvent extraction using an enzyme-membrane reactor (Giorno et al., 2018) [45].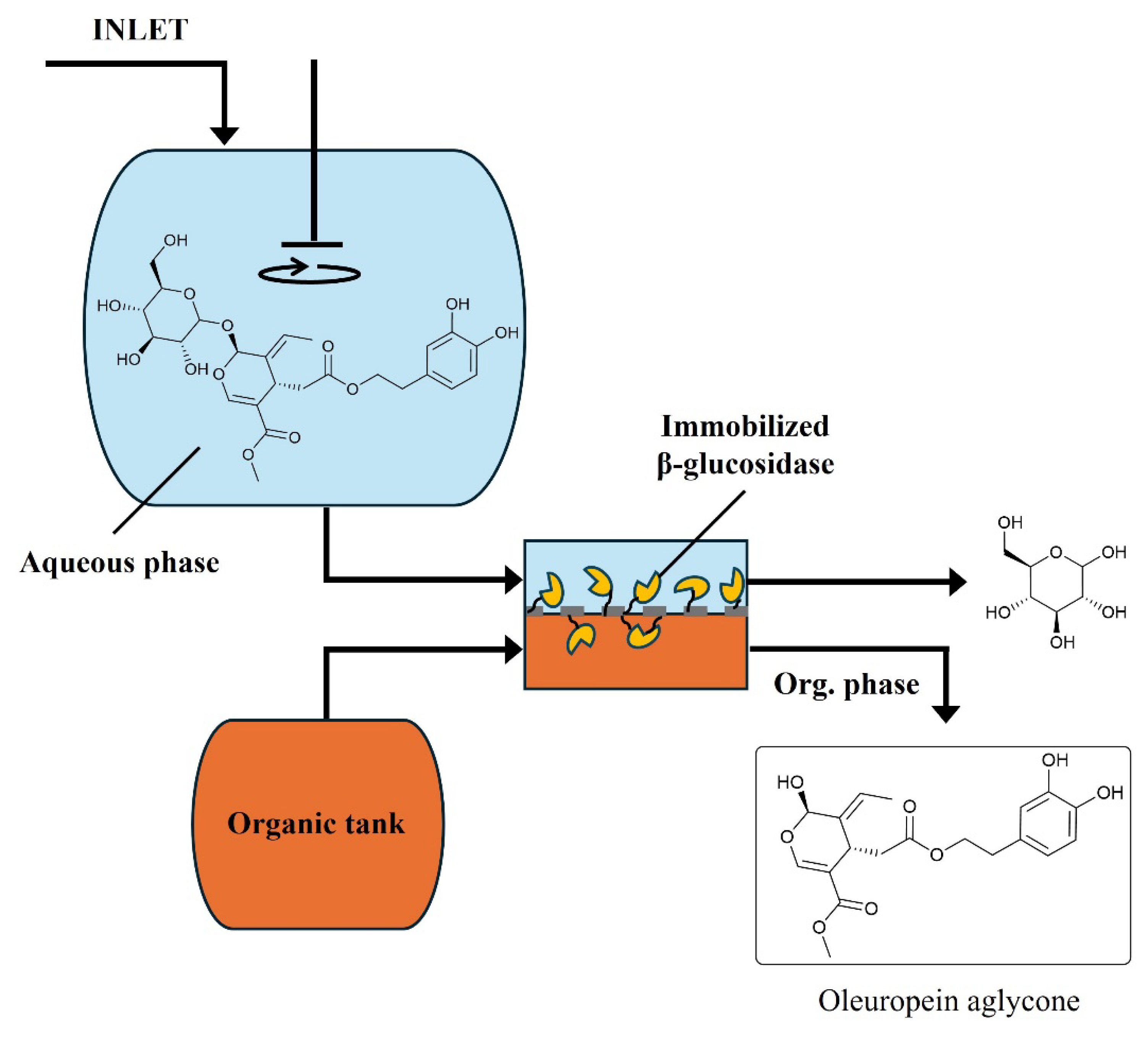
Another elegant example of membrane-immobilized enzyme reactor application is the in situ removal of water through pervaporation as in the intensified production of lauryl stearate (which is often used as an emollient and excipient in cosmetics and pharmaceuticals [159]) [68]. In this work, a “sandwich-like” membrane structure, made of a porous lipase–polyvinyl alcohol (PVA) catalytic layer coated on a PVA/polyethersulfone (PES) matrix, was employed as an enzymatic-pervaporation reactor. Interestingly, the immobilized lipase exhibited enhanced specific activity and stability compared to the soluble enzyme. This improvement was attributed to the hydrophilic microenvironment created by the hydrophilic PVA carrier, which probably absorbs the water produced during the enzymatic esterification, thereby shielding the lipase from adverse effects like enzyme inactivation. The implementation of this catalytically active membrane in a pervaporation reactor resulted in a substantial increase in conversion (from 60% to 83%) compared to the equilibrium-limited esterification process (conducted without pervaporation). A similar strategy was applied for the synthesis of ethyl lactate (a pharmaceutical-grade excipient [160]) in which a lipase entrapped in a sodium alginate membrane was the enzymatic-pervaporation unit [130].
Immobilizing enzymes such as β-galactosidase, peptidase, and dextranase on membranes featuring adequate MWCO is also a practical tool to intensify the production of value-added compounds with tailored molecular weight via size-exclusion operations. For example, the production of GOSs from lactose is hindered by hydrolytic side reactions, and by enzyme inhibition caused by such monosaccharide formation and accumulation. To improve reaction yield and productivity, continuous coupled GOS purification and monosaccharide elimination from the reaction mixture is of particular interest. To this end, UF membranes are employed to simultaneously host the enzymes and perform product separation [133, 134, 139]. However, considering the molecular weight distribution of the carbohydrate mixture obtained after enzymatic reaction with lactose, NF also appears as an effective operation for GOS purification. Bhattacharjee et al. [135] immobilized β-galactosidase on NF (0.4 kDa) and UF (5–50 kDa) membranes via covalent grafting and compared their catalytic performance toward continuous membrane-assisted GOS production. A higher GOS yield was obtained when using the enzyme-loaded NF membrane, which aligns with the enhanced permeation of monosaccharides and improved retention of GOS observed with the NF membrane (compared to enzyme-immobilized UF membrane). Furthermore, the NF process resulted in substantial retention of the lactose substrate, providing extended residence time and greater interaction between the substrate and the immobilized enzyme, which contributed to increased GOS production. Similarly, Pinelo et al. [145] leveraged the use of membrane-immobilized dextranase in order to selectively permeate the produced oligodextran featuring the desired molecular weight (5–8 kDa) and avoid overdegradation of products. In the latter study, the membrane unit consisted of a three-layer system, composed of polystyrene (PS) electrospun nanofibers placed between a pristine commercial membrane (PES, 30 kDa or cellulose, 10 kDa) and a macroporous support layer. To make them amenable to enzyme grafting, the PS nanofibers were functionalized with tannic acid and APTES prior to enzyme immobilization. This approach significantly improved the catalytic performance (i.e., constant productivity over time and controlled saccharide molecular weight in permeate) with respect to the EMR employing free enzymes (which showed rapid deactivation over time).
The use of an EMR in the enzymatic production of L-DOPA (which is a drug commonly used for the treatment of Parkinson’s disease [161]) from tyrosine offers numerous advantages. By continuously supplying L-tyrosine to the biocatalytic system at a controlled rate, the EMR helps prevent enzyme inhibition and maintain a constant substrate concentration. Additionally, it enables product separation, which is of particular interest as some by-products formed through L-DOPA spontaneous overoxidation (e.g., dopamine and dopaquinone) tend to polymerize and complicate purification. The EMR configuration facilitates the separation of these by-products via membrane filtration, ensuring continuous purification of L-DOPA on the permeate side. However, L-DOPA spontaneous oxidation remains problematic as it hampers the yields and productivity of such an enzymatic process. To address this, Donato et al. [151] attempted to continuously introduce ascorbic acid [162], a reducing agent, while simultaneously removing the biocatalytically produced L-DOPA. To this end, the authors immobilized tyrosinase on a polyamide tubular membrane sponge layer and implemented the EMR in cross-flow configuration. The result was that the continuous removal of L-DOPA from the reaction environment along with the antioxidant effect of ascorbic acid further enhanced L-DOPA productivity, reaching 1.60 U⋅mg−1, which is higher than that of other processes reported in the literature (where product separation and ascorbic acid addition were not applied).
4. Conclusion
Among the existing methods capable of intensifying biocatalytic processes in an efficient way, the coupling of enzymes with membrane technology in EMRs is emerging as a highly potential method. Such integrated systems, simultaneously combining biocatalytic reactions and membrane operations, allow for more productive flow processes displaying enhanced product yield (by increasing the conversion and/or the selectivity of the process, via, e.g., membrane-assisted product separation) and boosted enzyme kinetics (by preventing enzyme inhibition via controlled reagent introduction). Through this review, we aim at providing the recent trends and examples of the use of EMR for the intensified production of high-value chemicals and, in particular, APIs. Among the reviewed EMR configurations, we notably turn our attention toward membrane-immobilized enzymatic reactors. We argue that the implementation of such novel hybrid reactors that simultaneously host the immobilized enzymes and perform in situ product separation will catalyze further advances in the field of green fine chemical and pharmaceutical manufacturing. Arguably, merging the fields of biocatalysis and organic synthesis on the one hand and process engineering on the other hand, resulting in EMRs, is crucial to both academic and industrial developments.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.





 CC-BY 4.0
CC-BY 4.0