1. Introduction
The rapid growth in population with increased efforts on attaining sufficiency in food grains has pressurized Indian farmers to switch to the use of pesticides in considerable quantity. It has been estimated that predominantly for agricultural purposes, lakhs of tons of dichlorodiphenyltrichloroethane has been utilized in India alone due to its cost-effectiveness and excessive toxicity, making it effective in the control of pests [1]. However, certain features such as persistency, instability, and atmospheric distribution have led to the contamination of water, air, and soil [2]. Pesticides get washed away with water from fields and are drained off into rivers. This leads to a high toxic content in rivers. Hence, this causes pollution, which affects land and aquatic ecosystems in particular as the food for aquatic life becomes exposed to pesticides [3]. Pesticides enter the food chain, which affects our health. This has become a serious issue, which is certain to become more severe in the time to come.
Isoproturon 3-(4-isopropylphenyl)-1,1-dimethylurea is a selective herbicide that belongs to the family of substituted ureas. The herbicide is intended for use in cereals by controlling broadleaf weeds and annual grasses in them. The chemical is readily absorbed by the roots and leaves of weeds, thereby inhibiting photosynthesis. Various studies have confirmed the presence of isoproturon in fresh water due to production plant discharges, surface runoff, and runoff from agricultural sources [4].
More effective and efficient treatment techniques are required to reduce the environmental and potential effects of these pesticides containing effluents. Advanced oxidation processes (AOPs) are well cited in the literature to remove natural and inorganic toxins as well as for complete mineralization [5]. The AOP is an oxidative approach that involves the formation of a highly reactive intermediate radical (hydroxyl radical (HO∙)) capable of oxidizing a large number of organic pollutants [6]. The HO∙ can be produced through various techniques under the umbrella of AOPs, including photocatalysis, photo-Fenton, fixed-bed catalysis, and modified versions of these AOPs [7]. Although capable, they all pose certain operational drawbacks, which restrict their commercial-scale applications. The limitations of the Fenton process include the formation of iron sludge, which can be reduced by the application of ultraviolet irradiation or solar irradiation, the requirement of a high dose of hydrogen peroxide (H2O2), and the production of a large concentration of anions in the treated wastewater [8]. Certain pitfalls of photocatalysis include separation of the catalyst from the aqueous solution, thus generating an additional step in post-treatment. Recombination of electrons and holes is the other limitation that controls the rate of degradation [6]. Fixed-bed catalysis provides certain solutions for field-scale applications of AOPs. Studies related to the fixing of catalysts on supports are extensively cited in the literature [9]. Then again, some problems such as mass transfer limitations, inertness of supports, and time-consuming processes [10] pose impediments to field-scale applications.
Keeping in view the potential implications of all AOPs and field-scale applications, a hybrid process, called dual effect, has been studied, which can combine the pros and cons of the individual processes of photocatalysis and photo-Fenton [11]. The dual process prompts the generation of HO∙ in large numbers because of the combined effects of photo-Fenton and titanium dioxide (TiO2) photocatalysis. The basic mechanism involves the occurrence of photocatalysis on the surface and of the photo-Fenton in bulk [12, 13, 14]. It prevents the frequent recombination of electrons and holes by trapping the electrons from the conduction band for the production of Fe2+ ions, which are essential for the photo-Fenton reaction [15]. The investigation in this specific area of interest is always oriented toward reducing the time for treatment along with optimizing the process cost [16]. In the current study, the idea of incorporating the integrated effect (photo-Fenton and photocatalysis) was proposed to reduce the treatment time of the isoproturon. The high dose of the chemicals used as well as the increased cost of the involved process can be reduced considerably if both the processes (photocatalysis and photo-Fenton) are run together, which would simultaneously increase the rate of degradation along with reducing the degradation time [17]. There are limited studies on the incorporation of dual processes for wastewater treatment. Therefore, the proposed study would maximize the photocatalytic efficiency of the system by including the dual effect (heterogeneous and homogeneous photocatalysis).
Hence, in the current study, the main focus is on the degradation of isoproturon using an in situ dual effect of photo-Fenton and photocatalysis. For the present study, composites were prepared by using the waste materials of industries as a source of iron. The prepared composite with a coating of TiO2 enabled the dual effect to take place. The prepared support was also characterized using various techniques such as scanning electron microscopy–energy-dispersive spectroscopy (SEM–EDS), UV–diffuse reflectance spectroscopy (UV–DRS), and Fourier-transform infrared (FTIR) spectroscopy for the confirmation of TiO2 along with the oxides of iron. For the optimization and the analysis of responses, the Box–Behnken design (BBD) was used. The process was intensified by varying parameters such as the number of beads, time, and the dose of H2O2.
2. Materials and methods
Isoproturon, a selective herbicide that belongs to the substituted urea family, is used for controlling the growth of annual grasses and weeds, specifically, wheat and barley. It is present in freshwater bodies at low concentrations due to its application in agricultural activities. Moreover, it is highly stable possessing low biodegradability. It was bought from Pioneer Pesticides Pvt. Ltd, Chandigarh (India) and was used as such without any further purification. TiO2 P-25 was bought from Evonik (India). Clay, waste fly ash (FA), and foundry sand (FS) were collected as such from a nearby manufacturing unit (Patiala, India), and hydrogen peroxide (H2O2; 30% w/v), an oxidant, was acquired from Ranbaxy (India). For the preparation of acetate buffer for maintaining the pH, acetic acid (99.5% purity) and sodium acetate (98.5% purity) were procured from TCI Chemicals (India). For the preparation of all solutions, double distilled water was used.
Standard American Public Health Association (APHA) methods were followed for the monitoring and evaluation of nitrite [18], nitrate [19], and ammonium ions [20]. For estimating the concentrations of Fe(II) and Fe(III) leached from the beads under acidic conditions, the phenanthroline method was used according to APHA standards [21]. The chemical oxygen demand (COD) was estimated according to the closed reflux method and analyzed using a UV–Vis spectrophotometer (HACH, DR 5000, USA) [22]. The total organic carbon (TOC) content was monitored through a TOC analyzer (model Multi N/C 2100 BU, Analytic Jena AG Corporation, Germany). H2O2 interference was removed prior to COD and TOC determination by adding an adequate amount of concentrated solution of sodium carbonate followed by heating at 90 °C for 1 h as reported in the literature.
2.1. Fabrication of the support material
The materials used for preparing the composite beads were clay, FS, and FA. Bentonite and kaolin clay were used to provide binding to the composite beads. FS and FA have been proved to be a good blend of high-quality silica, aluminum oxides, iron oxides, calcium oxides, and some other oxides, which indicates their potential use in water/wastewater applications. The use of FA alone or FS alone does not provide the appropriate strength to the beads. Furthermore, clay alone does not yield the required amount of iron for the dual effect to take place.
Composite beads (spherical in shape) capable of introducing an integrated process of photo-Fenton and photocatalysis were prepared by mixing clay, FA, and FS in the ratio 2:1:1, respectively. These beads were calcined at 800 °C and were further immersed in water for 24 h to improve their strength. The average diameter of the bead was 25.5 mm as measured by taking its diameter from six to eight directions. The prepared composite was effectively coated with TiO2 by immersing the composite completely in a TiO2 slurry (2% w/v) using the standard dip coating technique [11]. The process of dip coating was performed twice for attaining the required thickness of the TiO2 film on the composite. The beads were soaked for 48 h at a revolution speed of 10 rev/min.The average thickness of the TiO2 film was 65 μm with the average amount of TiO2 on one bead being 1.6 mg.
2.2. Experimental procedure for degradation
For conducting the experiments, constant irradiation of a UV light source in a wooden chamber (36 W, seven tubes fitted at the top of chamber; UV-A, Philips) was carried out. A borosilicate glass reactor (8 cm × 2.6 cm) having a capacity of 500 mL was used for conducting the study (Figure S1, Supplementary Data). The initial concentration of isoproturon used for the degradation experiments was 25 mg⋅L-1. The optimized number of catalyst-coated beads covering the whole surface was kept in the reactor in an adequate volume (200 mL) of isoproturon solution. The pH was maintained up to 3–3.5, and quantified H2O2 (750 mg⋅L-1) was added. For the analysis of the results, at regular intervals of time, samples were withdrawn and analyzed using a Hitachi V-500 UV/VIS double-beam spectrophotometer at 239 nm. All the experiments were repeated thrice, and the mean of the results along with the standard deviation was reported (Table 2).
2.3. Characterization techniques and analysis
For depicting the morphology of the prepared composite, a scanning electron microscope (JSM-6510LV, JEOL, Japan) was used. The EDS assembly (INCAX-act, Oxford Instruments, UK) as an attachment to SEM was used for elemental analysis. An FTIR spectrometer was obtained from PerkinElmer (RX1) for the evaluation of the functional groups and linkages present in the prepared catalyst. UV–DRS (UV-2600, Shimadzu, Asia Pacific) was used for the evaluation of band gap energy. For the analysis of the organic transformation products of isoproturon, gas chromatography–mass spectroscopy (GC–MS) was carried out. A PerkinElmer Clarus 500 MS instrument was employed; its capillary columns were made of fused silica (25 m × 0.20 μm internal diameter) coated with 95% dimethyl polysiloxane/5% diphenyl. An injector temperature of 300 °C was used with the inlet line being set at 280 and 250 °C. Helium was used as the carrier gas, which had a flow rate of 1 mL/min at a run time of 30 min.
2.4. Modeling and optimization
Dual treatment of wastewater containing pesticides is quite complex as it involves the implementation of two different processes (photocatalysis and photo-Fenton) simultaneously. Hence, there are a large number of factors that would determine the rate of degradation of a particular compound such as mass transfer mechanisms, amount of HO∙ formed, and various other limiting factors, which would be complex to understand. Modeling of dual treatment processes includes a multivariate system. It is obvious that this multivariate system cannot be solved by simple linear multivariate correlation [23]. It is also essential to study the interaction of parameters at a specific time. Hence, optimization was performed by utilizing response surface methodology (RSM) by varying different operational parameters to attain a suitable optimized condition. To optimize the process parameters, the BBD was employed [24].
The relationship between the input factors and responses is expressed as a quadratic response model. In general, the model used fits best in a polynomial equation of second order (1).
| (1) |
3. Results and discussion
3.1. Characterization of the prepared composite used for the experiment
(a) SEM–EDS of uncoated FA + FS spherical beads. (b) SEM–EDS of freshly TiO2 coated FA + FS spherical beads.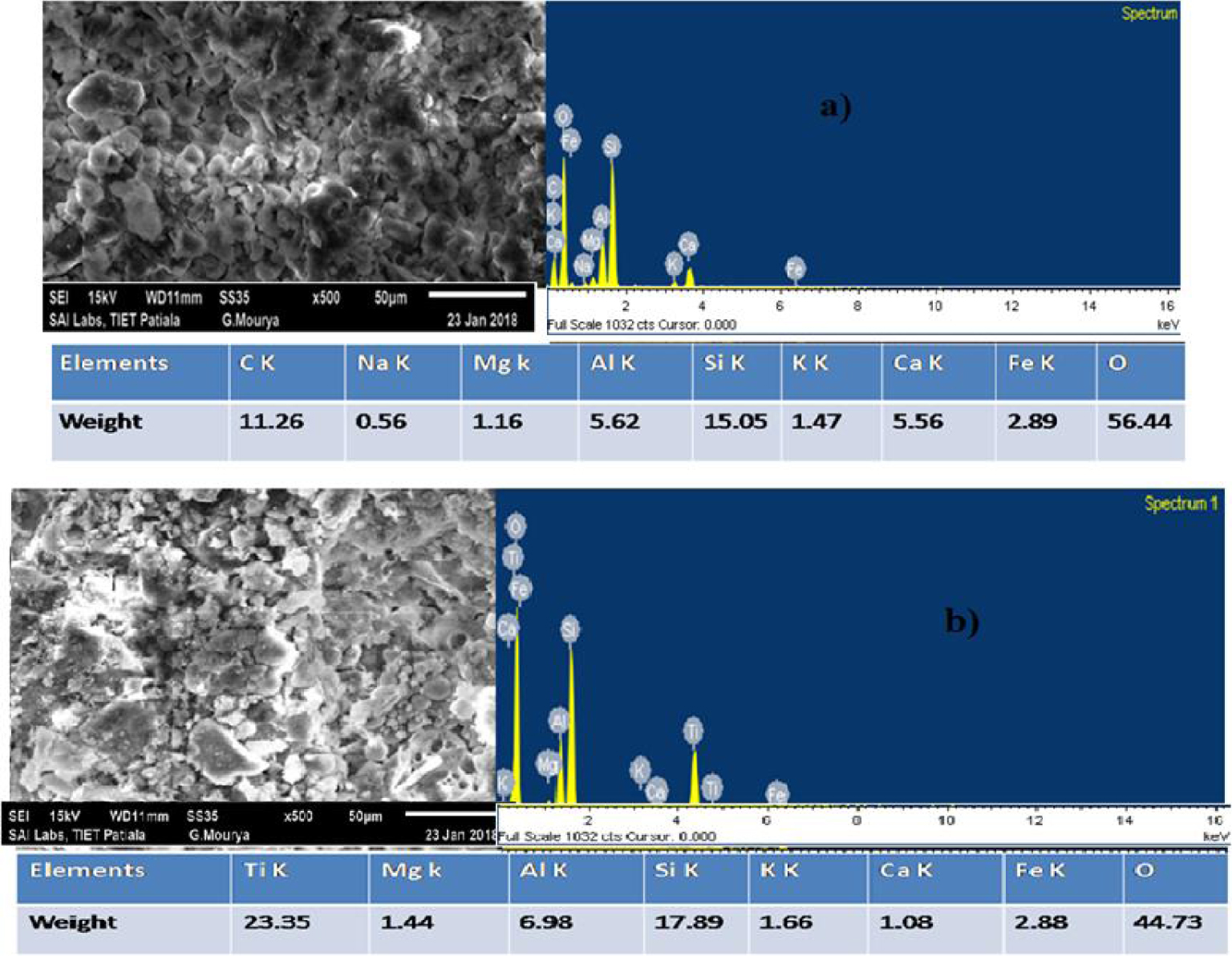
The prepared composite beads, uncoated and TiO2 coated, were analyzed using SEM. From Figure 1a, it is perceived that the surface of the composite is sufficiently rough for the coating to be stable and proper. Furthermore, the composition of the composite was proven by energy-dispersive X-ray spectrometry (EDS). The spectrum of the composite displays the signals of elements Fe, C, O, Al, Si, Ca, and Mg. The elements were present in the waste materials, and Al and Si were obtained from the clay used. The peaks of Fe confirmed that oxides of iron were present on the surface, thereby proving that the support was sufficiently suitable for the photo-Fenton reaction to take place. From Figure 1b TiO2, the coating of fresh beads is evaluated. The TiO2 coating was observed on the bead surfaces. The EDS analysis showed the occurrence of elements Ti, Fe, and O. This authenticates the presence of iron along with the coating of TiO2, which enables the hybrid effect to take place. The concentration of iron present on the surface of composite beads was evaluated to be approximately 2.89 wt% from the EDS analysis.
The FTIR spectra for confirming the modifications with the prepared Fe–TiO2 catalyst are shown in Figure 2. The analysis of the structure as well as the vibrations corresponding to the specific functional groups could be determined using FTIR spectroscopy. The peaks in the range 800–1200 cm-1 confirmed the presence of a Ti–O–Ti bond in the composite bead. The fingerprinting method could be applied for the analysis of the compounds present. Stretching vibrations related to the Si–O bond were also observed in the range of 550 cm-1. The stretching vibrations of the O–H groups were related to the occurrence of transmittance bands at 3407.91 cm-1 and 3410.82 cm-1 in the case of TiO2 and composite beads, respectively.
FTIR of TiO2, fresh composite.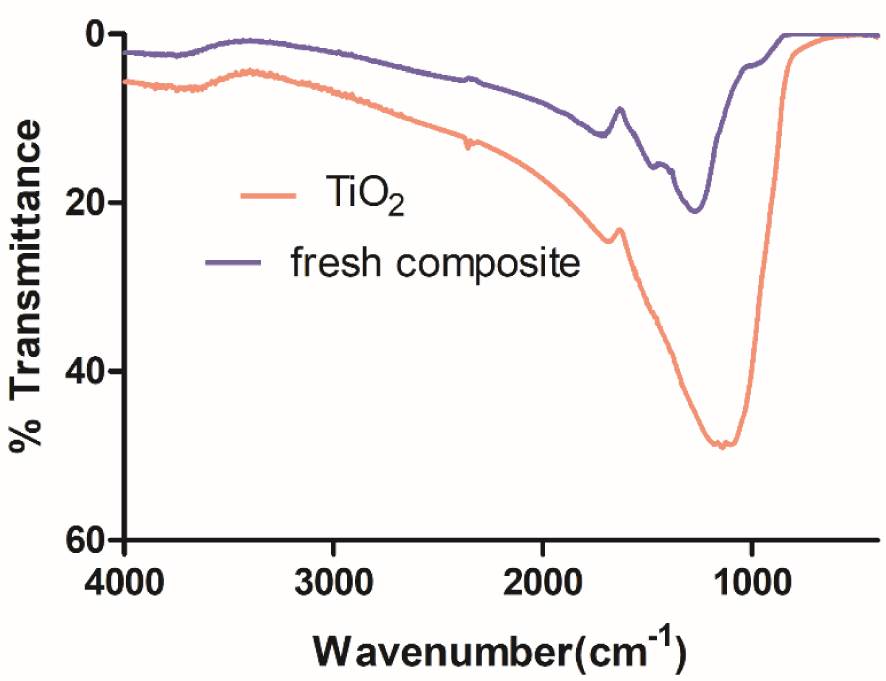
Owing to the increased activity of the prepared catalyst, the band gap energy was evaluated utilizing UV–Vis DRS. Basically, the reflectance of the sample and their light absorption capability were tested using UV–Vis DRS. A comparison was made between the band gap energy of P-25 TiO2 alone and the freshly TiO2 coated Fe composite (Figure 3). The scan was carried out ranging from 200 to 800 nm. There was a slight shift in the spectrum of the composite catalyst over TiO2 alone, thereby indicating the capability of the composite catalyst to absorb light in the visible spectrum as shown in (2).
| (2) |
UV–Vis DRS of TiO2 and the fresh composite.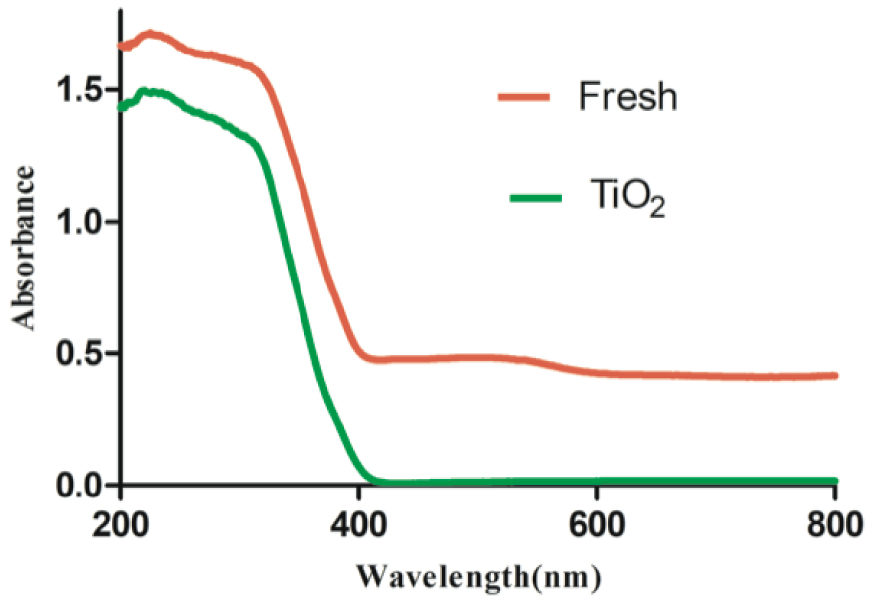
Accordingly, the value of the band gap energy was 3.2 eV for P-25 TiO2 and 3.15 eV for the fresh catalyst (beads), confirming the activity of the catalyst even after the coating of catalyst on the composite beads.
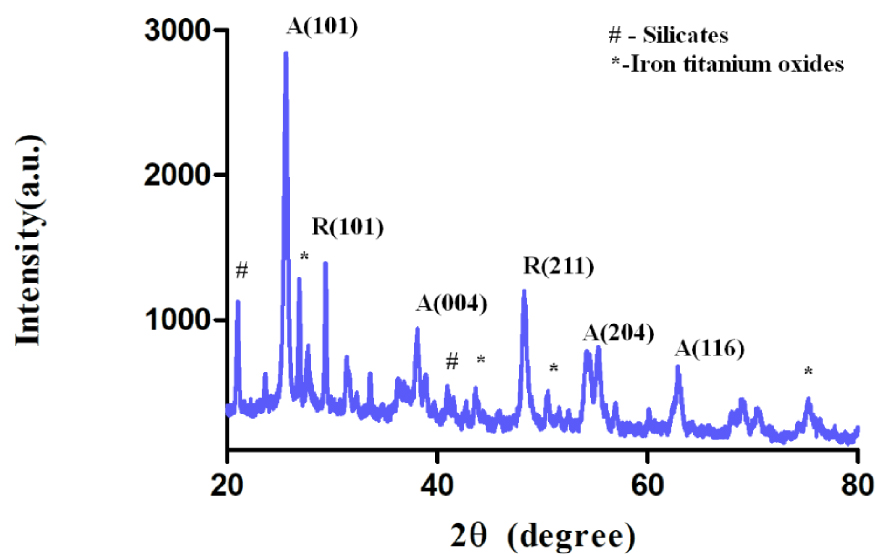
For the crystalline phase analysis of the prepared composite, X-ray diffraction (XRD) analysis was carried out (Figure 4). Along with the presence of the anatase and rutile phases of TiO2, the additional peaks corresponding to the oxides of iron with titanium were also observed. The occurrence of the dual effect, that is, photocatalysis and photo-Fenton taking place simultaneously, has been also proven.
3.2. Performance analysis of the prepared composite
For the application of the prepared composite of Fe and TiO2, isoproturon was used as the model compound. The catalyst exhibits the inherent properties of Fe2+ and Fe3+, indicating their presence as well as a layer of TiO2. This enables both photocatalysis and photo-Fenton reactions to take place simultaneously.
Variation in variables and coded levels
| Factor | Name | Low actual | High actual | Low coded | High coded |
|---|---|---|---|---|---|
| A | Variation in H2O2 (mg⋅L-1) | 375 | 1125 | − 1 | 1 |
| B | Number of beads | 30 | 90 | − 1 | 1 |
| C | Volume of solution (ml) | 150 | 400 | 1 | 1 |
| D | Time | 30.00 | 180 | 1 | 1 |
For proving the ability of the prepared catalyst, various preliminary experiments including adsorption, photolysis, UV + H2O2, photocatalysis, photo-Fenton, and combined photocatalysis and photo-Fenton (i.e., dual process) were performed (Figure 5a). For conducting the photo-Fenton experiments, 40 uncoated beads covering the whole surface were kept in a reactor at an adequate volume (200 mL). The pH was maintained up to 3–3.5, and quantified H2O2 (750 mg⋅L-1) was added. For the photocatalysis process, TiO2 coated beads were used under the same conditions as those for photo-Fenton. For adsorption, TiO2 coated beads were used after adding the beads to an isoproturon solution. The reactor was placed in the dark. Even after 180 min, only 2.05 ± 1% of isoproturon adsorption was observed, whereas photolysis contributed to a reduction of 11.25 ± 2.5% in isoproturon concentration after 180 min. Photocatalysis resulted in a reasonable degradation of isoproturon, 57.7 ± 5% in 180 min, as compared to photo-Fenton, which led to a reduction of 29.9 ± 5% in the concentration of the compound at the same time. However, the combined effect of both photocatalysis and photo-Fenton (Fe + UV + TiO2 + H2O2) produced the best results as a degradation of 80.96% was observed under the same conditions. This reveals that the occurrence of Fe with TiO2 leads to the acceleration of the reaction in the presence of H2O2. For practical real-life applications, this study can be a base on which further parameters can be optimized according to scale-up calculations. For validating the technology in real-life applications, authors have published few papers on real industry effluents [25]. The lower pH of the wastewater enabled the photo-Fenton reactions to take place. For practical and economic purposes, low-pH water could be procured from acid industries, which face the problem of disposing low-pH wastewater.
Full factorial BBD matrix with standard deviation
| Sl. No. | Variation in H2O2 (mg⋅L-1) | Number of beads | Volume of solution (mL) | Time (min) | % degradation (standard deviation calculated from triplicates; same beads were used) |
|---|---|---|---|---|---|
| 1 | 750 | 90 | 400 | 105 | 44.64 ± 2.573 |
| 2 | 750 | 30 | 275 | 30 | 20.34 ± 0.466 |
| 3 | 750 | 60 | 400 | 180 | 63.57 ± 1.110 |
| 4 | 375 | 60 | 150 | 105 | 60.09 ± 1.350 |
| 5 | 1125 | 90 | 275 | 105 | 21.56 ± 0.311 |
| 6 | 750 | 90 | 275 | 180 | 68.21 ± 1.562 |
| 7 | 1125 | 60 | 400 | 105 | 28.41 ± 1.704 |
| 8 | 1125 | 60 | 275 | 30 | 10.24 ± 0.537 |
| 9 | 750 | 30 | 275 | 180 | 68.05 ± 1.449 |
| 10 | 1125 | 60 | 275 | 180 | 79.12 ± 0.622 |
| 11 | 750 | 60 | 275 | 105 | 44.87 ± 0.091 |
| 12 | 1125 | 30 | 275 | 105 | 23.93 ± 1.463 |
| 13 | 750 | 90 | 150 | 105 | 15.49 ± 0.360 |
| 14 | 375 | 90 | 275 | 105 | 40.53 ± 1.039 |
| 15 | 375 | 60 | 400 | 105 | 41.53 ± 1.746 |
| 16 | 750 | 60 | 275 | 105 | 52.11 ± 1.491 |
| 17 | 375 | 30 | 275 | 105 | 44.87 ± 0.091 |
| 18 | 750 | 30 | 150 | 105 | 60.25 ± 1.237 |
| 19 | 750 | 60 | 150 | 30 | 24.19 ± 1.548 |
| 20 | 750 | 60 | 275 | 105 | 56.44 ± 1.018 |
| 21 | 750 | 60 | 275 | 105 | 50.44 ± 1.103 |
| 22 | 750 | 60 | 400 | 30 | 5.06 ± 0.749 |
| 23 | 750 | 30 | 400 | 105 | 27.53 ± 0.332 |
| 24 | 375 | 60 | 275 | 30 | 24.14 ± 0.806 |
| 25 | 1125 | 60 | 150 | 105 | 26.35 ± 0.954 |
| 26 | 750 | 90 | 275 | 30 | 18.97 ± 0.685 |
| 27 | 375 | 60 | 275 | 180 | 70.11 ± 0.629 |
| 28 | 750 | 60 | 275 | 105 | 48.99 ± 1.407 |
| 29 | 750 | 60 | 150 | 180 | 78.33 ± 0.940 |
Outcomes of different preliminary reactions to assess the advantage and effectiveness of each condition.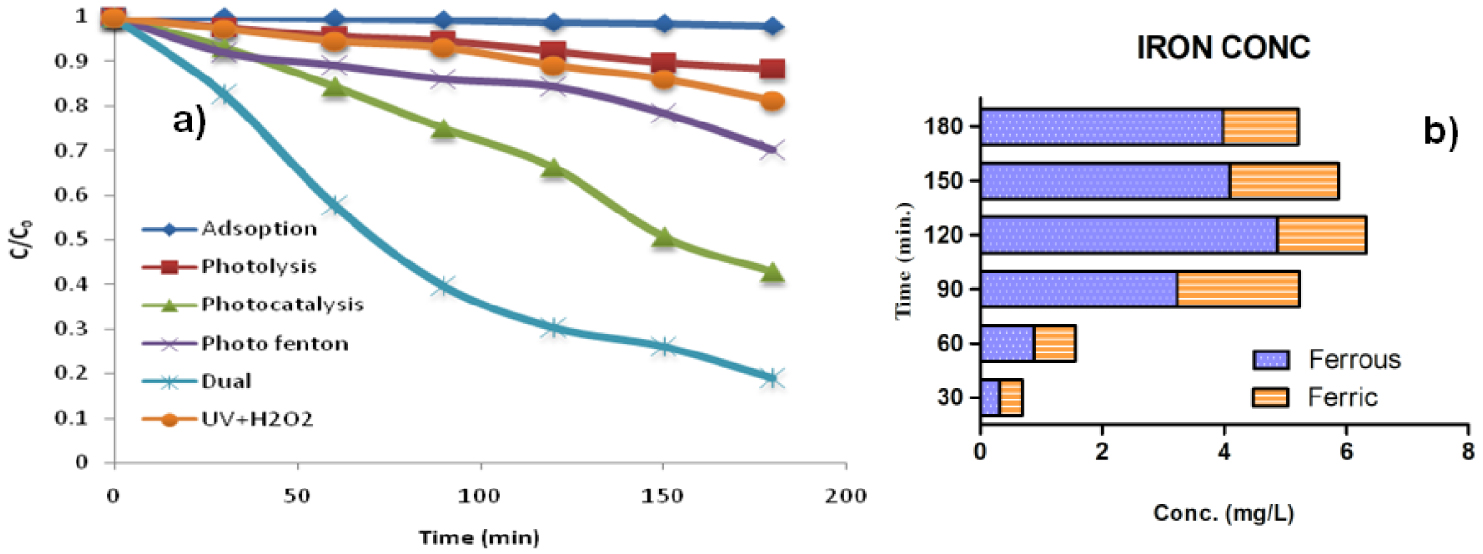
For further optimization of the degradation process, various parameters such as the number of beads, H2O2 dose, volume of the solution (wastewater), and degradation time were utilized. Table 1 presents the various optimum conditions and their maximum and minimum limits. Overall, 29 experiments were suggested by the BBD for optimizing the process parameters such as the H2O2 dose from 375 to 1125 mg⋅L-1, the number of beads from 30 to 90 in the 500 mL batch reactor depending on the exposed area of the reactor (i.e., 30 beads covering approximately 50%, 60 beads covering approximately 100%, and 90 beads covering 150% of the reactor surface area), the volume of the solution ranging from 150 to 400 ml (wastewater), and the degradation time from 30 to 180 min for isoproturon as shown in Table 2.
H2O2 plays a dual role in the proposed hybrid process of photo-Fenton as well as photocatalysis. It is consumed simultaneously in both the processes, leading to enhanced production of HO∙. H2O2 along with ferrous ions (leached from composite beads) leads to the formation of HO∙ (i.e., photo-Fenton); see Figure 5b). The H2O2 reacts with the superoxide radicals formed in the photocatalysis, leading to additional production of HO∙. The in situ H2O2 photolysis also adds to the HO∙ pool. Hence, HO∙ produced in abundance leads to an increased rate of degradation. In the dual as well as the individual processes of photocatalysis and photo-Fenton, H2O2 plays a very important role. The decomposition of H2O2 has been studied on the surface of various catalysts such as Fe, TiO2, CuO, and many more [26]. It was observed that in the dark, there was negligible change in the concentration of H2O2. However, here there was significant change in the concentration, that is, the decline in H2O2 concentration was observed in the dual process. This confirms the role of the dual process in the decomposition of H2O2 (Figure S2). A high consumption of H2O2 was observed in the case of the dual process. Thus, it was concluded that in the presence of light, along with TiO2 and iron, the decomposition of H2O2 is accelerated with greater production of ∙OH.
(a) 3D response surface graph for percentage degradation of isoproturon by variation in H2O2 dose and number of beads. (b) 3D response surface graph for percentage degradation of isoproturon by variation in H2O2 dose and volume of wastewater. (c) 3D response surface graph for percentage degradation of isoproturon by variation in H2O2 dose and time. (d) 3D response surface graph for percentage degradation of isoproturon by variation in time and number of beads.
The three-dimensional (3D) response graph plotted against H2O2 dose and number of beads for the degradation of isoproturon is depicted in Figure 6(a). The graph was plotted for an optimized time of 175 min. H2O2 was not completely used after the reactions. With increase in H2O2 concentration from 375 mg⋅L-1 to 750 mg⋅L-1, there is a slight increase in percentage degradation due to the production of an increased amount of HO radicals. On further increasing the concentration to 1125 mg⋅L-1, the degradation rate of isoproturon decreases, which may be attributed to the scavenging effect of H2O2. This involves the formation of excess radicals when HO∙ radicals combine with H2O2 as expressed in (3):
| (3) |
Sequential model sum of squares
| Source | Sum of squares | Degree of freedom | Mean square | -value | Prob > F | |
|---|---|---|---|---|---|---|
| Mean | 51177.00 | 1 | 51177.00 | |||
| Linear | 9820.31 | 4 | 2455.08 | 21.75 | <0.0001 | |
| 2FI | 1200.81 | 6 | 200.13 | 2.39 | 0.07 | |
| Quadratic | 848.82 | 4 | 212.21 | 4.51 | 0.01 | Suggested |
| Cubic | 513.70 | 8 | 64.21 | 2.65 | 0.12 | Aliased |
| Residual | 145.41 | 6 | 24.23 | |||
| Total | 63706.05 | 29 | 2196.76 | |||
Figure 6a shows that with increase in the number of beads from 30 to 60, the percentage degradation of isoproturon would undergo an increment. However, with further increase in the number of beads, there is a decrease in percentage degradation. As discussed, the number of beads was varied as per the available surface area of the reactor. In fact, more number of beads would eventually lead to an increase in the concentration of iron in the solution, which would hinder the reaction due to the blockage of active sites. Moreover, an excess number of beads would trap the solution in the void, thus leading to dead zones.
The 3D response graph plotted against H2O2 dose and volume of the solution for the percentage degradation of isoproturon is depicted in Figure 6b. The number of beads as shown in Figure 6b is 42. This indicates that with increase in volume of the solution from 150 mL to 275 mL, there is negligible effect on the percentage degradation. Moreover, with further increase in volume, the percentage degradation decreases considerably due to ineffective penetration of UV radiation as the volume of the solution ultimately causes lesser degradation. On the other hand, the change in the concentration of H2O2 from 375 mg⋅L-1 to 750 mg⋅L-1 causes a slight increase in percentage degradation, which eventually would decrease with further increase in concentration to 1125 mg⋅L-1 due to the scavenging effect expressed in (3). As we implement a dual process, the amount of H2O2 is used in both the processes simultaneously, that is, in photocatalysis as well as in photo-Fenton. At low concentrations of H2O2, the process takes place at a slow pace as this compound is utilized in both the reactions. The optimized dose of H2O2 used was 700 mg⋅L-1.
An analysis of the 3D response graph plotted against H2O2 dose and time for the percentage degradation of isoproturon shows that there is a sharp increase in percentage degradation with increment in time as depicted in Figure 6c. Clearly, with more exposure time experienced by the solution under UV light, there is enhanced production of HO∙. The variation in H2O2 dose causes a scavenging effect with increase in volume. From Figure 6d (H2O2 700 mg⋅L-1), it is observed that when the number of beads is increased up to 45 at the maximum time, the degradation of isoproturon increases, which remains almost constant up to 60 beads. This is basically due to the increase in the dose of the catalyst (i.e., both TiO2 and the iron content), leading to the process occurring at a faster pace. There is almost 80% degradation when the number of beads is 60, hence ideally covering 100% area of the reactor.
The optimum conditions for the dual treatment of isoproturon were verified experimentally. The experiment was run for 176 min at pH 3.7 with 42 beads, 190 mL of wastewater, and 700 mg⋅L-1 of H2O2 dose. At this optimum condition, the percentage degradation of isoproturon at a concentration of 25 ppm was estimated to be 80.96%. The model confirmed effective degradation of the contaminant.
3.3. Statistical analysis
For the selection of the best model, a sequence of F-test and other required measures was used. The p-valuefor the degradation of isoproturon was found to be less than 0.01 for the quadratic model evaluated by the sequential model sum of squares [27 ]. As regards the model summary statistics and the sequential model sum of squares, the adequacy of the model was tested. The result of the adequacy of the model is shown in Table 3 for the percentage degradation of isoproturon. For the percentage degradation of isoproturon, the quadratic model showed the perfect fit using the sequential model sum of squares.
For the quadratic model, the Prob > F-value was 0.0151, suggesting it to be significant. For the quadratic model, the coefficient of determination was 0.94. This indicates a good correlation between the predicted and observed values. The predicted values were quite close to the actual values. The difference between the predicted values and observed values divided by the standard error of the residual has a close relationship with the percentage normal probability.
The ANOVA suggested by BBD for the percentage degradation of isoproturon
| Source | Sum of squares | Degree of freedom | Mean square | F-value | Prob > F | ||
|---|---|---|---|---|---|---|---|
| Model | 11869.94 | 14 | 847.85 | 18.01 | <0.0001 | Significant | |
| Variation in H2O2 | 699.98 | 1 | 699.98 | 14.87 | 0.0017 | ||
| Number of beads | 105.38 | 1 | 105.38 | 2.24 | 0.15 | ||
| Volume of solution | 242.64 | 1 | 242.64 | 5.15 | 0.039 | ||
| Time | 8772.32 | 1 | 8772.32 | 186.33 | <0.0001 | Highly significant | |
| (Variation in H2O2)2 | 286.45 | 1 | 286.45 | 6.08 | 0.027 | ||
| (Number of beads)2 | 492.00 | 1 | 492.00 | 10.45 | 0.006 | ||
| (Volume of solution)2 | 239.32 | 1 | 239.32 | 5.08 | 0.040 | ||
| (Time)2 | 4.02 | 1 | 4.02 | 0.085 | 0.77 | ||
| Variation in H2O2 × Number of beads | 0.96 | 1 | 0.96 | 0.020 | 0.88 | ||
| Variation in H2O2 × Volume of solution | 106.30 | 1 | 106.30 | 2.26 | 0.15 | ||
| Variation in H2O2 × Time | 131.22 | 1 | 131.22 | 2.79 | 0.11 | ||
| Number of beads × Volume of solution | 956.97 | 1 | 956.97 | 20.33 | 0.0005 | ||
| Number of beads × Time | 0.59 | 1 | 0.59 | 0.012 | 0.91 | ||
| Volume of solution × Time | 4.77 | 1 | 4.77 | 0.10 | 0.75 | ||
| Residual | 659.11 | 14 | 47.08 | ||||
| Lack of fit | 587.24 | 10 | 58.72 | 3.27 | 0.13 | Not significant | |
| Pure error | 71.87 | 4 | 17.97 | ||||
| Corrected total sum of squares | 12529.05 | 28 | |||||
The analysis of variance (ANOVA) indicates that the F-value for the percentage degradation of isoproturon is 18.01 as shown in Table 4. The significance of the model is revealed by the F-value of 18.01. The chances for the occurrence of a large F-value are only 0.01%, which might be due to noise. The “Prob > F-values” larger than 0.100 indicate that the model is insignificant.
From the ANOVA, it is clear that the variation in H2O2, volume of solution, time, (variation in H2O2)2, (number of beads)2, (volume of solution)2, and interaction between the number of beads and the volume of solution are highly significant for the percentage degradation of isoproturon.
The second-order quadratic equation obtained in terms of significant interaction parameters and process parameters is presented in (4):
| (4) |
3.4. Kinetic study and synergistic calculations
Pseudo-first-order kinetics were used for the evaluation of the rate equations of the dual process as presented in (5)–(9):
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
Synergy is the term used to represent the efficacy of the improved process over the conventional processes. The rate constants calculated from the kinetics data are used to quantify the synergy [28]. The synergy is calculated using (10):
| (10) |
3.5. Scale-up studies
To visualize the field-scale applications, a scale-up experiment was performed in sunlight using a baffled glass reactor with dimensions (25 cm × 30 cm × 5 cm) designed to handle the total volume of 5 L. TiO2 immobilized composite beads were used to conduct in situ dual effect experiments. The synthetic wastewater from the pesticide, under acidic conditions, containing an optimized dose of H2O2 was pumped from a collection tank with the help of a pump. The flow rate was maintained at 2.5 L⋅h-1 (Figure 7a). The intensity was measured using a pyranometer (MP-100, Apogee) and was averaged to 920 W⋅m-2 for that period. From Figure 7b, it was observed that for 180 min initially, the degradation rate was fast. The rate increased to 71%, which might be basically due to the predominance of photocatalysis, that is, the presence of a TiO2 layer as well as Fe leaching, leading to homogeneous photocatalysis, as fresh beads come in contact with the effluent, resulting in faster degradation. With further increase in treatment time, there was a slight increase in the rate of degradation, that is, approximately 78% of degradation was observed after 250 min. The degradation rate remained mostly constant after this, which might be due to the stabilization of various products formed by the conversion of isoproturon. This study ensures the commercial-scale viability of the process.
(a) Image of fixed-bed reactor scale-up (V = 5 L). (b) Time vs. percentage degradation of isoproturon using fixed-bed reactor scale-up.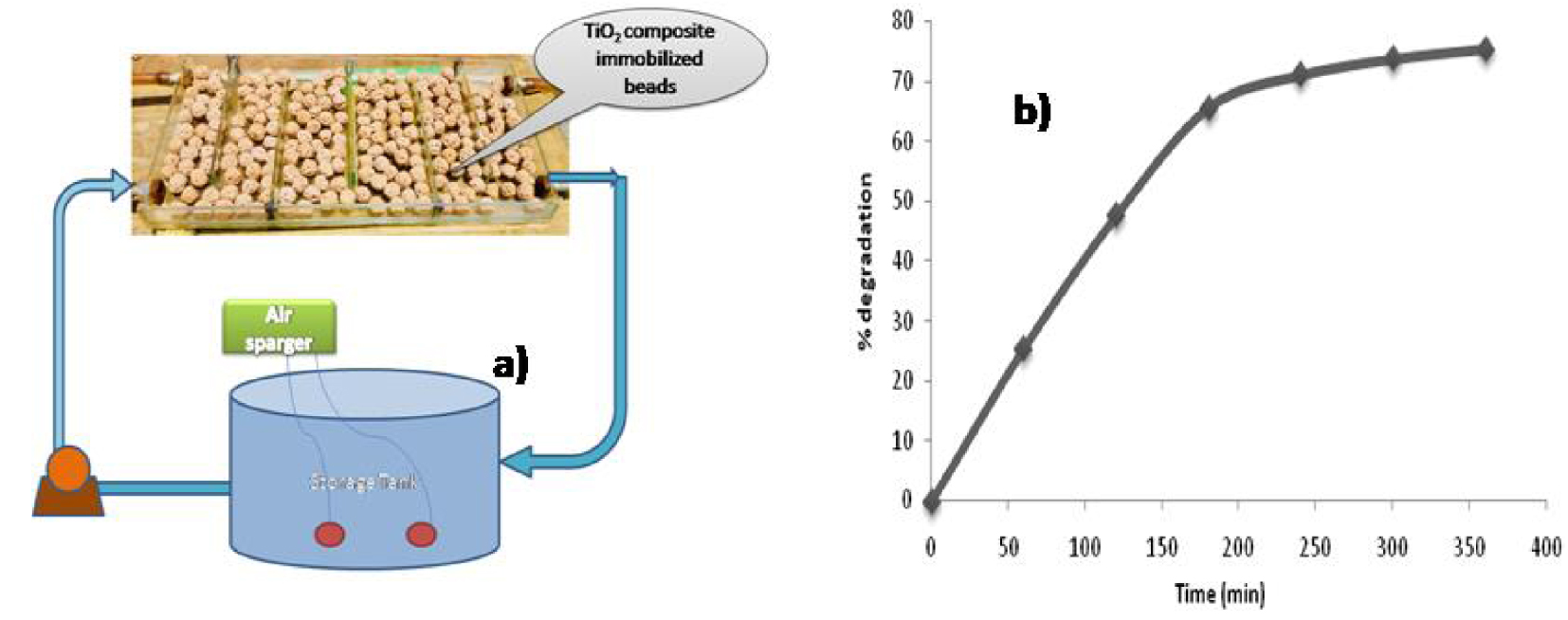
3.6. Energy consumption
A comparison can be drawn by measuring the consumption of energy (EEO) for the integrated effect on the percentage degradation of isoproturon and energy consumption in the case of individual processes of photo-Fenton and photocatalysis. EEO can be described as the requirement of electrical energy for the degradation of contaminant concentration (m3) of the wastewater. The value of EEO (kW⋅h⋅m−3⋅order-1) can be calculated by utilizing (11):
| (11) |
It can be observed that the EEO value was comparatively very less in the case of dual effect (Table 5) due to the intactness of TiO2 activity and simultaneous photo-Fenton owing to iron content leaching from the beads causing effective degradation.
Estimated value of EEO for degradation processes
| Degradation processes | Energy consumption (EEO) (MW⋅h⋅m-3) |
|---|---|
| Photo-Fenton | 25.30 |
| Photocatalysis | 10.39 |
| Dual effect | 5.28 |
3.7. Durability studies
The durability studies of supported catalysts are very important in the case of fixed-bed catalysis. These studies are even more complex in our reported dual process of photocatalysis and photo-Fenton. In fact, the process has to maintain the activity of coated TiO2 along with sustaining iron leaching. The activity of a catalyst decreases with every cycle, but it can be reactivated at high temperatures. However, this is not economically feasible [25]. We have made efforts to study the durability of TiO2 coated (clay + FS + FA) beads used for the degradation of isoproturon in wastewater. The same beads were used to prove the recyclability/durability of the composite. The durability was maintained in dual effect. That is, there was stable coating of TiO2 even after many recycling steps and subsequent leaching of iron with the recycling. Hence, the beads were adequately recycled for at least 40 to 50 times without any effective decrease in efficiency with respect to the percentage degradation of isoproturon as shown in Figure 8a. There was not much change in the iron concentration even after 35 recycling steps (Figure 8b). After every cycle, the beads were reactivated by placing them in a heating oven at 100 °C for a duration of 1 h. A small amount of reduction in the photocatalytic activity is due to the loss of TiO2 coating with every cycle. The gradual reduction in dual effect (10–12%) after 30 cycles may be attributed to the loss of the catalyst and less amount of iron leaching into the solution during consecutive cycles of the reaction. The stability of the catalyst and the presence of iron in the beads were determined by SEM–EDS of the fresh beads, coated beads, and recycled immobilized beads. It was found that the catalyst was intact and iron was still actively leaching even after 35 cycles.
(a) Durability studies of fresh and recycled beads for percentage degradation of isoproturon using dual effect. (b) Estimation of the iron content in terms of ferrous and ferric content after recycling.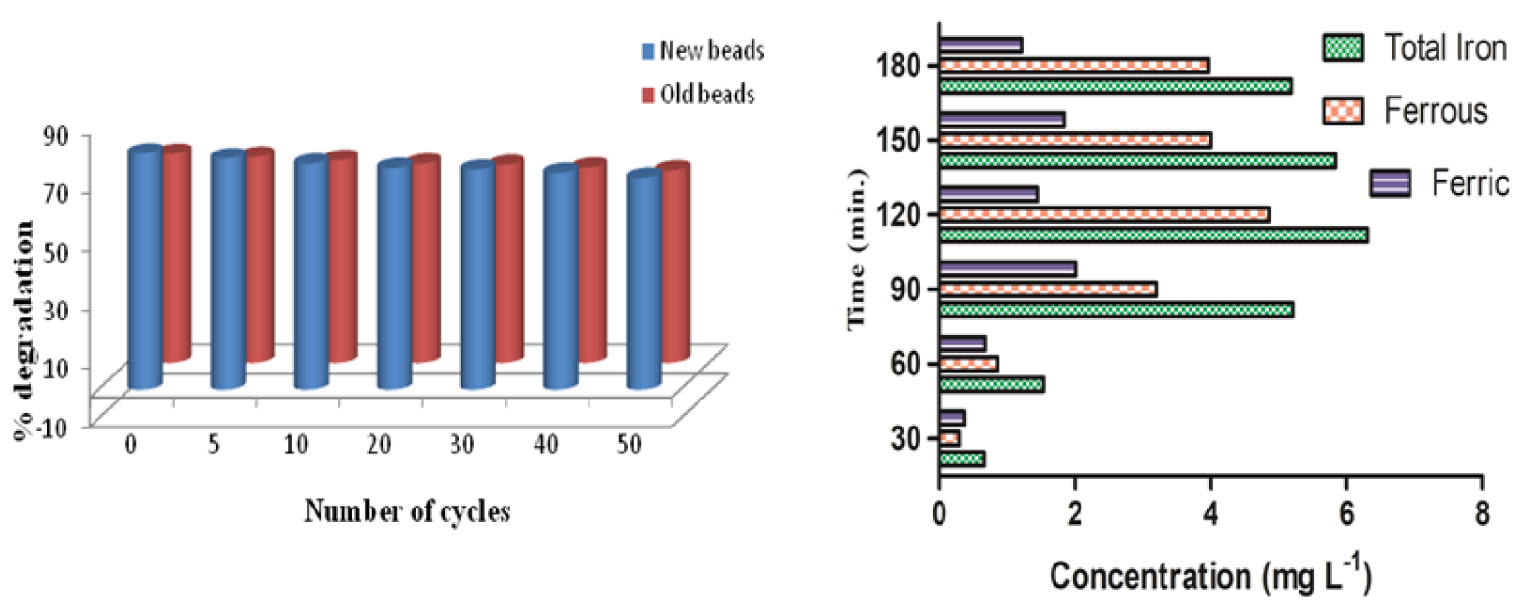
3.8. Characterization of recycled beads
The SEM–EDS image of the recycled beads (40 cycles) shows the presence of TiO2 on the bead surface with a little loss of TiO2 coating after repeated cycles (Figure 9a). The presence of Fe, Ti, and O was confirmed by the appearance of distinct peaks in EDS.
(a) SEM–EDS of recycled TiO2 coated FA + FS spherical beads. (b) UV–Vis DRS of TiO2 and recycled composite.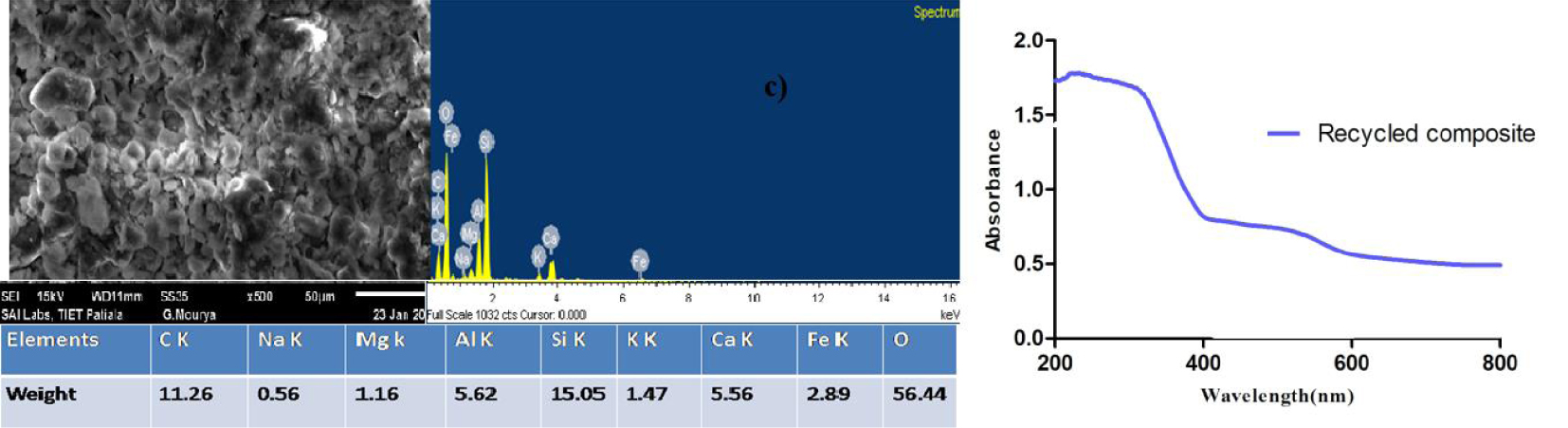
The evaluation of the band gap energy by UV–Vis DRS of the recycled-catalyst-coated Fe composite yielded an energy of 3.10 eV for the recycled catalyst (beads), confirming the activity of the catalyst even after repeated use (Figure 9b).
The recycled composite was further evaluated using FTIR (Figure 10a). The graph followed the same pattern as that of the unused composite beads. The scan depicts the appearance of peaks in the range 800–1200 cm-1 for both the fresh and recycled spectra, verifying the presence of Ti–O–Ti bonds in both the samples. This proves the retention of TiO2 lattices in the case of immobilized beads. In the initial few cycles, TiO2 weathering off from the support. At the same time, the coating may not be uniform for all the beads. Furthermore, there are fewer dense pockets of TiO2 at some scattered locations on the beads.
For the structural analysis of the recycled catalyst, XRD analysis was carried out (Figure 10b). Along with the peaks indicating the basic characteristics of TiO2 (i.e., anatase and rutile phases), the peaks of iron oxides were also observed, confirming the dual nature of the catalyst even after recycling.
3.9. Mineralization
The mineralization study of isoproturon was carried out to determine the presence of anions such as nitrite and nitrate. There was reduction in COD during the treatment process, thereby verifying the increase in degradation as shown in Figure 11c. The reduction in TOC was also studied for confirming the mineralization of isoproturon (Figure S3). There is decrease in the concentration of nitrate ions for the duration of the experiment as there is a possibility of these ions being converted into nitrite ions. This is indicated by the effective increase in the concentration of nitrite ions as shown in Figure 11a,b,d. The relation between the degradation of isoproturon and the formation of various ions of nitrate, nitrite, and ammonium has also been studied (Figure S4).
(a) FTIR of TiO2, fresh composite. (b) UV–Vis DRS of TiO2 and fresh composite.
Mineralization analysis of isoproturon in terms of (a) nitrate, (b) nitrite, (c) COD reduction, and (d) ammonical nitrogen.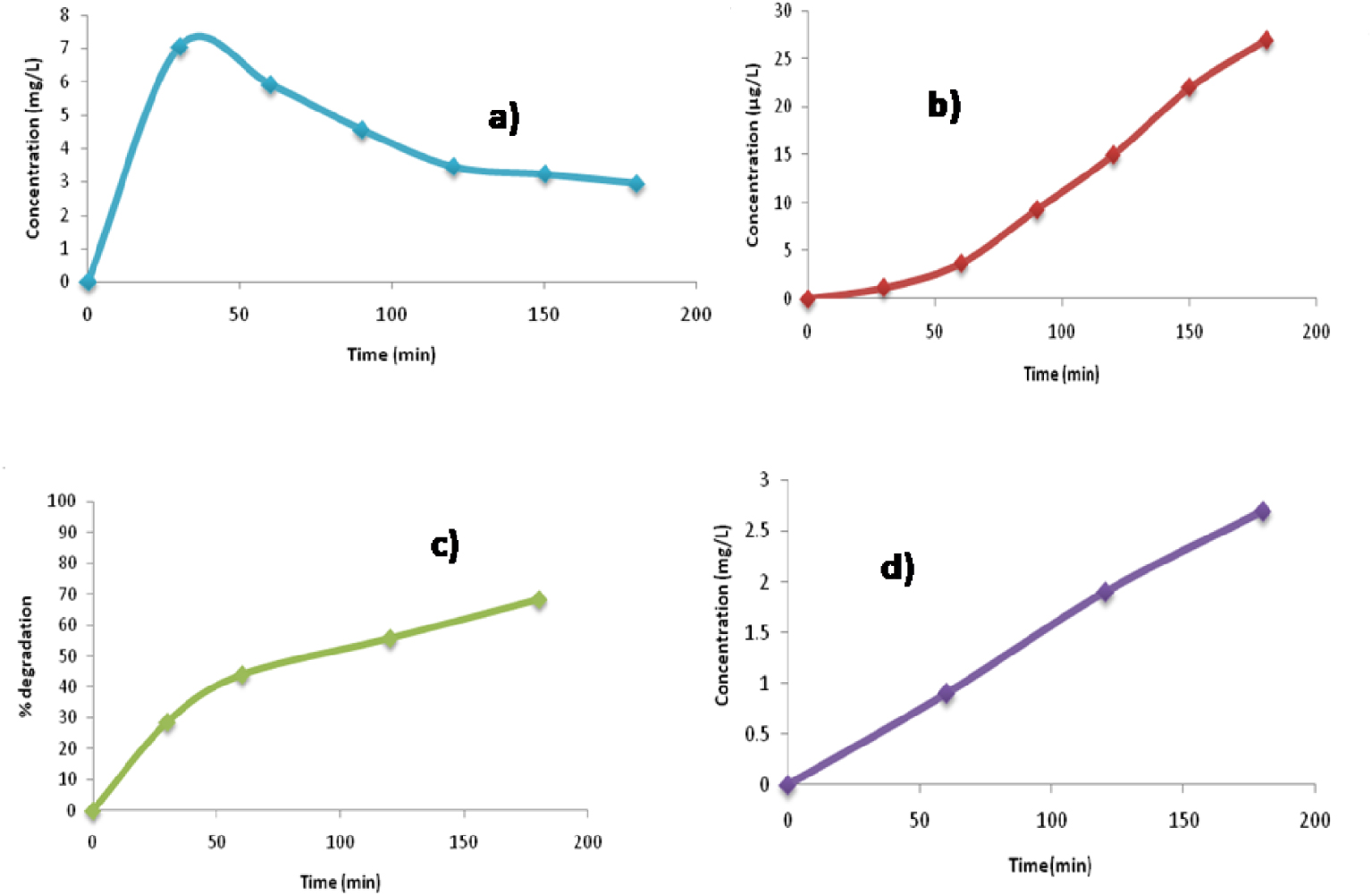
The complete mineralization of the compound could be proved by GC–MS analysis for identification of the compounds formed during degradation. The proposed degradation mechanism (Figure 12) can follow two distinct routes of hydroxylation. Further fragmentation occurs by hydrodenitrogenation and yields 2-amino-5-isopropylbenzene-1,4-diol. The intermediate formed undergoes final fragmentation to produce acetaldehyde, 2-methyl butane, nitrate, nitrite, nitrogen, carbon dioxide, and water.
Proposed mechanism for degradation of isoproturon.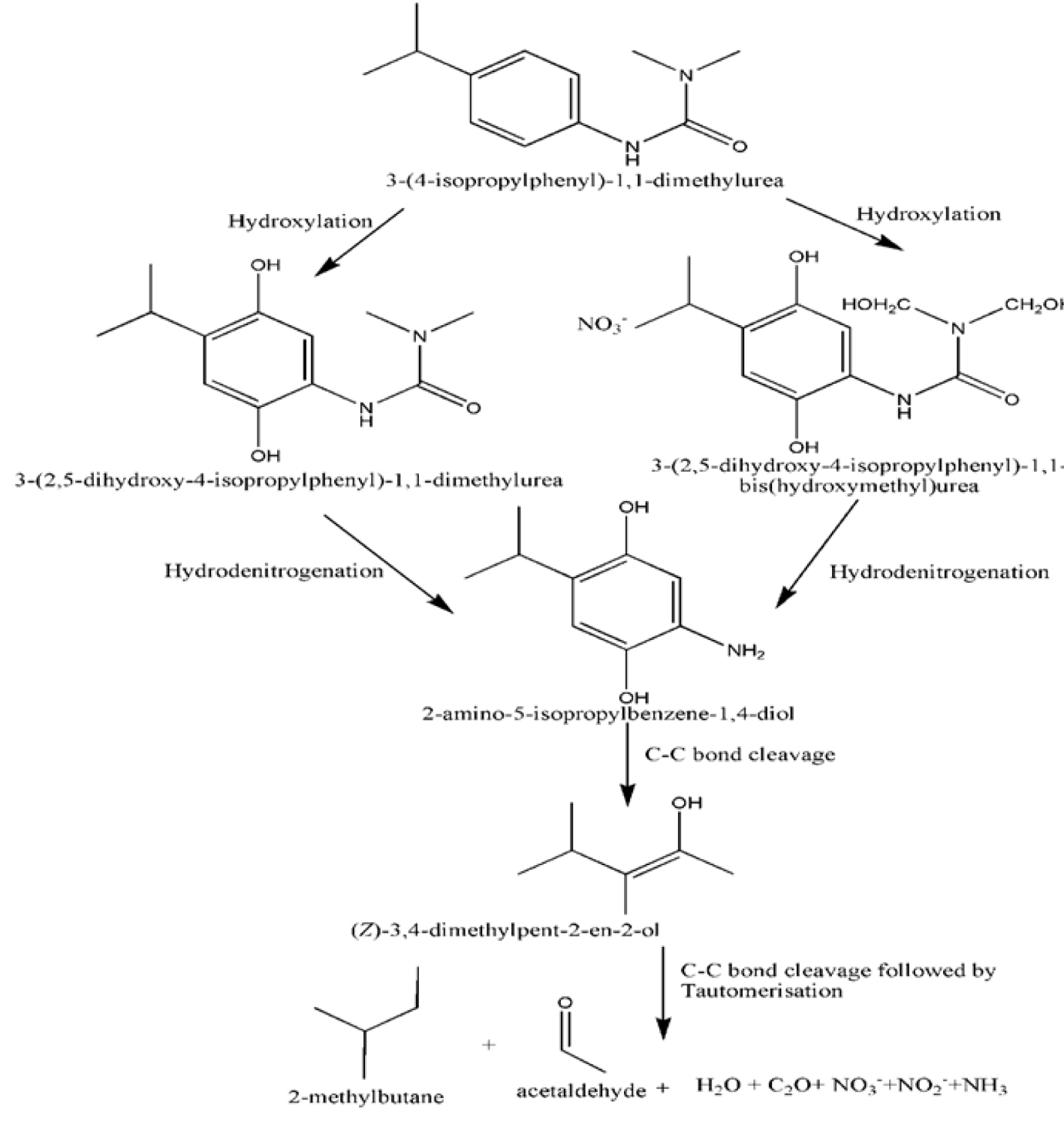
4. Conclusion
A hybrid concept of integrating an in situ dual effect of photo-Fenton and photocatalysis utilizing waste materials such as FS and FA has been successfully established for degradation of the compound isoproturon. The optimized conditions were specified using RSM based on BBD, which included H2O2 dose (700 mg⋅L-1), number of composite beads (42) (i.e., covering 75% of the reactor’s area), volume of wastewater (190 mL), and treatment time (176 min) showing degradation up to 80.96%. The integrated binary process followed pseudo-first-order kinetics, and overall 1.8 times synergy was obtained over the respective individual processes. Through the characterization of composite beads using SEM–EDS, UV–DRS, XRD, and FTIR, the presence of iron along with TiO2 on the surface of beads was confirmed, hence proving the stability and utility of the integrated process. Mineralization of isoproturon by the production of nitrate, nitrite, and ammonia along with reduction in COD verified the degradation of the compound. The degradation mechanism was determined by identifying intermediates through GC–MS analysis. Hence, the integrated binary process using composite beads would provide a better alternative for treating wastewater containing pesticides.



 CC-BY 4.0
CC-BY 4.0