1. Introduction
The total global yield of olive oil for the 2019/2020 season is estimated to reach 3.67 million tons as compared to 3.13 million tons for the previous season [1]. More specifically, according to reports by the European Union [2], recent forecasts indicate that “Spanish olive oil production is projected to reach 1.76 million tons at the end of 2018/19 season, up from 1.39 million tons the preceding season”. 226,000 tons is expected to be produced by Italy (which is 50% less from the previous year’s production). This is less than Greece’s projected 248,000 tons (which is 35% less compared with the previous year’s production for Greece). The main issues faced by Italy are attributed to climate change and Xylella fastidiosa infestation in some olive groves in the east.
According to many researchers [3, 4, 5, 6, 7, 8], olive mill waste (OMW) management is an issue that has been of concern to the global research community for many years. This is because OMW management has significant environmental, social, and economic implications. Among the main issues for the treatment of OMW is the fact that OMW contains hazardous wastes having high concentrations of phenolic compounds (up to 10 g⋅L−1 depending on the type and origin of the effluent) that are difficult to biodegrade. Due to strong seasonality (between October and March), OMW cannot be transferred in a central unit due to the size of olive mills, which usually are spread close to agricultural areas. Moreover, the mills consist of plants with a daily OMW flow rate between 10 and 100 m3 and are distributed over large areas [3, 4].
In recent years, apart from the significant achievements regarding different management technologies [9, 10], there has been great improvement in the awareness level of citizens, especially of olive mill owners and farmers, in terms of rational management, minimization of environmental impacts, and reuse of OMW in agriculture. Moreover, the legislative framework of all Mediterranean countries, which are the main olive oil producers globally, permits the use of olive mill wastewater (OMWW) and also the remaining sludge after OMWW preliminary treatment for fertigation and use as a soil additive, respectively. For example, the Greek Common Ministerial Decision No. 3924 of 7th December 2016 sets the framework for OMW application on soils and defines the terms and preconditions for landspreading. According to this decision, and considering that all preconditions are fulfilled, the maximum permitted waste amount is 80 m3/ha/y. Furthermore, for Italy and according to Law No. 574 of 1996 with regard to the agronomic use of sewage sludge and other wastes such as OMWW, the maximum amount is 50 m3/ha/y for OMWW generated by traditional mills (discontinuous extraction systems) and 80 m3/ha/y for vegetable water generated by centrifugal extraction (continuous extraction systems).
Through research studies, it became clear that there are significant benefits from the reuse of OMW in agriculture [11, 12, 13, 14]. However, despite the proven significant benefits in terms of environmental problems caused by nonrational OMW management (e,g., uncontrolled discharge into aquatic systems and soil as discussed in the technical study of the LIFE PROSODOL project [15]), it should be clear that it is ultimately waste reuse. For this reason, if landspreading has been decided for cultivation purposes, care should be taken not only to avoid phytotoxic effects on cultivated plants but also for the protection of soil and underground and surface water bodies [16, 17]. As mentioned by several researchers [13, 14, 18, 19], OMW can be a valuable source of nutrients, which has a direct effect on improving soil quality [20] affected due to the presence of phenol compounds, pH variations, and salts. López-Piñeiro et al. [21] suggested that OMW contains more than 90% organic matter (OM) and is free from heavy and toxic metals as well as pathogenic microorganisms. As a result, it enhances soil properties, especially those of soils containing limited OM. Moreover, it increases humified fractions, which constitute a major source of phytonutrients. Other studies have shown that the disposal of OMW on soils affect all soil properties, while more severe impacts have been observed for soil electrical conductivity (EC), OM, nitrogen, phosphorus, potassium, polyphenols, and iron [13, 18, 19, 22]. These properties have also been proposed by Doula et al. [22] as parameters that can be used as indicators for soil quality assessment in areas where OMWs are discharged. They were also considered for soil monitoring in Greek Common Ministerial Decision No. 3924 of 7th December 2016 (except iron). However, Di Bene et al. [23] stated that OMW application may affect the biological and chemical properties of soil. However, OMW can be considered nontoxic after a certain period of application, especially on healthy soil. It is therefore important that all necessary measures are taken to avoid soil burden even from elements that are not considered to be pollutants in the classical sense of the term as for example, potassium and iron. A protective measure is the calculation of the appropriate waste amount to be distributed on soil by considering soil composition and nutritional needs of cultivated plants [24]. This is, however, a stricter but a safer measure than the process imposed by the laws of the Mediterranean countries, which define a specific amount of OMW for landspreading (e.g., 80 m3/ha/y).
Another protective measure is the use of active materials as soil additives, such as natural zeolites, which can mitigate the effects of waste addition to soil. The use of natural zeolites as soil additives has been extensively studied over the past several years by many researchers worldwide [13, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35]. Its use has been proven not only beneficial in soils contaminated by heavy metals but also as a slow-release fertilizer [36]. Its ability to bind and release potassium and ammonium according to crop requirements and also to bind toxic elements, such as heavy metals, may be applicable in the case of OMW reuse in agriculture. However, as Doula et al. [37] has reported, besides the positive effects, sodium release during the first few weeks after its application on soil may cause severe problems such as phytotoxicity and overload of sodium ions in the soil if these issues are not taken into consideration during use.
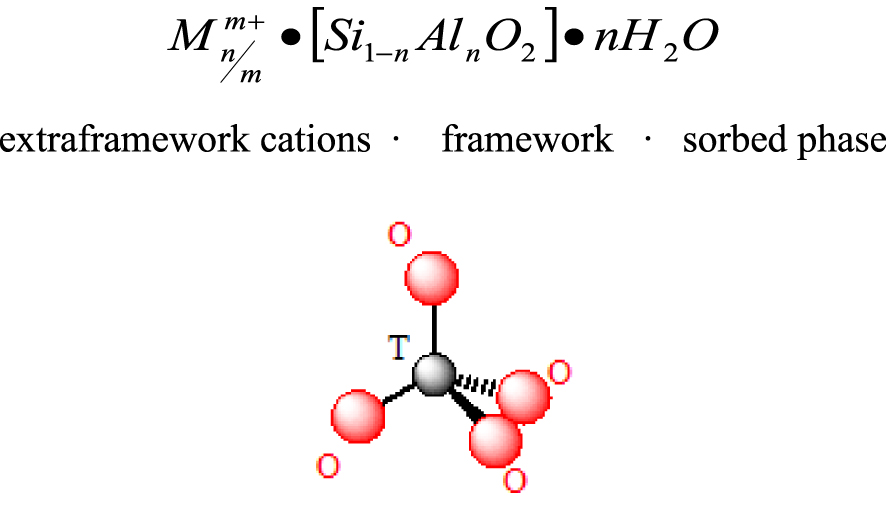
Primary building unit of the zeolite framework.
The aim of this study is to identify the positive and negative effects of clinoptilolite as a soil additive together with residual sludge from OMW for the cultivation of pepper plants. The research focused on the effects on basic soil properties caused by the presence of these two materials in the cultivation substrate. This work also aims at determining the optimum mixing ratio that would be of low cost and ensuring high production without soil degradation and with a low risk of nutrient loss through leaching.
2. Materials and methods
2.1. Experimental design
To study the effects of OMW sludge and clinoptilolite addition on cultivated soil, an 11-week pot experiment was conducted under greenhouse conditions. The greenhouse system is considered a protected plant growth environment and further was chosen to avoid weather conditions such as rain and wind. No control of temperature, ventilation or relative humidity was integrated into the experimental process to better simulate field conditions. Pepper plant (Capsicum annuum) was selected for conducting the experiment, first because it is a species not so commonly found in the waste management research area and second because it is a rather nondemanding vegetable in terms of soil and management requirements. During the experiment, pepper seedlings were transplanted and grown onto different substrates containing combinations of 0%, 2.5%, and 5.0% zeolite and 0%, 2.5%, and 5.0% of OMW sludge (v/v) (Table 1). Each treatment was composed of 12 replicates arranged in a split-plot design using zeolite as the main treatment material. Plants were irrigated twice a week, and leachates were collected on a weekly basis and further analyzed. After estimating the nutrient requirements, the plants were fertilized during the 10th week of the experiment by adding 3.6 g of nitrogen and phosphorus and 5.4 g of potassium to each pot and by using a commercially available fertilizer. After the completion of the experiment, the substrates were collected and analyzed to evaluate OMW and clinoptilolite impacts on soil properties after harvesting.
Sample preparation
| OMW (% v/v) | Clinoptilolite addition (% v/v) | |
|---|---|---|
| Z0AP0 | 0 | 0 |
| Z0AP1 | 2.5 | 0 |
| Z0AP2 | 5 | 0 |
| Z1AP0 | 0 | 2.5 |
| Z1AP1 | 2.5 | 2.5 |
| Z1AP2 | 5 | 2.5 |
| Z2AP0 | 0 | 5 |
| Z2AP1 | 2.5 | 5 |
| Z2AP2 | 5 | 5 |
2.2. Clinoptilolite
The natural zeolite used in this study was clinoptilolite (Na0.2K0.6Mg0.7Ca2.0Al6.2Si29.8O72 ⋅ 19.6H2O), which originates from northern Greece. The material has also been used for experiments in previous studies. Moreover, its formula, X-ray diffraction spectrum, and physicochemical properties are well known and have been already published [25, 33, 37, 38].
2.3. OMW sludge
The OMW sludge was obtained in February 2017 from a three-phase olive mill located in Peloponnese, Greece. The sludge was collected after the precipitation of OMW for 2 weeks in a tank. The main chemical parameters of OMW, namely pH, EC, total N, K, P, Na, Mg, Fe, Cu, and Zn, were determined in triplicate by using established methodologies [39, 40, 41]. The total phenol content was determined using the Folin–Ciocalteu method [42].
2.4. Analyses of soil and leachates
Leachates were collected on a weekly basis in plastic containers. They were further analyzed directly in the liquid phase after filtration for pH, EC, polyphenols, Na, and K. Analyses of soil and substrates were carried out using standard methodologies. The particle size distribution was estimated using the Bouyoucos method [43]; pH and EC were measured from a paste extract [44]; OM was determined by dichromate oxidation [45]; carbonates were estimated by using the Bernard calcimeter [44]; the total N was calculated by the Kjeldahl method [46]; the available phosphorus was estimated using sodium hydrogen carbonate extraction [47], exchangeable K, Na, Ca, and Mg using BaCl2 extraction [48], and available Mn, Fe, Cu, and Zn by DTPA extraction [49]. Soil B was extracted by boiling water using the azomethine-H method [44]. Furthermore, methanol-extractable phenol compounds were quantified through the Folin–Ciocalteu colorimetric method [42].
2.5. Instrumentation
For the measurement of K and Na, a Sherwood (Corning) 410 Flame Photometer was used. For the estimation of Ca, Mg, Cu, Fe, and Zn, a Varian SpectrAA 220 Atomic Absorption Spectrometer was utilized. Polyphenols and phosphorus were estimated by using a Cary UV-Vis Spectrophotometer.
3. Results and discussion
Table 2 presents the physical and chemical properties of the material. This material is very rich in OM. Moreover, it has a low pH, and it is also rich in K, N, Fe, Cu, and Zn. The OMW contains OM up to 97 ± 1.17%. It has a pH of 4.10 ± 0.12 (slightly acidic). The concentrations of polyphenols are 0.32 ± 0.08%. Table 3 lists physicochemical properties of the soil used as the basic material for the formation of different substrates in this study. The soil is characterized by a low OM concentration (5.65 ± 1.12%) and a pH of 7.39 ± 0.05 (neutral). The exchangeable capacity of K, Ca, Mg, and Na is very good.
Physical and chemical properties of the OMW sludge
| Parameters | Value |
|---|---|
| Organic matter (%) | 97 ± 1.17 |
| Electrical conductivity (mS/cm) | 2.6 ± 0.05 |
| pH | 4.10 ± 0.12 |
| Polyphenols (%) | 0.32 ± 0.08 |
| Nitrogen (%) | 0.51 ± 0.12 |
| Potassium (%) | 1.10 ± 0.04 |
| Calcium (%) | 0.11 ± 0.02 |
| Magnesium (%) | 0.05 ± 0.02 |
| Sodium (%) | 0.04 ± 0.02 |
| Phosphorus (%) | 0.05 ± 0.03 |
| Iron (mg/kg) | 41.12 ± 3.41 |
| Copper (mg/kg) | 12.02 ± 1.33 |
| Zinc (mg/kg) | 4.30 ± 0.91 |
Physical and chemical properties of soil used for the formation of substrates
| Parameters | Value |
|---|---|
| Texture | Sandy loam |
| pH | 7.39 ± 0.05 |
| Electrical conductivity (mS/cm) | 1.22 ± 0.11 |
| CaCO3 (%) | 28.32 ± 5.61 |
| Organic matter (%) | 5.65 ± 1.12 |
| Total nitrogen (mg/g) | 3.47 ± 0.58 |
| Exchangeable K (cmol(+)/kg) | 0.80 ± 0.22 |
| Exchangeable Ca (cmol(+)/kg) | 30.59 ± 4.92 |
| Exchangeable Mg (cmol(+)/kg) | 1.87 ± 0.45 |
| Exchangeable Na (cmol(+)/kg) | 0.11 ± 0.02 |
| Available P (mg/kg) | 33.19 ± 7.82 |
| Available Fe (mg/kg) | 8.86 ± 2.23 |
| Available Cu (mg/kg) | 1.27 ± 0.32 |
| Available Zn (mg/kg) | 18.02 ± 4.31 |
| Polyphenols (mg/kg) | 114.98 ± 14.91 |
Pepper cultivation on substrates consisting of soil, clinoptilolite (Z), and sludge from olive processing (AP) causes changes in the substrate’s physicochemical properties. Results obtained from this study clearly demonstrate the effect of each of the substrate’s components as well as their combined synergistic effects on the substrate.
Variations in physicochemical characteristics of treated samples
| Samples | EC (mS/cm) | Total N (mg/kg) | Organic matter (%) | Polyphenols (mg/kg) | Available | Exchangeable | ||
|---|---|---|---|---|---|---|---|---|
| B (mg/kg) | Fe (mg/kg) | Na (cmol(+)/kg) | K (cmol(+)/kg) | |||||
| Z0AP0 | 1.29 ± 0.03 | 2.55 ± 0.39 | 4.12 ± 0.36 | 118.02 ± 13.23 | 1.10 ± 0.08 | 9.41 ± 2.12 | 0.11 ± 0.02 | 0.91 ± 0.07 |
| Z0AP1 | 1.04 ± 0.02 | 2.88 ± 0.41 | 5.98 ± 0.22 | 169.85 ± 19.96 | 1.57 ± 0.11 | 14.79 ± 3.15 | 0.77 ± 0.08 | 1.12 ± 0.11 |
| Z0AP2 | 1.38 ± 0.05 | 3.02 ± 0.63 | 7.02 ± 0.64 | 185.11 ± 21.07 | 1.62 ± 0.23 | 16.80 ± 2.09 | 0.89 ± 0.06 | 1.54 ± 0.18 |
| Z1AP0 | 1.91 ± 0.07 | 2.81 ± 0.27 | 4.10 ± 0.19 | 127.56 ± 17.94 | 1.21 ± 0.15 | 9.61 ± 2.08 | 0.99 ± 0.07 | 1.15 ± 0.13 |
| Z1AP1 | 1.76 ± 0.07 | 2.73 ± 0.58 | 6.22 ± 0.21 | 167.05 ± 22.12 | 1.47 ± 0.20 | 17.03 ± 3.11 | 1.01 ± 0.08 | 1.61 ± 0.17 |
| Z1AP2 | 1.79 ± 0.08 | 2.95 ± 0.64 | 7.30 ± 0.66 | 189.29 ± 27.81 | 1.69 ± 0.19 | 18.49 ± 4.09 | 1.14 ± 0.05 | 1.89 ± 0.23 |
| Z2AP0 | 2.39 ± 0.11 | 2.17 ± 0.29 | 4.19 ± 0.31 | 129.96 ± 17.33 | 1.21 ± 0.11 | 7.92 ± 1.98 | 1.62 ± 0.12 | 1.67 ± 0.12 |
| Z2AP1 | 2.27 ± 0.07 | 2.91 ± 0.33 | 6.23 ± 0.47 | 178.21 ± 26.33 | 1.53 ± 0.18 | 17.88 ± 3.51 | 1.44 ± 0.11 | 1.80 ± 0.21 |
| Z2AP2 | 2.32 ± 0.12 | 2.63 ± 0.19 | 7.49 ± 0.55 | 187.01 ± 30.15 | 1.76 ± 0.22 | 18.93 ± 3.01 | 1.40 ± 0.08 | 2.22 ± 0.34 |
3.1. Substrates with soil and clinoptilolite
In the case of Z1AP0 and Z2AP0, it is clear from Table 4 that an increase in the Z percentage of the substrate led to an increase in the EC. For the case of 5% Z addition, the EC exceeded the threshold of 2 mS/cm [11, 16]. The increase in EC recorded for these two treatments was the highest compared to all other treatments with the same Z percentage (i.e., Z1AP1, Z1AP2 and Z2AP1, Z2AP2), indicating that the addition of AP improves the substrate and positively addresses the issue of salt increase.
The increase in the EC of the substrates is due to the ions present in zeolite exchangeable sites and mainly Na+ as seen from the results in Table 4. The table lists the concentrations of exchangeable Na for all treatments.
Compared to the substrate consisting of only soil (Z0AP0), exchangeable Na was approximately 8 and 13 times higher for 2.5% (Z1AP0) and 5% (Z2AP0) of clinoptilolite addition, respectively. The increased sodium concentrations were also recorded during a soil remediation field experiment conducted with the addition of clinoptilolite to the soil at different percentages up to 10% [13]. In this case, it was observed that exchangeable sodium was significantly increased in the first two months after the zeolite application. After this period, limited Na amounts were detected in the pilot soils.
About plant harvesting (Figure 2), the Z2AP0 treatment yielded the worst result, probably due to sodium. As is known, the concentration of excessive salts may restrict plant growth and productivity and lead to plant death [50]. Two mechanisms have an impact on plant growth: osmotic stress and ionic toxicity. In the presence of excessive salt content, the osmotic pressure of the soil solution becomes higher than that in the plant cells, inhibiting water uptake by plants. Furthermore, ionic toxicity may arise when the salt concentration is imbalanced inside cells, inhibiting cellular processes and metabolism [51]. Sodium ions at the root zone inhibit nutrient uptake (e.g., potassium) as well as the enzymatic activities within cells. It was reported that sodium may cause stress at concentrations higher than 10 mM and that it is an inhibitor of many enzymes, thereby affecting metabolic processes [50, 52].
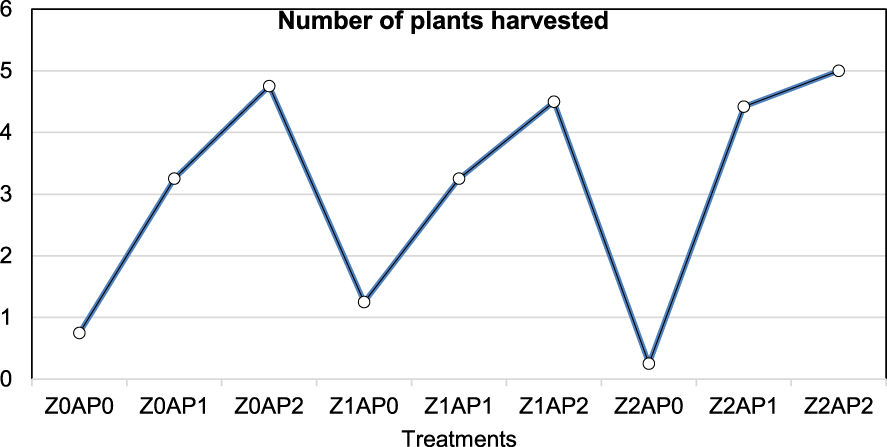
Plants harvested for different treatments. Z: zeolite; AP: sludge from olive processing; 0: no addition; 1: 2.5% addition; 2: 5% addition.
The initial soil used for the cultivation experiments was rich in nitrogen (Table 3). However, during plant growth, it was found that the addition of supplementary nitrogen was necessary. Therefore, nitrogen measured in the substrates after crop harvesting (Table 4) came, apart from the soil and AP, from the fertilizer added in the 10th week of the experiment. A slight increase in the substrate’s nitrogen content can be seen for the case of 2.5% Z addition, while a decrease of almost 0.5 mgN/g substrate is seen for 5% Z, indicating that zeolite addition does not improve the N-holding capacity of the substrate. Nitrogen can be retained by the zeolite mainly as NH4+. However, no significant activity is expected in retaining NO3- due to the negative charge of the ions, which are leached during irrigation if they are not used by plants. Leachates in the case of Z2AP0 had the highest EC (data not shown).
The capability of the substrate to retain nitrogen is strongly associated with OM, which for the case of only zeolite addition (Table 4) was the lowest among all other treatments and, as expected, similar to the Z0AP0 treatment (i.e., substrate consisting of only soil). As previous studies mention, when OMW is applied to the soil, the leaching of nitrogen is avoided as it is present mainly in organic form or as ammonium, which is adsorbed by soil colloids. Thereafter, ammonium is oxidized to nitrate (negatively charged), which can easily be transported to soil solutions and to greater soil depths through leaching.
On the other hand, and as Kavvadias et al. [11, 16] mentioned, the presence of AP in soils may lead to the immobilization of available nitrogen forms, resulting in an increased need for supplementary nitrogen addition as is also the case in this experiment.
The concentration of exchangeable potassium was increased with increase in zeolite percentage (Table 4) as expected. This is due to the characteristic property of clinoptilolite to retain cations at the exchangeable sites of its framework, preventing leaching and making them available to plants slowly on demand [13]. In this case, potassium came from the fertilizer and also from the zeolite itself and increased with the percentage of Z in the substrate. No significant (Table 4) effects and changes were observed for B, available Fe, and available Cu and Zn (data not shown).
3.2. Substrates with soil and sludge from olive processing
For the cases of only AP addition, which are Z0AP1 and Z0AP2, a significant improvement of 50% and 75% in substrate OM, respectively, was recorded. The percentage of OM content for both cases exceeds the value of 5%, which characterizes soils as very rich in OM [11, 16]. This increase could be beneficial to soils and especially to Mediterranean soils, which are very poor in OM. However, it has to be mentioned that accumulation of insufficiently stable OM, which could be the case for part of the OM content of this waste type, may cause various negative effects on soil properties, including the generation of anaerobic conditions and release of phytotoxic substances [13, 53]. Moreover, Pezzolla et al. [54] reported that the addition of a not well-stabilized organic fertilizer/additive to soil might cause a significant increase in dissolved organic carbon and therefore CO2 emissions due to a rapid rise in microbial respiration after soil amendment.
Compared to Z0AP0 and also to the Z1AP0 and Z2AP0 treatments, an increase in the capability of substrates to retain nitrogen was also observed (Table 4) due to the increase in organic-nitrogen forms. Similarly, B content (Table 4) was increased by almost 45%, however, without significant differences between the two AP treatments. Exchangeable K (Table 4) was also increased by approximately 22% for 2.5% AP content (which is similar to the increase caused by 2.5% zeolite) and by 72% for 5% AP content (which is slightly lower than the increase recorded for 5% zeolite addition; i.e., Z2AP0). A significant improvement in the substrate’s available Fe content (Table 4) was also recorded, which is a known effect of AP addition to soils. This was confirmed also during the LIFE PROSODOL project [15] and by the work of Doula et al. [22] as regards the soil parameters that are mostly affected by OMW discharge into soils. The advantage of this type of treatment (i.e., only soil and AP) in comparison to only Z addition can be also confirmed by the improvement of the substrate’s EC (Table 4). For the case of the 2.5% AP content, the EC was lower than that for the Z0AP0 treatment (corresponding to cultivation on only soil), while for the treatment of 5% AP, it was slightly higher than that for Z0AP0. Exchangeable Na (Table 4) was increased in the substrate compared to that in the Z0AP0 treatment. However, the increase was significantly lower compared to the treatments of only Z addition. No significant effects were observed for available Cu and Zn content (data not shown).
3.3. Substrates with soil, clinoptilolite, and sludge from olive processing
As regards harvesting, the Z2AP2 treatment (i.e., 5% Z and 5% AP) resulted in the largest mean number of harvested plants among all studied treatments (Figure 2). Organic matter content (Table 4) seems to depend solely on the percentage of the sludge content since no improvement of substrates’ OM was observed with the increase in zeolite content. As regards nitrogen, no substantial effect of Z was observed (Table 4) because the AP content is the factor that determines the N-holding capacity of the substrate.
In the present work, in temporal terms, harvesting denoted the end of the experimental process in the greenhouse environment. In particular, the harvest did not concern tasks such as cutting the upper plant part and separating the leaves, the stems, and the fruits, which would have led to further tissue analyses, as these did not fall within the scope and objectives of the study. However, what was measured and referred to as “yield” (as indicated in Figure 2) was plant survival in terms of the number of plants that was actually collected per treatment at the end of the experiment. In this study, plant stress and mortality were only discussed in correlation to soil characteristics such as salt concentration and to the soil additives used. Finally, in Figure 2, the term “crops” is substituted by the term “plants”.
Among all treatments, higher amounts of exchangeable K were measured for the treatments with Z and AP. The highest K content was recorded for the case of Z2AP2 treatment (Table 4). Although the K concentration exceeded the threshold of 2 cmol (+)/kg according to Kavvadias et al. [11, 16], no phytotoxic effects were observed. This is mainly due to zeolite activity to retain cations at exchangeable sites and supply them to the substrate solution on plant demand. Therefore, the zeolite acts as a slow-release fertilizer. Further improvement in the Fe content (Table 4) compared to the results obtained for Z1AP0, Z2AP0, Z0AP1, and Z0AP2 was observed, which is attributed to the synergistic effect of the two materials.
Electrical conductivity (Table 4) was also affected, and it was found that the presence of AP decreased the ion concentration in the substrate solution in comparison to the cases of only Z addition. Even if the reduction in EC is not substantial, it is indicative of the improvement of substrates due to AP content. Similar behavior was observed for exchangeable Na (Table 4).
3.4. Polyphenols in substrates and leachates
The polyphenol content in soils amended using OMW is a well-known problem [12]. It has been reported [55] that the residual levels of polyphenols could remain significant even 6 years after OMW application on the soil. On the contrary, Chartzoulakis et al. [18] mentioned that despite a temporal increase in phenolic compound concentrations in soil soon after OMW application, their concentration is rapidly reduced with a few months. The same authors also mentioned that polyphenols do not move rapidly across the soil profile even if OMWs are applied on irrigated soil or during the rainy season. In addition, negligible leaching of polyphenols is expected from soils rich in carbonates and clay materials [56, 57]. These findings were confirmed in this study also since no polyphenols were detected in the leachates, which is a very significant result that provides further assurance for the protection of groundwater and deeper soil profiles.
As presented in Table 4, there was an increase in polyphenol content due to the AP application, while Z did not affect polyphenol concentration significantly. It is worth noting, however, the disproportionate increase in the concentration of phenols when the amounts of AP and OM of the substrates are doubled (the cases of 5% AP). This could be due to the higher concentration of OM since, as Sierra et al. [58] and Saadi et al. [59] reported, decomposition or incorporation of phenolics into the humic fraction of OM might take place.
As regards polyphenol concentration, it is higher for all treatments with AP than the Z0AP0 treatment. However, it still can be characterized as medium compared to the findings of Sierra et al. [58], who have reported values up to 9926 mg/kg, and of Nikolaidis et al. [60], who measured a lower total phenol content (481 mg/kg) when only pre-treated OMW was applied on soils.
3.5. Leaching of Na and K
Leaching of Na and K can be observed in Figures 3 and 4, which demonstrate the important role of both materials, Z and AP. As regards K leaching, the absence of Z (Figure 3a) resulted in high leaching of the ion, which was affected by the AP percentage. That is, leaching increased with increase in AP percentage in the substrate. On the contrary, even for the highest percentage of AP, the presence of clinoptilolite restricted K leaching. However, for the cases of Z1AP2 and Z2AP2 also, potassium leaching was higher than the other treatments shown in Figures 3b and 3c. Among all treatments with Z and AP, Z1AP1 exhibited the optimum behavior as regards K leaching. It is also important to highlight the increase in K leaching detected during the 11th experimental week owing to plant fertilization, which indicates that fertilization may have a more significant impact with respect to nutrient leaching and consequently their loss and degradation of aquifers. However, in this case also, the presence of Z and AP (Figures 3b and 3c) could limit leaching compared to the case of Z0AP0 (Figure 3a).
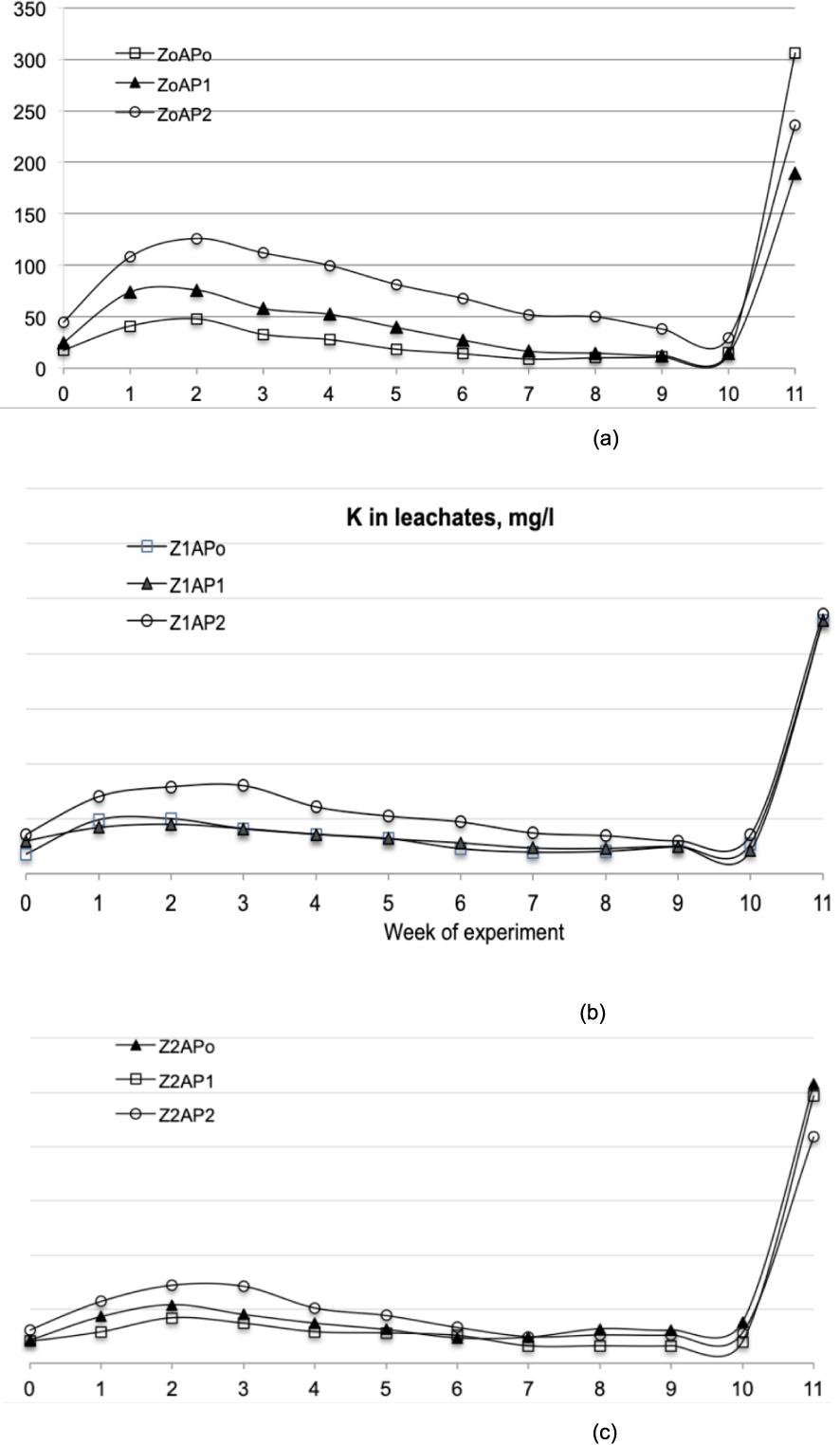
Potassium measured in leachates for each experimental week and for all treatments.
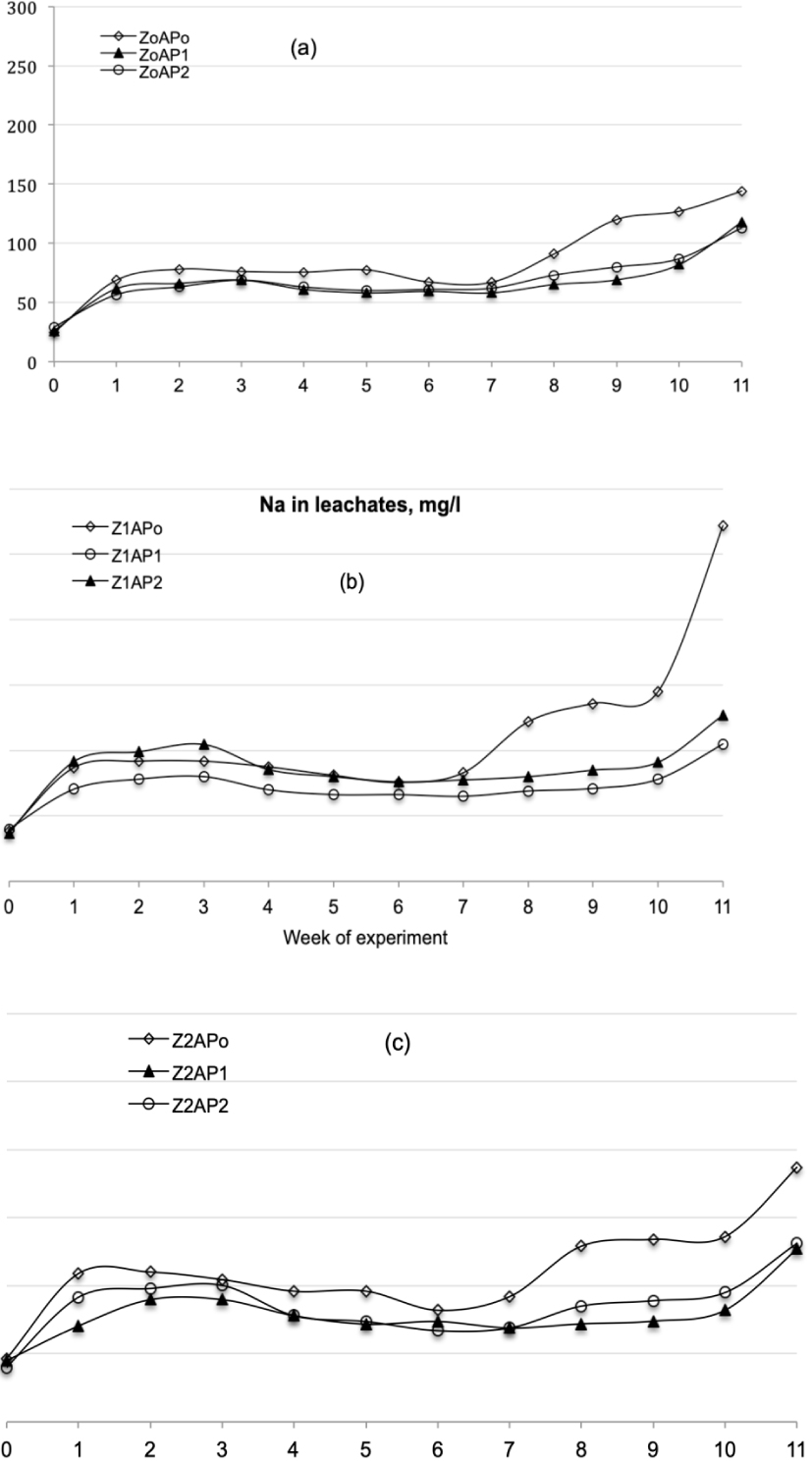
Sodium measured in leachates for each experimental week and for all treatments.
According to Figure 3a, the addition of AP restricted Na leaching in comparison to the Z0AP0 treatment. This occurred due to the improvement in the cation-holding capacity of the substrate, resulting from the increase in OM. With an increase in Z percentage (Figures 3b and 3c), Na leaching was enhanced and higher concentration values were measured in the leachates. However, among treatments of the same Z percentage, higher leaching was recorded for the cases where the AP percentage was not increased, confirming the positive impact of the OMW in inhibiting Na leaching. Among treatments with Z and AP, the optimum performance, as regards Na leaching, was exhibited by Z1AP1.
Finally, the increase in Na leaching during the first 5 weeks of the experiment is owing to the already reported problem of Na release by clinoptilolite [13], which however was limited after that period. The treatments with 2.5% Z (Z1AP1 and Z1AP2) showed the optimum behavior of smooth Na release from Z.
3.6. Other changes in substrate properties
Other parameters measured in the substrate were pH and exchangeable Ca and Mg. The pH measured for Z0AP0 was 7.39 ± 0.12, while for all other treatments, the pH values measured were within the range 7.00 ± 0.05 and 7.67 ± 0.11. Although there are differences in pH values, this is not considered important for a pot experiment in which soil resilience and buffering capacity are limited. As regards exchangeable Ca, it was measured to be between 40.05 ± 1.22 and 42.11 ± 1.05 cmol(+)/kg for all treatments (for Z0AP0, exchangeable Ca was 41.02 ± 1.08 cmol(+)/kg). An increase in exchangeable Mg was recorded for all treatments, where the concentrations were between 2.80 ± 0.09 and 3.29 ± 0.12 cmol(+)/kg (for Z0AP0, exchangeable Mg was 2.41 ± 0.16 cmol(+)/kg).
The addition of clinoptilolite increased the EC; however, soil amendment by OMW equilibrated this increase. In any case, special caution should be exercised in monitoring and controlling salt concentration in amended substrates to avoid extreme values that lead to a potential burden in plant growth or even plant mortality. Furthermore [26, 27, 29, 30, 35], clinoptilolite may improve the availability of exchangeable potassium, thereby acting as a slow-release fertilizer. Moreover, the addition of OMW increased OM concentration in the substrate, indicating that poor- or low-fertility soils can be enriched using nutrient compounds. Attention should also be paid so that phytotoxic conditions do not arise at the root zone and consequently in the plants. It is reported that the clinoptilolite acts as a means of effective amendment for the remediation of heavy-metal-polluted soils [61], which is not dealt with in the present study. This activity in conjunction with its capacity to reduce leaching phenomena may serve as a mitigation feature in soil-polluted or soil-degraded sites.
4. Conclusions
Olive mill wastes are still considered as a major problem in the Mediterranean region although many research works provide solutions for OMW treatment and production of clean or almost clean effluents that can be further used for irrigation or discharge. However, the issue that remains unresolved is the high cost for establishing such a treatment facility, which prevents the adoption of these solutions. The distribution of OMWs in soils has been extensively studied during the past years. In addition, it has been reported that such practices could provide a sustainable alternative methodology for OMW management provided that all appropriate measures are taken to avoid soil degradation.
This study demonstrated the benefits of using OMW sludge in the cultivation of pepper plants with respect to increase in OM, N, K, B, and Fe of the substrate and without serious effects on Cu and Zn and also pH, thereby resulting in higher yields. On the contrary, the addition of clinoptilolite to soil did not appear to have a positive effect on the above properties (except in the case of K). Moreover, it was found to cause phytotoxic effects due to increased Na concentration in the soil solution and increased sodium leaching.
In addition to the positive effects of the OMW sludge, the zeolite’s synergistic effect improves the stability of the substrate by reducing loss of potassium through leaching and enhancing the soil’s ability to retain cations. Therefore, the zeolite acts as a slow-release fertilizer and also reduces the effect of soil salinity after fertilization, thereby reducing nutrient loss through leaching.
Even though polyphenols increased for all treatments with OMW sludge, they did not cause any adverse effect on plants and they were not detected in the leachates after irrigation. In this study, the overall best performance in terms of yield and substrate and leachate properties was observed for the Z1AP1 treatment, which corresponds to the addition of 2.5% zeolite and 2.5% sludge to the soil. However, further studies are needed for the assessment of more substrate parameters and the nutritional status of plant parts (roots, stems, and leaves) to finalize a potential cultivation protocol.




 CC-BY 4.0
CC-BY 4.0